Self-assembled monolayer coated gold-nanoparticle catalyzed aerobic oxidation of α-hydroxy ketones in water: an efficient one-pot synthesis of quinoxaline derivatives†
Tamalika
Bhattacharya
,
Tridib K.
Sarma
* and
Sampak
Samanta
*
Department of Chemistry, Indian Institute of Technology Indore, Indore-452017, India. E-mail: tridib@iiti.ac.in; sampaks@iiti.ac.in; Fax: +91-731-2364182; Tel: +91-731-243-8706
First published on 4th July 2012
Abstract
For the first time, 4-aminothiophenol self-assembled monolayer-coated gold-nanoparticles (Au-NPs) which catalyze the aerobic oxidation of aryl substituted α-hydroxy ketones to aryl 1,2-diketones are reported. In addition, a one-pot synthesis of quinoxalines has been successfully achieved via in situ oxidation of α-hydroxy ketones and subsequent condensation with aryl 1,2-diamines in water. This method offers the potential for simple self-assembled monolayer-coated Au-NPs to exhibit catalytic activity for the aerobic oxidation reaction in a green and efficient manner.
Introduction
The exploration of gold nanoparticles (Au-NPs) as catalysts has attracted tremendous attention in recent years in the context of developing environmentally friendly and sustainable routes to a myriad of important organic transformations.1 This development is fuelled by the benign character of Au-NPs and their simplistic synthesis with controlled sizes and compositions. The high activity of Au in the nanometer dimension has led to several reports of catalytically active Au-NP systems prepared in the presence of supports, such as poly(N-vinyl-2-pyrrolidone), polyaniline, coordination polymers, dendrimers and solid oxide surfaces such as TiO2, CeO2, SiO2, Al2O3etc.2 In these systems, investigations related to the catalytic activity of Au has been oriented towards the morphology of the nanoparticles and the nature of the support, where the Au-NPs are bound by weak coordination to the supported polymeric or solid surfaces. However, there is no report which studies the catalytic properties of Au-NPs, governed mainly by the intrinsic surface properties of the nanoparticles, while stabilized with only a self-assembled monolayer, e.g. surface bound via the strong Au–S bond. Undoubtedly, understanding the catalytic behaviour of self-assembled monolayer-coated Au-NPs would be helpful in establishing the guiding principles for the rational design of active Au-NP-based catalytic systems.As part of our continuing interest in the development of environmentally friendly protocols in organic transformations3 as well as the synthesis of nanoparticle-based composite materials,4 we have been trying to develop Au-NP-based mild catalytic systems for the oxidation of alcohols to give carbonyl compounds. In this regard, developing a simple self-assembled monolayer-coated Au-NP-based catalytic system for the oxidation of α-hydroxy ketones and the synthesis of biologically significant quinoxaline derivatives in water has great significance. Aryl substituted 1,2-diketones and quinoxaline derivatives are utilized as intermediates in the synthesis of chiral ligands and biologically active compounds.5,6 In addition to their medicinal use, these derivatives have also found technological importance in dyes, semiconductors, anticorrosion in mild steels, as photosensitive coatings in photocurable agents etc.7 Investigations, such as oxalyl chloride with organostannanes,8a oxidation of alkynes,8b aldehyde condensation8c and oxidation of alcohols8d,e,f on the preparation of benzil derivatives have been reported. Similarly, for the preparation of quinoxaline derivatives, generally acid or transition metal (Mn, Ru, Pd, Cu, Ce etc.) catalyzed condensations of an aryl 1,2-dicarbonyl compound with a 1,2-diamine have been reported.9 There are several other approaches reported for the synthesis of quinoxaline compounds, e.g. the combination of phenyl epoxides or phenacyl bromides with o-phenylenediamines, using catalysts such as Bi(0),10a HClO4/SiO2,10b β-cyclodextrin (β-CD)10c and TMSCl.10d Recently, a few reports of one pot syntheses of quinoxaline from α-hydroxy ketones using solid supports, such as KF/Al2O3,11a Ru/C in the presence of β–CD,11b manganese oxide octahedral molecular sieves (OMS-2),11c HgI2,11d RuCl2(PPh3)3–TEMPO,11e MnO211fetc. have been published. However, they often suffer from one or more disadvantages, such as the use of organic solvents, unsatisfactory product yields, tedious experimental procedures, non-catalytic, multi step, harsh reaction conditions, difficult operation etc. To overcome all these disadvantages, the development of an effective nanoparticle-based catalytic system is highly desirable for the synthesis of benzils and quinolaxine derivatives that leads to better yields under mild reaction conditions, specifically in water, which is very significant in the context of green chemistry.
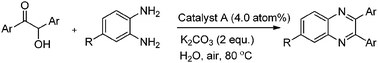
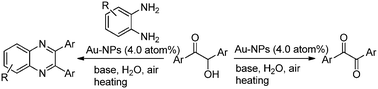
Herein, we report an efficient, simple and green procedure for the aerobic oxidation of aryl substituted α-hydroxy ketones to afford 1,2-diketones in water (Scheme 1), catalyzed by 4-aminothiophenol self-assembled monolayer-coated Au-NPs. We also extended this procedure for the one-pot direct synthesis of aryl substituted quinoxalines via in situ oxidation and condensation reactions between aryl substituted α-hydroxy ketones with aryl 1,2-diamines (Scheme 1).
 | ||
| Scheme 1 Syntheses of aryl 1,2-diketones and quinoxaline derivatives. | ||
Results and discussion
The 4-aminothiophenol monolayer-coated Au-NPs (A) were prepared in a one-pot synthetic procedure using 4-aminothiophenol as both the reducing and stabilizing agent, according to a literature procedure.12 The Au-NPs were stabilized by thiolates adsorbed onto the surfaces of the nanoparticles. Fig. 1a depicts a representation of the self-assembled monolayer formation of 4-aminothiophenol on the surfaces of the Au-NPs. The appearance of a plasmon resonance band at 530 nm (as shown in Fig. 1b) confirmed the formation of Au-NPs. The formation of Au-NPs was further confirmed from the powder X-ray diffraction (XRD) pattern, where the intense peaks corresponding to the (111), (200) and (220) Braggs' reflections are in good agreement with those reported for Au nanoparticles4a (Fig. 1c). The TEM images taken of the synthesized Au-NPs from the DMF–water mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), as shown in Fig. 1d, indicated the formation of Au-NPs with an average diameter of 10 ± 3 nm (see the histogram in the ESI†). In order to compare the catalytic properties of the self-assembled monolayer systems which have terminal thiolate functional groups on the Au surface, we also synthesized undecanethiol-coated Au-NPs (catalyst B) (details in the ESI†).
1), as shown in Fig. 1d, indicated the formation of Au-NPs with an average diameter of 10 ± 3 nm (see the histogram in the ESI†). In order to compare the catalytic properties of the self-assembled monolayer systems which have terminal thiolate functional groups on the Au surface, we also synthesized undecanethiol-coated Au-NPs (catalyst B) (details in the ESI†).
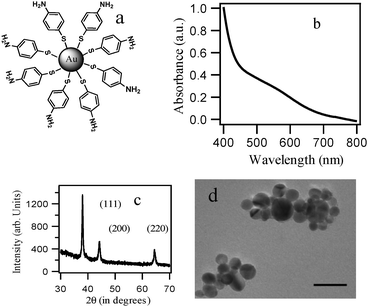 | ||
| Fig. 1 (a) Schematic presentation of 4-aminothiophenol-coated Au-NPs (catalyst A). (b) UV-visible spectra of the Au-NPs formed using 4-aminothiophenol as both the reducing and stabilizing agent in a DMF–water mixture. (c) XRD pattern of the Au-NPs. The corresponding lattice planes are marked. (d) TEM images of the Au-NPs in a DMF–water mixture, scale bar 20 nm. | ||
We chose the model reaction between benzoin (1.0 mmol), K2CO3 (2.0 mmol) and water (10 mL) in the presence of catalyst B (2.5 atom%) at 60 °C for 4 h under air. Although the reaction did not progress well, we were able to isolate the oxidized product in a 15% yield (Table 1, entry 5). As a consequence, this significant result prompted us to investigate the aerobic oxidation of benzoin in detail. It was observed that the reaction proceeded very slowly in the absence of base, catalyst and air (entries 1–3 and 12, Table 1). To improve the catalytic activity of this reaction, catalyst A was examined. As shown in Table 1, with a 2.5 atom% loading of catalyst A, the desired oxidized product benzil was obtained in a 22% yield after reacting at 40 °C for 4 h (entry 6). For this catalyst, on further increasing the reaction temperature to 80 °C, the yield improved dramatically from 22% to 83% for the same reaction time (entry 8). Among the bases, K2CO3 was the best choice compared to Na2CO3 and Cs2CO3 in terms of reactivity under similar reaction conditions (entries 14 and 15). In particular, there was a substantial enhancement in the yield when the amount of catalyst was increased from 2.5 to 4 atom%. From various reaction conditions, as shown in Table 1, it is obvious that a superior result was obtained under the conditions mentioned in entry 11 (91% yield). In particular, there was no significant improvement in yield when the reaction was carried out in the presence of oxygen instead of air (entry 13).
| Entry | Catalysts | Base | Temp (°C) | Yielda (%) |
|---|---|---|---|---|
| Unless otherwise specified, all reactions were carried out with benzoin (1.0 mmol), base (2.0 mmol) and water (10 mL) in the presence of air at the specified temperature and catalysts.a Isolated product after column chromatography.b Reaction was carried out in an argon atmosphere.c Reaction was carried out in an oxygen atmosphere. | ||||
| 1 | Nil | Nil | 60 | 2 |
| 2 | Nil | Na2CO3 | 60 | 6 |
| 3 | Nil | K2CO3 | 60 | 10 |
| 4 | A (4.0 atom% ) | Nil | 80 | 3 |
| 5 | B (2.5 atom%) | K2CO3 | 60 | 15 |
| 6 | A (2.5 atom%) | K2CO3 | 40 | 22 |
| 7 | A (2.5 atom%) | K2CO3 | 60 | 38 |
| 8 | A (2.5 atom%) | K2CO3 | 80 | 83 |
| 9 | A (0.8 atom%) | K2CO3 | 80 | 41 |
| 10 | A (1.6 atom%) | K2CO3 | 80 | 59 |
| 11 | A (4.0 atom%) | K2CO3 | 80 | 91 |
| 12 | A (4.0 atom%) | K2CO3 | 80 | 7b |
| 13 | A (4.0 atom%) | K2CO3 | 80 | 92c |
| 14 | A (4.0 atom%) | Na2CO3 | 80 | 27 |
| 15 | A (4.0 atom%) | Cs2CO3 | 80 | 83 |
To understand the scope and limitation of this novel aerobic oxidation reaction, we studied several aryl substituted α-hydroxy ketones using the self-assembled monolayer-coated Au-NPs, catalyst A (4 atom%), at standard reaction conditions and the results are compiled in Table 2. As is evident from Table 2, aryl α-hydroxy ketones with various substituents on the aromatic ring all produced the desired oxidized product in good to excellent yields (entries 2–4). It should be pointed out that electron withdrawing substituents (entry 4) on the aromatic ring increased the yield when compared to electron donating groups (entries 2 and 3). Hetero-aryl groups also afforded the desired oxidized products in good yields at 60 °C. The reaction conditions were mild enough to tolerate furan, thiophene and pyridine rings. The isolated products were fully characterized from their spectral data and by direct comparison with the reported data. In addition, the bench-scale preparation of the oxidized product of benzoin under our conditions was investigated. Catalyst A (80 atom%) was added to a stirred reaction mixture containing benzoin (20 mmol), K2CO3 (40 mmol) and water (200 mL) and heated at 80 °C for 6 h. The benzil product was isolated with a 79% yield. This exciting result reveals that our present conditions can be applied for milligram to gram scale syntheses.
| Entry | Ar (Substrate) | t (h) | Yielda (%) |
|---|---|---|---|
| Unless otherwise specified, all reactions were carried out with α-hydroxy ketone (1.0 mmol), base (2.0 mmol), water (10 mL) and catalyst A under air at the specified temperature.a Isolated product after column chromatography.b Reactions were carried out at 60 °C. | |||
| 1 | Ph | 4 | 91 |
| 2 | 4-MeC6H4 | 5 | 71 |
| 3 | 4-MeOC6H4 | 5 | 70 |
| 4 | 4-FC6H4 | 3.5 | 95 |
| 5 | 2-Pyridyl | 4 | 90b |
| 6 | 2-Thiophenyl | 5 | 93b |
| 7 | 2-Furyl | 6 | 66b |
| 8 |
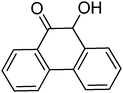
|
4 | 82 |
The reusability of the Au-NPs catalysts was investigated. In a typical experiment, the catalyst was reused twice (recovery amount was 71% after 1st run and the 53% after the 2nd run for entry 1, Table 2). The recovered nanoparticles after the 1st and 2nd oxidations were examined by TEM measurements, which showed substantial agglomeration of the particles after the 2nd run (as shown in Fig. 2). The agglomeration which took place may be due to several factors, such as interactions among self-assembled monolayers,12 effect of salt (base)13 and temperature.
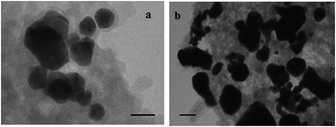 | ||
| Fig. 2 TEM images of the 4-aminothiophenol-protected Au-NP catalyst after the 1st (a) and 2nd (b) oxidation of benzoin. The Au-NPs were deposited from the separated and concentrated aqueous layer after the removal of the organic products. Scale bar is 50 nm. | ||
We propose that the probable mechanism of this reaction follows a similar path to that reported earlier for the Au-NP-catalyzed aerobic oxidation reactions of alcohol.2i,k,l At first, the absorption of the oxy anion onto the Au surface enhanced the electron density that facilitated the absorption of oxygen molecules, probably in the superoxo-type form. The ketone is formed due to the abstraction of hydrogen by the O2− species (Fig. 3). From the reaction mechanism, the lower catalytic efficiency of the 1-undecanethiol-protected Au-NPs when compared to the 4-aminothiophenol-coated Au-NPs was not surprising. The formation of a highly dense monolayer in the case of the 1-undecanethiol-coated Au-NPs14 probably retarded the absorption of bulky molecules onto the Au surface.
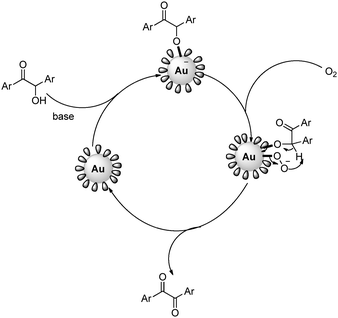 | ||
| Fig. 3 Proposed mechanism for the aerobic oxidation of α-hydroxy ketones by the self-assembled monolayer-protected Au-NPs. | ||
After successfully developing a simple, green and efficient catalytic system for the aerobic oxidation of α-hydroxy ketones to aryl α-diketones, catalyzed by Au-NPs, we then applied the same procedure to a one-pot tandem oxidation and subsequent condensation reactions of aryl α-hydroxy ketones with aryl 1,2-diamines for the synthesis of functionalized quinoxaline derivatives using catalyst A in water. As shown in Table 3, a wide range of structurally varied aryl substituted and un-substituted α-hydroxy ketones with aryl 1,2-diamine worked very well in this procedure to provide the corresponding quinoxaline derivatives in high to excellent yields. Several sensitive functional groups, such as OMe, Cl, F and CO2H, remained unaffected under the present reaction conditions. It is noteworthy that for the first time, 3,4-diaminobenzoic acid has been successfully used for this condensation reaction, providing excellent yields (entries 8, 10, and 13). Our Au-NP catalytic system is very efficient for the synthesis of quinoxaline derivatives when compared to established procedures.
| Entry | Ar (Substrate) | R | t (h) | Yielda (%) |
|---|---|---|---|---|
| Unless otherwise specified, all reactions were carried out with α-hydroxy ketones (1.0 mmol), 1,2-diamine (1.1 mmol), K2CO3 (2.0 mmol), water (10 mL) and catalyst A (4.0 atom%) under air at 80 °C.a Yield of the isolated product. | ||||
| 1 | Ph | H | 4 | 92 |
| 2 | Ph | Me | 4 | 93 |
| 3 | Ph | Cl | 4 | 88 |
| 4 | 4-FC6H4 | Me | 2 | 89 |
| 5 | 4-FC6H4 | Cl | 2 | 91 |
| 6 | 4-MeOC6H4 | Me | 4 | 89 |
| 7 | 4-MeOC6H4 | Cl | 4 | 84 |
| 8 | 4-MeOC6H4 | CO2H | 5 | 85 |
| 9 | 2-Thiophenyl | Cl | 3 | 88 |
| 10 | 2-Thiophenyl | CO2H | 4 | 90 |
| 11 | 2-Furyl | H | 2 | 86 |
| 12 | 2-Furyl | Me | 2 | 82 |
| 13 | 2-Furyl | CO2H | 2 | 87 |
| 14 |
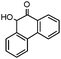
|
Me | 4 | 91 |
Conclusion
In this manuscript, we have investigated the catalytic activity of self-assembled monolayer-coated Au-NPs for the aerobic oxidation of aryl substituted α-hydroxy ketones to aryl 1,2-diketones and extended this reaction for a one-pot synthesis of highly biologically significant quinoxaline derivatives in water. Our current methods avoid the use of acid, highly toxic reagents, hazardous organic solvents, multisteps etc. In addition, the simplistic synthesis of the catalyst, operational simplicity, high yields, catalytic and environmentally friendly reaction conditions make them attractive. This result should encourage new applications for self-assembled monolayer-coated Au-NPs in organic syntheses as efficient catalysts. In the present system, the reusability of the catalyst was limited after few cycles of oxidation due to agglomeration of the nanoparticles. However, the development of suitable stabilizers to prevent agglomeration as well as enhance the catalytic efficiency of the nanoparticles would offer significant applications for self-assembled monolayer-coated nanoparticles in organic syntheses.Experimental section
Materials and reagents
Hydrogen tetrachloroaurate(III) hydrate, 4-aminothiophenol and 1-undecanethiol were purchased from Aldrich Chemicals. N,N-dimethylformamide (DMF) and hydrochloric acid (HCl) were purchased from Merck India. All these chemicals were used without further purification. Milli Q water was used throughout the experiment. The starting materials were either purchased from commercial sources or synthesized by known literature procedures.Synthesis of the catalysts
The details of the syntheses of the 4-aminothiophenol-coated Au-NPs (catalyst A) and the 1-undecanethiol-coated Au-NPs (catalyst B) are reported in the ESI.† The characterization of the nanoparticles was performed using UV-visible spectroscopy and X-ray diffraction. The particle size of the nanoparticles was evaluated using TEM measurements. TGA experiments were performed to estimate the Au content in the catalyst. Details of the analyses are reported in the ESI.†Oxidation of benzoin
K2CO3 (276 mg, 2.0 mmol) and catalyst A (28 mg, 4.0 atom%) were added to a stirred heterogeneous mixture of benzoin (212 mg, 1.0 mmol) in water (10 mL) at room temperature. The reaction mixture was then heated at 80 °C for 4 h under air. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was extracted with ethyl acetate (3 × 10 mL), washed with water and brine respectively and dried with Na2SO4. The organic phase was evaporated on a rotary evaporator under reduced pressure to give the crude product. The crude product was purified by column chromatography over silica gel to furnish the pure product (191 mg, 91% yield). The product was characterized by the corresponding spectroscopic data, which was in good agreement with the reported values.One-pot synthesis of 2,3-diphenylquinoxaline
A mixture containing benzoin (212 mg, 1.0 mmol), o-phenylenediamine (108 mg, 1.0 mmol), K2CO3 (276 mg, 2.0 mmol) and catalyst A (28 mg, 4.0 atom%) in 10 ml of water was stirred and heated at 80 °C under air. After completion of the reaction (monitored by TLC), the reaction mixture was extracted with ethyl acetate (3 × 10 mL). The organic layer was dried over anhydrous Na2SO4, followed by evaporation of the solvent to obtain the crude product which was purified by column chromatography over silica gel to give the pure product (259 mg, 92% yield). The product was characterized by the corresponding spectroscopic data, which was in good agreement with the reported data. The spectroscopic data of the unknown organic compounds is reported in the ESI.†Acknowledgements
We acknowledge financial support from DST, the Govt. of India (Project No. SR/S1/PC-32/2010). We are also thankful to SAIC IIT Bombay for the TEM measurements and the UGC-DAE Inter-University Consortium Indore for the powder XRD measurement facilities.Notes and references
- (a) S. Biella and M. Rossi, Chem. Commun., 2003, 378 RSC; (b) C. Milone, R. Ingoglia, M. Tropeano, G. Neri and S. Galvango, Chem. Commun., 2003, 868 RSC; (c) M. Haruta, T. Kobayashi, H. Sano and N. Yamamda, Chem. Lett., 1987, 405 CrossRef CAS; (d) M. Haruta, Nature, 2005, 437, 1098 CrossRef CAS; (e) C. D. Pina, E. Falletta, L. Prati and M. Rossi, Chem. Soc. Rev., 2008, 37, 2077 RSC; (f) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896 CrossRef; (g) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS; (h) E. Jimenez-Nunez and A. M. Echavarren, Chem. Commun., 2007, 333 RSC.
- (a) M. Haruta, Catal. Today, 1997, 36, 153 CrossRef CAS; (b) T. Ishida and M. Haruta, Angew. Chem., Int. Ed., 2007, 46, 7154 CrossRef CAS; (c) B. K. Min and C. M. Friend, Chem. Rev., 2007, 107, 2709 CrossRef CAS; (d) G. J. Hutchings and H. Brust, Chem. Soc. Rev., 2008, 37, 1759 RSC; (e) A. Corma and H. Garcia, Chem. Soc. Rev., 2008, 37, 2096 RSC; (f) M. S. Chen and D. W. Goodman, Science, 2004, 306, 252 CrossRef CAS; (g) W. Fang, J. Chen, Q. Zhang, W. Deng and Y. Wang, Chem.–Eur. J., 2011, 17, 1247 CrossRef CAS; (h) A. A. Herzing, C. J. Kiely, A. F. Carley, P. Landon and G. J. Hutchings, Science, 2008, 321, 1331 CrossRef CAS; (i) H. Tsunoyama, N. Ichikuni, H. Sakurai and T. Tsukuda, J. Am. Chem. Soc., 2009, 131, 7086 CrossRef CAS; (j) H. Liu, Y. Liu, Y. Li, Z. Tang and H. Jiang, J. Phys. Chem. C, 2010, 114, 13362 CrossRef CAS; (k) T. Ishida, M. Nagaoka, T. Akita and M. Haruta, Chem.–Eur. J., 2008, 14, 8456 CrossRef CAS; (l) T. Tsukuda, H. Tsunoyama and H. Sakurai, Chem.–Asian J., 2011, 6, 736 CrossRef CAS.
- (a) B. C. Ranu, S. Samanta and S. K. Guchhait, J. Org. Chem., 2001, 66, 4102 CrossRef CAS; (b) B. C. Ranu, A. Das and S. Samanta, J. Chem. Soc., Perkin Trans. 1, 2002, 1520 RSC; (c) B. C. Ranu, S. Samanta and S. K. Guchhait, Tetrahedron, 2002, 58, 983 CrossRef CAS; (d) Y. Hayashi, S. Samanta, H. Gotoh and H. Ishikawa, Angew. Chem., Int. Ed., 2008, 47, 6634 CrossRef CAS.
- (a) T. K. Sarma, D. Chowdhury, A. Paul and A. Chattopadhyay, Chem. Commun., 2002, 1048 RSC; (b) G. Majumdar, M. Goswami, T. K. Sarma, A. Paul and A. Chattopadhyay, Langmuir, 2005, 21, 1663 CrossRef CAS; (c) T. K. Sarma and A. Chattopadhyay, Langmuir, 2004, 20, 4733 CrossRef CAS.
- (a) E. J. Corey, D. H. Lee and S. Sarshar, Tetrahedron: Asymmetry, 1995, 6, 3 CrossRef CAS; (b) A. Ryoda, N. Yajima, T. Haga, T. Kumamoto, W. Nakanishi, M. Kawahata, K. Yamaguchi and T. Ishikawa, J. Org. Chem., 2008, 73, 133 CrossRef CAS; (c) L. R. Hillis and R. C. Ronald, J. Org. Chem., 1985, 50, 470 CrossRef CAS.
- (a) A. Gomtsyan, E. K. Bayburt, R. G. Schmidt, G. Z. Zheng, R. J. Perner, S. Didomenico, J. R. Koenig, S. Turner, T. Jinkerson, I. Drizin, S. M. Hannick, B. S. Macri, H. A. McDonald, P. Honore, C. T. Wismer, K. C. Marsh, J. Wetter, K. D. Stewart, T. Oie, M. F. Jarvis, C. S. Surowy, C. R. Faltynek and C. H. Lee, J. Med. Chem., 2005, 48, 744 CrossRef CAS; (b) R. Sarges, H. R. Howard, R. G. Browne, L. A. Label, P. A. Seymour and B. K. Koe, J. Med. Chem., 1990, 33, 2240 CrossRef CAS; (c) L. E. Seitz, W. J. Suling and R. C. Reynolds, J. Med. Chem., 2002, 45, 5604 CrossRef CAS; (d) A. Jaso, B. Zarranz, I. Aldana and A. Monge, J. Med. Chem., 2005, 48, 2019 CrossRef CAS; (e) W. He, M. R. Myers, B. Hanney, A. P. Spada, G. Bilder, H. Galzcinski, D. Amin, S. Needle, K. Page, Z. Jayyosi and M. H. Perrone, Bioorg. Med. Chem. Lett., 2003, 13, 3097 CrossRef CAS; (f) G. Sakata, K. Makino and Y. Kurasawa, Heterocycles, 1988, 27, 2481 CrossRef CAS.
- (a) E. D. Brock, D. M. Lewis, T. I. Yousaf and H. H. Harper, U. S. Patent WO9951688, 1999 Search PubMed; (b) S. Dailey, J. W. Feast, R. J. Peace, I. C. Sage, S. Till and E. L. Wood, J. Mater. Chem., 2001, 11, 2238 RSC; (c) B. I. Ita and O. E. Offiong, Mater. Chem. Phys., 2001, 70, 330 CrossRef CAS; (d) M. R. Ams and C. S. Wilcox, J. Am. Chem. Soc., 2007, 129, 3966 CrossRef CAS.
- (a) T. Kashiwabra and M. Tanaka, J. Org. Chem., 2009, 74, 3958 CrossRef; (b) A. Giraud, O. Provot, J. Franc, O. Peyrat, M. Alami and J. D. Brion, Tetrahedron, 2006, 62, 7667 CrossRef CAS; (c) Y. Shimakawa, T. Morikawa and S. Sakaguchi, Tetrahedron Lett., 2010, 51, 1786 CrossRef CAS; (d) C. Joo, S. Kang, S. M. Kim, H. Han and J. W. Yang, Tetrahedron Lett., 2010, 51, 6006 CrossRef CAS; (e) J. M. Khurana and B. M. Kandpal, Tetrahedron Lett., 2003, 44, 4909 CrossRef CAS; (f) D. Saio, T. Amaya and T. Hirao, Adv. Synth. Catal., 2010, 352, 2177 CrossRef CAS.
- (a) R. S. Bhosale, S. R. Sarda, S. S. Ardhapure, W. N. Jadhav, S. R. Bhusare and R. P. Pawar, Tetrahedron Lett., 2005, 46, 7183 CrossRef CAS; (b) S. V. More, M. N. V. Sastry, C. C. Wang and C. F. Yao, Tetrahedron Lett., 2005, 46, 6345 CrossRef CAS; (c) H. R. Darabi, S. Mohandessi, K. Aghapoor and F. Mohsenzadeh, Catal. Commun., 2007, 8, 389 CrossRef CAS; (d) M. M. Heravi, S. Taheri, K. Bakhtiari and H. A. Oskooie, Catal. Commun., 2007, 8, 211 CrossRef CAS; (e) T. K. Huang, R. Wang, L. Shi and X. X. Lu, Catal. Commun., 2008, 9, 1143 CrossRef CAS; (f) C. Srinivas, C. N. S. S. P. Kumar, V. J. Rao and S. Palaniappan, J. Mol. Catal. A: Chem., 2007, 265, 227 CrossRef CAS; (g) S. S. Katkar, P. H. Mohite, L. S. Gadekar, Arbad, B. R. Lande and M. K. Cent, Eur. J. Chem., 2010, 8, 320 CAS; (h) H. Alinezhad, M. Tajbakhsh and P. Biparva, Bull. Korean Chem. Soc., 2011, 32, 3720 CrossRef CAS; (i) M. M. Heravi, K. Bhaktiari, F. F. Bamoharram and M. H. Tehrani, Monatsh. Chem., 2007, 138, 465 CrossRef CAS.
- (a) S. Antoniotti and E. Donach, Tetrahedron Lett., 2002, 43, 3971 CrossRef CAS; (b) B. Das, K. Venkateswarlu, K. Suneel and A. Majhi, Tetrahedron Lett., 2007, 48, 5371 CrossRef CAS; (c) B. Madhav, S. N. Murthy, V. P. Reddy, K. R. Rao and Y. V. D. Nageswar, Tetrahedron Lett., 2009, 50, 6025 CrossRef CAS; (d) J. P. Wan, S. F. Gan, J. M. Wu and Y. J. Pan, Green Chem., 2009, 11, 1633 RSC.
- (a) S. Paul and B. Basu, Tetrahedron Lett., 2011, 52, 6597 CrossRef CAS; (b) V. K. Akkilagunta, V. P. Reddy and R. R. Kakulapati, Synlett, 2010, 2571 CAS; (c) S. Sithambaram, Y. Ding, W. Li, X. Shen, F. Gaenzler and S. L. Suib, Green Chem., 2008, 10, 1029 RSC; (d) S. A. Kotharkar and D. B. Shinde, Bull. Korean Chem. Soc., 2006, 27, 1466 CrossRef CAS; (e) R. S. Robinson and R. J. K. Taylor, Synlett, 2005, 1003 CAS; (f) S. A. Raw, C. D. Wilfred and R. J. K. Taylor, Chem. Commun., 2003, 2286 RSC.
- J. Sharma, S. Nahima, B. A. Kakade, R. Pasricha, A. B. Mandale and K. Vijayamohanan, J. Phys. Chem. B, 2004, 108, 13280 CrossRef CAS.
- C. A. Mirkin, Inorg. Chem., 2000, 39, 2258 CrossRef CAS.
- (a) J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103 CrossRef CAS; (b) D. Samanta and A. Sarkar, Chem. Soc. Rev., 2011, 40, 2567 RSC; (c) M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cy20438j |
| This journal is © The Royal Society of Chemistry 2012 |