A new green catalyst: 1,3,5-triazine-functionalized bisimidazolium dichloride tethered SPION catalyzed Betti synthesis†
Mehdi
Shafiee
,
Ahmad R.
Khosropour
*,
Iraj
Mohammadpoor-Baltork
*,
Majid
Moghadam
,
Shahram
Tangestaninejad
and
Valiollah
Mirkhani
Catalysis Division, Department of Chemistry, University of Isfahan, 81746-73441, Isfahan, Iran. E-mail: khosropour@chem.ui.ac.ir; imbaltork@sci.ui.ac.ir; Fax: +98 3116689732; Tel: +98 3117932700
First published on 24th July 2012
Abstract
A unique dicationic ionic liquid tethered to superparamagnetic iron oxide nanoparticles as a green and powerful catalyst for the efficient synthesis of Betti bases in high to excellent yields has been evaluated. Due to the high magnetization of the catalyst, it can be satisfactorily recovered by a simple external magnet. The catalyst could then be recycled and reused at least six times without any loss of activity.
Introduction
Since the first reports on task specific ionic liquids (TSILs) have appeared in the past decade,1 a wide variety of these materials with tailored compositions which possess different properties have been synthesized.2–5 The chemical properties of TSILs can be modified with different chemical groups, by which their functionality and textural properties could be changed.6 Within this context, these compounds have attracted increasing amounts of attention in both academic and industrial fields owing to their unique properties, such as catalytic activity.7The advantage of utilizing TSILs as environmentally friendly catalysts can be higher if efficient recovery and reuse of the catalyst can be performed, so improving the greenness of the process. In this context, one possible route is the immobilization of catalytic TSILs onto inorganic or polymer supports, which produces a new class of catalyst systems.8 In these systems, a thin film of TSIL is dispersed over the large internal surface of the support. Utilizing nano-supports has attracted considerable interest in recent years due to their high activity and environmental acceptability.9 Although these materials offer a high specific surface area for the active component, which enhances the contact between the reactants and supported catalyst, they are easily agglomerated and are difficult to separate. Therefore, to overcome this problem, it is essential to design an easily recoverable catalyst.
Currently, various nano-catalysts which employ magnetic separation properties have been developed in a number of organic transformations.10 These magnetic nanoparticles (MNPs) show excellent catalytic activities. The magnetic separation method presents many advantages over conventional ones. It can be considered environmentally benign as the filtration steps are omitted in the reaction. For instance, the catalyst can be recovered with an external magnetic field.
To date, although TSILs moieties are widely used in organic synthesis, there are only a few examples of ionic liquids anchored to MNPs which are used as catalysts in organic transformations.11 In 2009, Zhao and co-workers reported the first example of an imidazole-based ionic liquid grafted onto SPION as a catalyst for the Knoevenagel condensation reaction.12 SPION-decorated ionic liquids have also been used as catalysts for the cycloaddition of CO2 and epoxides to produce cyclic carbonates.13 Very recently, Lue et al. reported a magnetic nanoparticle-supported acidic IL as a catalyst for the synthesis of benzoxanthenes.14 As a result, the ionic liquid based-catalysts bearing imidazolium groups are apparently more effective than catalysts with other groups.15 However, to the best of our knowledge, multicomponent reactions promoted by dicationic ionic liquids based on imidazolium cations anchored to MNPs as a new class of eco-friendly catalysts has not been reported.
A one-pot multicomponent reaction is a useful method for the synthesis of complex chemotypes which saves time, allows instantaneous access to large compound libraries with diverse functionalities and avoids costly purification processes by the systematic variation of the starting material, which could be either commercially available or easily prepared.16
One of the classic multicomponent reactions is the Betti bases synthesis.17 The classical Betti reaction is a three-component reaction between an aldehyde, ammonia and β-naphthol.18 This reaction was a milestone in organic synthesis and was the method used to obtain amidoalkyl naphthols17 which, in turn, are very important precursors for the synthesis of important bioactive 1-aminomethyl-2-naphthols, their bradycardia and hypotensive effects in humans having been evaluated.19 These are attractive compounds as chiral ligands in enantioselective reactions.20 Moreover, they can be used as chiral shift reagents for carboxylic acids or chiral auxiliaries for the synthesis of α-aminophosphonic acids.21 Several studies have concentrated on the catalysis of the reaction, using different catalytic systems.22 Nevertheless, in the most recent report, separation of the catalyst from the reaction mixture was carried out via conventional methods, which lead to leaching of the utilized catalysts into the environment and the use of toxic organic solvents, which are critical disadvantages of these protocols.
Now, encouraged by previous results and our interest in developing efficient, sustainable and greener pathways for organic transformations,23 we report herein a simple synthesis for a new dicationic ionic liquid with a 1,3,5-triazine core anchored to nano-superferromagnetic particles and its application in the Betti reaction to synthesize β-amidoalkylnaphthols via a one-pot multicomponent reaction.
The synthetic path for the catalyst is depicted in Scheme 1.
 | ||
| Scheme 1 The synthesis path of 1,3,5-triazine-functionalized bisimidazolium dichloride tethered to SPIONs. | ||
The free-dicationic ionic liquid (Cl-ACl2) was first prepared via the reaction of 1,3,5-trichlorotriazine (TCT) with two equimolar N-methylimidazole in dry dioxane. The key factor in the synthesis was a nucleophilic substitution reaction between TCT and N-methylimidazole to generate the desired bisimidazolium salt. Finally, the ionic liquid was anchored to the SPIONs by an ipso-substitution reaction with the OH group of silica-encapsulated Fe3O4 nanoparticles.
SPION-ACl2 was characterized by means of fourier transform infrared spectroscopy (FTIR), nuclear magnetic resonance (NMR), thermal gravimetric analysis (TG), vibrating sample magnetometry (VSM) and transmission electron microscopy (TEM).
Fig. S1 (see ESI†) illustrates the FTIR spectra of Fe3O4 (a), silica-encapsulated Fe3O4 (b), the free-dicationic ionic liquid (Cl-ACl2) (c) and the SPION-ACl2 nanocatalyst (d), respectively.
The spectral features of ACl2 are observed for SPION-ACl2 by the presence of bands at 3141, 2953, 1720, 1440, 805 and 620 cm−1. These bands are absent in the spectrum of the silica-encapsulated Fe3O4 nanoparticles and confirm the presence of ACl2 on SPION.
The thermal stability of SPION-ACl2 was also evaluated by TGA-DTG and the thermograms are given in Fig. S2 (see ESI†). These curves show about 5.04% weight loss below 120 °C, suggesting that 1 mg of SPION-ACl2 still contained about 0.0028 mmol of H2O, which it is due to the removal of adsorbed water. The decomposition temperature (Td) was also calculated from the TGA-DTG curves. As exhibited in Fig. S2 (ESI†), the Td value was in the range of ∼200–460 °C with about 33 wt.% loss for ACl2. The remaining ca. 60% weight is revealed to be the silica-encapsulated Fe3O4 nanoparticles, which illustrates that the ratio between the organic moiety (ACl2) and the inorganic phase is 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60% approximately.
60% approximately.
The dc magnetic characterization of SPION-ACl2 and the neat silica-encapsulated Fe3O4 nanoparticles were examined at room temperature in an external field range of ±10 kOe (Fig. S3, ESI†).
In this investigation, due to the functionalization of the silica-encapsulated Fe3O4 by ACl2, Ms (the saturation magnetization) is found to be 20.2 emu g−1, which is considerably lower than that of the bulk magnetite (40.6 emu g−1, Fig. S3, ESI†).
To study the morphology characteristics of SPION-ACl2, TEM images were also investigated (Fig. 1).
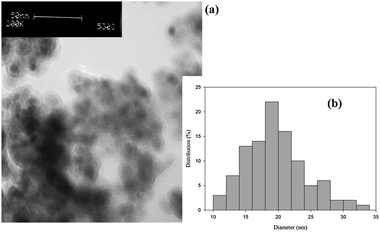 | ||
| Fig. 1 (a) TEM image of SPION-ACl2 and (b) SPION-ACl2 particle size distribution histogram. | ||
The TEM images of SPION-ACl2 revealed that it appears to have an almost spherical structure with a narrow size distribution (the average particle size is 10–35 nm, Fig. 1b), thereby maintaining a nanocrystalline appearance. Thus, the enormous active sites of this nanoparticle may allow for excellent activity in organic transformations, even with a low catalyst loading.
In order to show the catalytic activity of SPION-ACl2 in a Betti reaction, a comparison between the type and texture of the catalyst was examined in a condensation reaction of 4-nitrobenzaldehyde (1 mmol), β-naphthol (1 mmol) and acetamide (1.2 mmol) as a template reaction under solvent-free conditions (Table 1, entries 1–9).
| Entry | Catalyst | Temp. (°C) | Yielda (%) |
|---|---|---|---|
| Reaction conditions: 4-nitrobenzaldehyde (1 mmol), β-naphthol (1 mmol), acetamide (1.2 mmol) and catalyst after 20 min.a Isolated yield. | |||
| 1 | SPION-ACl2 (50 mg, 6 mol%) | 100 °C | 94 |
| 2 | SPION-ACl2 (40 mg, 4.8 mol%) | 100 °C | 81 |
| 3 | — | 100 °C | N.R. |
| 4 | Nano-SiO2 | 100 °C | 2 |
| 5 | Fe3O4 | 100 °C | 7 |
| 6 | SPION-A(PF6)2 (50 mg, 6 mol%) | 100 °C | 83 |
| 7 | SPION-A(NTf2)2 (50 mg, 6 mol%) | 100 °C | 77 |
| 8 | [bmim]Cl (6 mol%) | 100 °C | <15 |
| 9 | Cl-ACl2 (6 mol%) | 100 °C | 65 |
| 10 | β-naphthol-ACl2 (6 mol%) | 100 °C | 37 |
| 11 | CH3CONH-ACl2 (6 mol%) | 100 °C | 44 |
| 12 | SPION-ACl2 (50 mg, 1.7 mol%) | 100 °C | 40 |
| 13 | SPION-ACl2 (50 mg, 2.5 mol%) | 100 °C | 60 |
| 14 | SPION-ACl2 (50 mg, 5 mol%) | 100 °C | 85 |
| 15 | SPION-ACl2 (50 mg, 6 mol%) | 110 °C | 94 |
| 16 | SPION-ACl2 (50 mg, 6 mol%) | 120 °C | 95 |
| 17 | SPION-ACl2 (50 mg, 6 mol%) | 90 °C | 80 |
Surprisingly, in the presence of SPION-ACl2 (50 mg, 6 mol%), quantitative conversion was registered in 20 min and 94% of the Betti adduct, 2h, was isolated (Table 1, entry 1). Notably, lower yields were obtained when the same reaction was carried out while utilizing a lower catalyst loading (Table 1, entry 2).
No reaction occurred in the absence of a catalyst (Table 1, entry 3) and no valuable yield was obtained in the presence of nano-SiO2 or Fe3O4 without ACl2 (Table 1, entries 4 and 5). In contrast, using ionic liquids with different counter anions,25 such as A(PF6)2 or A(NTf2)2 instead of ACl2, evidently gave the product in lower yields (Table 1, entries 6 and 7). It is surprising that the desired product was obtained in small amounts in the presence of 1-butyl-3-methyl-imidazolium chloride (<15%), while the conversion of 2h while utilizing Cl-ACl2 was 65% (Table 1, entries 8 and 9). It can be attributed to the fact that Cl-ACl2 is not stable in the reaction and part of it is introduced into the reaction with the substrates (and was then consumed) and β-naphthol-ACl2 and CH3CONH-ACl2, two of the byproducts, were detected. A closer examination illustrated that when the Cl-moiety of Cl-ACl2 was replaced with functionalities such as β-naphthol or CH3CONH and these were used as the catalyst instead of SPION-ACl2, the yield was drastically reduced (Table 1, entries 10 and 11). On the other hand, the catalyst loading on the support was also investigated (Table 1, entries 12–14). As shown, the best result was obtained for 6 mol% loading. After further investigation, we also found that the reaction temperature can also be greatly affected in this transformation.
The obvious improvement in the conversion (94%) was achieved for the reaction under solvent-free conditions at 100 °C. A higher reaction temperature (110–120 °C) made no obvious difference to the yield of the products but using a lower reaction temperature (90 °C), sharply decreased the conversion to approximately 80%, even with longer reaction times (Table 1, entries 15–17). Accordingly, performing the reaction at 100 °C under solvent-free conditions is the optimal condition for the Betti reaction in the presence of SPION-ACl2.
After the successful generation of 2h, we considered introducing more diversities into the β-amidoalkylnaphthol scaffold via the one-pot reaction. Therefore, with the optimized reaction conditions in hand, we examined several aryl aldehydes and found that SPION-ACl2 is an efficient catalyst for the synthesis of a large spectrum of Betti bases through this reaction (Table 2).
| Entry | Ar | R | Time (min) | Product | Yielda (%) | TOF (h−1) |
|---|---|---|---|---|---|---|
| TOF = (mmol of product/mmol of catalyst)/time (h).a Isolated yield. | ||||||
| 1 | C6H5 | CH3 | 30 | 2a | 85 | 28.4 |
| 2 | 2-ClC6H4 | CH3 | 25 | 2b | 87 | 34.8 |
| 3 | 4-ClC6H4 | CH3 | 20 | 2c | 95 | 47.5 |
| 4 | 2-BrC6H4 | CH3 | 25 | 2d | 90 | 36.0 |
| 5 | 3-BrC6H4 | CH3 | 20 | 2e | 94 | 47.0 |
| 6 | 4-BrC6H4 | CH3 | 25 | 2f | 89 | 35.6 |
| 7 | 4-FC6H4 | CH3 | 15 | 2g | 83 | 55.4 |
| 8 | 4-NO2C6H4 | CH3 | 20 | 2h | 94 | 48.5 |
| 9 | 3-NO2C6H4 | CH3 | 20 | 2i | 92 | 47.0 |
| 10 | 4-CNC6H4 | CH3 | 20 | 2j | 92 | 46.0 |
| 11 | 4-CH3C6H4 | CH3 | 35 | 2k | 85 | 24.3 |
| 12 | 4-CH3OC6H4 | CH3 | 35 | 2l | 82 | 23.4 |
| 13 | C6H5 | C6H5 | 30 | 2m | 86 | 28.7 |
| 14 | 2-ClC6H4 | C6H5 | 25 | 2n | 89 | 35.6 |
| 15 | 4-ClC6H4 | C6H5 | 20 | 2o | 90 | 45.0 |
| 16 | 2-BrC6H4 | C6H5 | 25 | 2p | 88 | 35.2 |
| 17 | 3-BrC6H4 | C6H5 | 25 | 2q | 94 | 37.6 |
| 18 | 4-BrC6H4 | C6H5 | 25 | 2r | 97 | 38.8 |
| 19 | 4-FC6H4 | C6H5 | 15 | 2s | 92 | 61.4 |
| 20 | 4-NO2C6H4 | C6H5 | 20 | 2t | 95 | 47.5 |
| 21 | 3-NO2C6H4 | C6H5 | 20 | 2u | 92 | 46.0 |
| 22 | 4-CNC6H4 | C6H5 | 20 | 2v | 95 | 47.5 |
| 23 | 4-CH3C6H4 | C6H5 | 30 | 2w | 80 | 26.7 |
| 24 | 4-CH3OC6H4 | C6H5 | 35 | 2x | 81 | 23.1 |
It was generally observed that high to excellent yields of the products were obtained in all cases almost irrespective of the type of substituent present in the aromatic ring of the aryl aldehyde, and the β-amidoalkylnaphthol derivatives, 2a–2x, were exclusively obtained with turnover frequencies (TOF) of 23.1–61.4 h−1 (Table 2). It was found that aryl aldehydes carrying electron-donating groups could be smoothly converted to the desired products (Table 2, entries 11 and 12).
The coupling of aryl aldehydes containing electron-withdrawing groups also afforded the corresponding Betti bases in high to excellent isolated yields (85–98%, Table 2, 2b–2j). Moreover, this reaction works well with phenylacetamide (Table 2, entries 13–24). The successful production of the Betti base derivatives indicated that the one-pot reaction is a general route for this transformation. Thus, based on these considerations, we explored a new and facile one-pot procedure for the synthesis of β-amidoalkylnaphthols derivatives.
The SPION-ACl2 catalyst prepared in this assay could be easily recovered by an applied magnetic field at the end of the reaction. The investigation of a solvent-free Betti reaction using SPION-ACl2 was repeated six times to evaluate the catalyst's recyclability and stability (Fig. 2). After each run, the catalyst was separated from the reaction mixture by an applied magnetic field, washed with absolute ethanol and reused in the next cycle, which indicates the high stability of the catalyst under these reaction conditions (Fig. 2).
 | ||
| Fig. 2 Reuse of SPION-ACl2 in the reactions: 4-nitrobenzaldehyde 1h (1 mmol), β-naphthol (1 mmol), acetamide (1.2 mmol) and SPION-ACl2 (50 mg, 6 mol%) stirred at 100 °C for 20 min. | ||
On the basis of these results, we propose a plausible pathway for the reaction (Scheme 2). The carbonyl group of aldehyde 1 may be activated by the coordination of the imidazolium cations of the catalyst, which may subsequently be attacked by the amide to afford the intermediate B. The reaction of Bvia β-naphthol affords the corresponding Betti adduct.
 | ||
| Scheme 2 Proposed mechanism. | ||
In conclusion, we have demonstrated that SPION-ACl2 acts as a unique and robust nano-catalyst for the efficient synthesis of Betti bases. This catalysis reaction furnishes a green, useful and practical procedure for the direct synthesis of β-amidoalkylnaphthols owing to the following profits: (a) the catalyst is very powerful, inexpensive and highly recyclable, (b) the highly efficient synthesis and separation processes of β-amidoalkylnaphthols, based on the present catalytic system, are promising for large-scale applications in an eco-friendly manner, (c) to the best of our knowledge, the synthesis of β-amidoalkylnaphthols in the presence of SPIONs as a modern nano-catalyst has not been achieved to date. This new bis-IL tethered SPION catalyst is a unique type of nano-superferromagnetic catalyst and may be applied to develop new catalytic systems for environmentally-friendly organic syntheses.
Experimental
All chemicals were of commercial reagent grade and were purified before use. All known organic products were identified by a comparison of their physical and spectral data with those of authentic samples. SPIONs (silica-encapsulated Fe3O4) were prepared according to a previously reported method.24 Thin layer chromatography (TLC) was performed on UV-active aluminium-backed plates of silica gel (TLC Silica gel 60 F254). FT–IR spectra were obtained as potassium bromide pellets in the range of 400–4000 cm−1 with a Nicolet Impact 400D instrument. 1H, and 13C NMR spectra were measured on a Bruker DPX 400 MHz. The TEM images were taken with a Philips CM120 unit operating at 200 kV. The TGA curve was obtained with a heating rate of 10 °C per min on a TG 50 Mettler thermogravimetric analyzer. The magnetic measurements were performed with a vibrating sample magnetometer (VSM) at Meghnatis Daghigh Kavir Co.Synthesis of Cl-ACl2
A mixture of 1,3,5-trichlorotriazine (184 mg, 1 mmol) and N-methylimidazole (164 mg, 2 mmol) in 10 mL of dry dioxane was magnetically stirred for 5 h under reflux conditions. At the end of the reaction, the white precipitate was filtered, washed three times with THF (3 × 5 ml) and dried under reduced pressure at 60 °C. The 1H and 13C NMR spectra are given below:1H-NMR (400 MHz, D2O) δH (ppm) 3.98 (s, N-CH3), 7.58 (t, 1H), 8.20 (t, 1H), 9.73 (s, 1H). 13C-NMR (100 MHz, D2O) δC (ppm) 36.70, 119.00, 124.99, 136.47, 161.06, 170.59.
Synthesis of SPION-ACl2
The SPIONs (silica-encapsulated Fe3O4), (60 mg) were first dispersed in 4 mL of dry THF by sonication in an ultrasonic bath for 15 min to form a brownish solution. The building block Cl-ACl2 (40 mg, 0.12 mmol) was then added. After purging the mixture with N2 for 5 min, the flask was sealed under a N2 atmosphere with a rubber stopper. The mixture was sonicated for 1 h. The nanoparticles were then separated and collected by an external magnet, washed three times with THF (3 mL) and dried under reduced pressure at 80 °C. 50 mg of SPION-ACl2 (3 mmol per ACl2) was obtained.General procedure for the synthesis of Betti bases catalyzed by SPION-ACl2
Aryl aldehyde (1 mmol), amide (1.2 mmol) and β-naphthol (1 mmol) were added to a reaction tube charged with a magnetic stirrer bar and SPION-ACl2 (50 mg, 6 mol%). The reaction mixture was stirred at 100 °C for 15–35 min (as indicated by the TLC). Most of the nanoparticles were adsorbed onto the magnetic stirrer bar when the stirring was stopped. After cooling to room temperature, the catalyst was collected by an external permanent magnet, washed two times with absolute ethanol (2 × 1 mL), air-dried and used directly for the next round of the reaction without further purification. After the separation of the catalyst, the collected solution was added to the residue of the reaction mixture and the volatiles were removed in vacuo. Recrystallisation of the solid product from ethanol–water afforded the pure corresponding product in 80–97% yields.All the products are known in the literature and were identified by a comparison of their FT-IR, 1H, and 13C NMR spectra with the literature data.22 As a sample, the characterization data for 2K is given below.
Acknowledgements
The support of this work by the Center of Excellence of Chemistry of the University of Isfahan (CECUI) is acknowledged.Notes and references
- (a) J. D. Holbrey and K. R. Seddon, Clean Prod. Processes, 1999, 1, 223 Search PubMed; (b) J. H. Davis, Chem. Lett., 2004, 33, 1072 CrossRef CAS; (c) T. Welton, Coord. Chem. Rev., 2004, 248, 2459 CrossRef CAS.
- N. V. Plechkovaa and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123 RSC.
- (a) S. Yu, S. Lindeman and C. D. Tran, J. Org. Chem., 2008, 73, 2576 CrossRef CAS; (b) R. Giernoth, Angew. Chem., Int. Ed., 2010, 49, 2 CrossRef.
- Q. Zhang, S. Zhang and Y. Deng, Green Chem., 2011, 13, 2619 RSC.
- (a) A. B. Pereiro, J. M. M. Araujo, J. M. S. S. Esperanca, I. M. Marrucho and L. P. N. Rebelo, J. Chem. Thermodyn., 2012, 46, 2 CrossRef CAS; (b) T. Yu and R. G. Weiss, Green Chem., 2012, 14, 209 RSC.
- (a) W. L. Hough, M. Smiglak, H. Rodńguez, R. P. Swatloski, S. K. Spear, D. T. Daly, J. Pernak, J. E. Grisel, R. D. Carliss, M. D. Soutullo, J. H. Davis and R. D. Rogers, New J. Chem., 2007, 31, 1429 RSC; (b) N. Liu, C. Liu and Z. Jin, Green Chem., 2012, 14, 592 RSC.
- (a) T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206 CrossRef CAS; (b) X. Ding, W. Tang, C. Zhu and Y. Cheng, Adv. Synth. Catal., 2010, 352, 108 CrossRef CAS; (c) C. B. Yue, D. Fang, L. Liu and T. F. Yi, J. Mol. Liq., 2011, 163, 99 CrossRef CAS.
- (a) A. Riisager, R. Fehrmann, M. Haumann and P. Wasserscheid, Top. Catal., 2006, 40, 91 CrossRef CAS; (b) Q. Gong, J. Klankermayer and B. Bluemich, Chem.–Eur. J., 2011, 17, 13795 CrossRef CAS; (c) R. A. Watile, D. B. Bagal, K. M. Deshmukh, K. P. Dhake and B. M. Bhanage, J. Mol. Catal. A: Chem., 2011, 351, 196 CrossRef CAS; (d) M. Haumann, M. Jakuttis, R. Franke, A. Schonweiz and P. Wasserscheid, ChemCatChem, 2011, 3, 1822 CrossRef CAS; (e) H. Hagiwara, K. Sato, T. Hoshi and T. Suzuki, Synlett, 2011, 2545 CrossRef CAS; (f) C. Fehér, E. Kriván, J. Hancsókb and R. Skoda-Földes, Green Chem., 2012, 14, 403 RSC.
- (a) Y. Hu, H. M. Yang, Y. C. Zhang, Z. S. Hou, X. R. Wang, Y. X. Qiao, H. Li, B. Feng and Q. F. Huang, Catal. Commun., 2009, 10, 1903 CrossRef CAS; (b) S. S. Moganty, N. Jayaprakash, J. L. Nugent, J. Shen and L. A. Archer, Angew. Chem., Int. Ed., 2010, 49, 9158 CrossRef CAS; (c) M. E. Mahmoud and M. H. Al-Bishri, Chem.–Eng. J., 2011, 166, 157 CrossRef CAS.
- (a) S. Shylesh, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef CAS; (b) E. Rafiee and S. Eavani, Green Chem., 2011, 13, 2116 RSC; (c) R. Arundhathi, D. Damodara, P. R. Likhar, M. L. Kantam, P. Saravanan, T. Magdaleno and S. H. Kwon, Adv. Synth. Catal., 2011, 353, 1591 CrossRef CAS; (d) R. Hudson, C.-J. Li and A. Moores, Green Chem., 2012, 14, 622 RSC; (e) C. Lian, H. Liu, C. Xiao, W. Yang, K. Zhang, Y. Liu and Y. Wang, Chem. Commun., 2012, 48, 3124 RSC.
- Q. Zhang, H. Su, J. Luo and Y. Wei, Green Chem., 2012, 14, 201 RSC.
- Y. Zhang, Y. Zhao and C. Xia, J. Mol. Catal. A: Chem., 2009, 306, 107 CrossRef CAS.
- X. Zheng, S. Luo, l. Zhang and J.-P. Cheng, Green Chem., 2009, 11, 455 RSC.
- Q. Zhang, H. Su, J. Luo and Y. Wei, Green Chem., 2012, 14, 201 RSC.
- C. B. Yue, D. Fang, L. Liu and T. F. Yi, J. Mol. Liq., 2011, 163, 99 CrossRef CAS.
- (a) T. J. J. Mueller, Beilstein J. Org. Chem., 2011, 7, 960 CrossRef CAS; (b) L. H. Choudhury and T. Parvin, Tetrahedron Lett., 2011, 67, 8213 CAS; (c) F. De Moliner, S. Crosignani, A. Galatini, R. Riva and A. Basso, ACS Comb. Sci., 2011, 13, 453 CrossRef CAS; (d) N. Isambert, M. D. S. Duque, J. C. Plaquevent, Y. Genisson, J. Rodriguez and T. Constantieux, Chem. Soc. Rev., 2011, 40, 1347 RSC; (e) E. Ruijter, R. Scheffelaar and R. V. A. Orru, Angew. Chem., Int. Ed., 2011, 50, 6234 CrossRef CAS.
- C. Cardellicchio, M. A. M. Capozzi and F. Naso, Tetrahedron: Asymmetry, 2010, 21, 507 CrossRef CAS.
- M. Betti, Org. Synth. Collect., 1941, 1, 381 Search PubMed.
- A. Y. Shen, C. T. Tsai and C. L. Chen, Eur. J. Med. Chem., 1999, 34, 877 CrossRef CAS.
- (a) C. Cimarelli, A. Mazzanti, G. Palmieri and E. Volpini, J. Org. Chem., 2001, 66, 4759 CrossRef CAS; (b) C. Cimarelli, G. Palmieri and E. G. Volpini, Tetrahedron: Asymmetry, 2002, 13, 2417 CrossRef CAS; (c) C. Cimarelli, G. Palmieri and E. Volpini, J. Org. Chem., 2003, 68, 1200 CrossRef CAS.
- K. E. Metlushka, B. A. Kashemirov, V. F. Zheltukhin, D. N. Sadkova, B. Büchner, C. Hess, O. N. Kataeva, C. E. McKenna and V. A. Alfonsov, Chem.–Eur. J., 2009, 15, 6718 CrossRef CAS.
- (a) M. M. Khodaei, A. R. Khosropour and H. Moghanian, Synlett, 2006, 916 CrossRef CAS; (b) H. R. Shaterian, H. Yarahmadi and M. Ghashang, Bioorg. Med. Chem. Lett., 2008, 18, 788 CrossRef CAS; (c) P. Zhang and Z. H. Zhang, Monatsh. Chem., 2009, 140, 199 CrossRef CAS; (d) M. Lei, L. Ma and L. Hu, Tetrahedron Lett., 2009, 50, 6393 CrossRef CAS; (e) A. R. Hajipour, Y. Ghayeb, N. Sheikhan and A. E. Ruoho, Tetrahedron Lett., 2009, 50, 5649 CrossRef CAS; (f) G. Rashinkar and R. Salunkhe, J. Mol. Catal. A: Chem., 2010, 316, 146 CrossRef CAS; (g) Q. Zhang, J. Luo and Y. Wei, Green Chem., 2010, 12, 2246 RSC; (h) J. Luo and Q. Zhang, Monatsh. Chem., 2011, 142, 923 CrossRef CAS; (i) B. Karmakar and J. Banerji, Tetrahedron Lett., 2011, 52, 4957 CrossRef CAS; (j) C. Cimarelli, D. Fratoni, A. Mazzanti and G. Palmieri, Eur. J. Org. Chem., 2011, 2094 CrossRef CAS.
- (a) M. Rostami, A. R. Khosropour, V. Mirkhani, I. Mohammadpoor-Baltork, M. Moghadam and S. Tangestaninejad, Synlett, 2011, 1677 CAS; (b) M. Rostami, A. R. Khosropour, V. Mirkhani, I. Mohammadpoor-Baltork, M. Moghadam and S. Tangestaninejad, Monatsh. Chem., 2011, 142, 1175 CrossRef CAS; (c) M. Rostami, A. R. Khosropour, V. Mirkhani, M. Moghadam, S. Tangestaninejad and I. Mohammadpoor-Baltork, Appl. Catal., A, 2011, 397, 27 CrossRef CAS; (d) E. Ranjbakhsh, A. K. Bordbar, M. Abbasi, A. R. Khosropour and E. Shams, Chem.–Eng. J., 2012, 179, 272 CrossRef CAS.
- (a) W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62 CrossRef; (b) X. Q. Liu, Z. Y. Ma, J. M. Xing and H. Z. Liu, J. Magn. Magn. Mater., 2004, 270, 1 CrossRef CAS; (c) J. Park, K. J. An, Y. S. Hwang, J. G. Park, H. J. Noh, J. Y. Kim, J. H. Park, N. M. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891 CrossRef CAS.
- A(PF6)2 and A(NTf2)2 were synthesized via the “ionic liquids ion cross-metathesis” process, see: V. Zgonnik, C. Zedde, Y. Génisson, M.-R. Mazières and J.-C. Plaquevent, Chem. Commun., 2012, 48, 3185 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cy20187a |
| This journal is © The Royal Society of Chemistry 2012 |