Poly(4-vinylpyridinium) hydrogen sulfate: an efficient catalyst for the synthesis of xanthene derivatives under solvent-free conditions†
Nader
Ghaffari Khaligh
*
Department of Chemistry, College of Science, University of Guilan, Rasht, 41335-19141, Iran. E-mail: ngkhaligh@gmail.com; ngkhaligh@guilan.ac.ir; Fax: +9866934046; Tel: +982166431738
First published on 18th June 2012
Abstract
A simple and convenient procedure for the synthesis of xanthene derivatives is described through a one-pot condensation of 2-naphthol {2-hydroxynaphthalene-1,4-dione or the mixture of 2-naphthol and 2-hydroxynaphthalene-1,4-dione} with aryl aldehydes in the presence of poly(4-vinylpyridinium)hydrogen sulfate as an efficient, cheap, easily synthesised and eco-friendly catalyst under solvent-free conditions. Further, the catalyst can be reused and recovered several times without any variations in the yield of the product.
In recent years, the development of heterogeneous catalysts has become a major area of research in synthetic organic chemistry due to the potential advantages of these materials over homogeneous systems such as simplified recovery, reusability, enhanced selectivity and reactivity, easy product isolation and incorporation in continuous reactors and microreactors.1,2 This can lead to novel and environmentally benign chemical procedures for academia and industry.3 Application of solid acids in organic transformations is important because these reagents do less harm to the environment and have no corrosion or disposal problems. As economically and ecologically benign catalysts, their research and application have attracted much attention in chemistry and industry.4–9 There are more than 100 industrial processes using over 103 solid acids as catalysts.10
It is clear that green chemistry not only requires the use of environmentally benign reagents and solvents, but also the recovery and reuse of the catalyst. One way to overcome the problem of recyclability of the traditional acid catalyst is to chemically anchor the reactive center onto a large surface area solid carrier.11 In these types of solids, the reactive centers are highly mobile similar to homogeneous catalysts and at the same time these species have the advantage of being recyclable in the same fashion as heterogeneous catalysts. Functional polymers have the potential advantages of small molecules with the same functional groups.12 Poly(4-vinylpyridine) is an interesting material because of its stable pyridyl group and ability to form charge transfer complexes with acidic dopants.13 Poly(4-vinylpyridine) seems to be an attractive support to immobilize mineral acids because of the basic nature of the pyridyl group.
The synthesis of xanthene derivatives, especially benzoxanthenes, has emerged as a powerful tool in organic synthesis due to their wide range of biological and therapeutic properties such as antibacterial,14 antiviral15 and anti-inflammatory activities16 as well as in photodynamic therapy17 and for antagonism of the paralyzing action of zoxazolamine.18 Furthermore, due to their useful spectroscopic properties, they are have been used as dyes,19 in laser technologies,20 and in fluorescent materials for visualization of biomolecules.21 Many procedures describe the synthesis of xanthenes and benzoxanthenes including cyclodehydrations,22 alkylations γ to the heteroatoms,23 trapping of benzynes by phenols,24 cyclocondensation between 2-hydroxyaromatic aldehydes and 2-tetralone,25 the reaction of 2-naphthol with aldehydes or acetals under acidic conditions and intramolecular phenyl carbonyl coupling reactions of benzaldehydes and acetophenones.26 In addition, 14H-dibenzo[a.j]xanthenes and related products were prepared by reaction of 2-naphthol with formamide,27 2-naphthol-1-methanol,28 carbon monoxide29 and sulfonic acid.30 Even though various procedures were reported, disadvantages including low yields, prolonged reaction times, use of an excess of reagents/catalysts and use of toxic organic solvents necessitate the development of an alternative route for the synthesis of xanthene derivatives.
In this paper, we investigate the synthesis of xanthene derivatives in the presence of poly(4-vinylpyridinium) hydrogen sulfate [P(4-VPH)HSO4] under solvent-free conditions. The reported route is an efficient, convenient and novel method for condensation of aldehydes with 2-naphthol and 2-hydroxynaphthalene-1,4-dione or the mixture of 2-naphthol and 2-hydroxynaphthalene-1,4-dione in the presence of P(4-VPH)HSO4 as solid acid (Scheme 1).
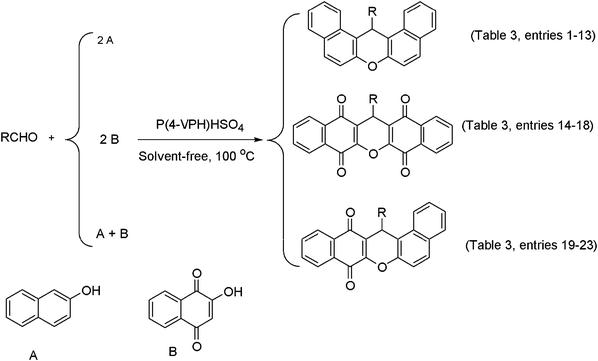 | ||
| Scheme 1 The synthesis of xanthene derivatives in presence P(4-VPH)HSO4 under solvent-free conditions. | ||
In continuation of our ongoing research program on the development of new catalysts and methods for organic transformations,31–34 an earlier report from our laboratory described the environmentally benign synthesis of poly(4-vinylpyridinium) hydrogen sulfate P(4-VPH)HSO4 and the application in 1,1-diacetylation of aldehydes.33 The structure of P(4-VPH)HSO4 assured us to accept that this reagent can act as an efficient catalyst in reactions that need mild acidic reagents to speed up.
In an initial study, in order to examine the catalytic activity of catalyst, we examined the reaction benzaldehyde (1 mmol) with 2-naphthol (A, 2 mmol) under different conditions including refluxing in various solvents (MeOH, EtOH, THF, MeCN, EtOAc, and toluene) and also under solvent-free classical heating conditions. In refluxing solvents, after 6 h, the yields of products were low (< 45%). We found that the best results were obtained under solvent-free conditions in the presence of P(4-VPH)HSO4 2 mol% at 100 °C (Table 1). This may be explained due to the decreased diffusion of the reactant molecules in the presence of the solvent and the interference of solvent molecules with the active sites of P(4-VPH)HSO4. When the reaction was carried out at higher temperature, TLC and 1H NMR spectra of the reaction mixture showed a combination of numerous products, the yield of the expected product was slightly low.
| Entry | Amount of catalyst/mg | T/°C | Yielda (%) | Time/min |
|---|---|---|---|---|
| 1 | 0 | 100 | — | 360 |
| 2 | 5 | 100 | 65 | 120 |
| 3 | 10 | 100 | 94 | 60 |
| 4 | 10 | 110 | 94 | 60 |
| 5 | 10 | 120 | 88 | 60 |
| 6 | 15 | 80 | 78 | 60 |
| 7 | 15 | 100 | 94 | 60 |
| 8 | 20 | 100 | 92 | 60 |
The condensation of 2-naphthol and aryl aldehydes (carrying both electron-withdrawing and electron-donating groups), in the presence of P(4-VPH)HSO4 10 mg as a solid acid catalyst under solvent-free conditions using conventional heating at 100 °C, yielded desired 14H-dibenzo[a.j]xanthenes in high purity with excellent yields. The reactions required 52–78 min with conventional heating at 100 °C (Table 2, enteries 1–13). The nature of the functional group on the aromatic ring of the aldehyde exerted a slightly influence on the reaction time and the yield of dibenzo[a,j]xanthene. A decrease of the reaction rate was observed with arylaldehyde bearing electron-withdrawing (such as –NO2) and electron-donating (such as –OCH3) group in the para-position (Table 2, entry 7,10), in comparison to the unsubstituted arylaldehyde. The presence of a halogen (such as –Br and –Cl) group in the same position (Table 2, entries 4,13) enhanced both the rate and the yield of product. This slight difference is also seen in ortho-substituted benzaldehydes (Table 2, entries 5,8,11).
| Entry | R | Time/min | Yield (%)b | Mp °C | |
|---|---|---|---|---|---|
| Found | Reported [Ref.] | ||||
| a Products were characterized by 1H NMR, IR and melting point and also by comparison with the reported in literature data. Due to the very low solubilities of products 17 and 18, 13C NMR spectra for these products were not reported. b Isolated yields. | |||||
| 1 | C6H5– | 55 | 94 | 184–185 | 185–18735 |
| 2 | 4-CH3–C6H4– | 72 | 93 | 230–231 | 228–22935 |
| 3 | 3-Br–C6H4– | 78 | 94 | 196–198 | — |
| 4 | 4-Br–C6H4– | 65 | 96 | 297–298 | 296–29836 |
| 5 | 2-NO2–C6H4– | 72 | 94 | 295–260 | 29330 |
| 6 | 3-NO2–C6H4– | 72 | 92 | 213–215 | 210–21135 |
| 7 | 4-NO2–C6H4– | 70 | 96 | 312–314 | 312–31335 |
| 8 | 2-CH3O–C6H4– | 78 | 94 | 259–260 | 26022c |
| 9 | 3-CH3O–C6H4– | 76 | 92 | 179–180 | 179–18037 |
| 10 | 4-CH3O–C6H4– | 72 | 90 | 204–205 | 203–20635 |
| 11 | 2-Cl–C6H4– | 57 | 92 | 214–216 | 216–21835 |
| 12 | 3-Cl–C6H4– | 54 | 93 | 210–213 | 184–18537 |
| 13 | 4-Cl–C6H4– | 52 | 96 | 290–292 | 290–29235 |
| 14 | C6H5– | 70 | 94 | 306–308 | 305–30738 |
| 15 | 4-CH3–C6H4– | 68 | 90 | 305–306 | 304–30738 |
| 16 | 4-Cl–C6H4– | 62 | 96 | > 320 | 330–33238 |
| 17 | Isatin | 132 | 82 | > 320 | 350 dec39 |
| 18 | Ninhydrin | 140 | 78 | 230 dec | 230 dec39 |
| 19 | C6H5– | 40 | 90 | 320 | 319–32040 |
| 20 | 4-CH3–C6H4– | 25 | 91 | 256–258 | 255–25640 |
| 21 | 4-Cl–C6H4– | 40 | 94 | 300–311 | 305–30640 |
| 22 | 4-CH3O–C6H4– | 26 | 88 | 280–282 | 279–28040 |
| 23 | 4-NO2–C6H4– | 28 | 94 | > 320 | 332–33340 |
After successfully synthesizing a series of 14H-dibenzo[a,j]xanthenes in good yields, we turned our attention toward the synthesis of 5H-dibenzo[b,i]xanthene-tetraones under similar conditions. We replaced the 2-hydroxynaphthalene-1,4-dione compound instead of 2-naphthol in same conditions (Scheme 1). We next examined the reaction with 2 equivalents of 2-hydroxynaphthalene-1,4-dione compound with various aldehydes. As expected, these substrates underwent smooth, one-pot conversion to give the corresponding 5H-dibenzo[b,i]xanthene-tetraones in good yields (Table 2, entries 14–18). From the results in Table 1, the slight variations in the yield of dibenzo[b,i]xanthene-tetraones was likely due to the presence of the different aromatic substituents on the aldehydes, although it must be noted that in general, the yield of most xanthene products was greater than 90%. Thus, 4-chlorobenzaldehyde (Table 1, entry 16), afforded slightly better xanthene yields over benzaldehyde and 4-methyl benzaldehyde (Table 2, entries 14,15). It was also observed that a more significant decrease in the yield of product with longer reaction times occurred when simple or substituted benzaldehydes were replaced with polycyclic aromatic precursors such as isatin and ninhydrin (Table 2, entries 17,18).
Finally, we have developed this synthetic method for one pot efficient synthesis of 14-aryl-14H-dibenzo[a,i]xanthene-8,13-diones by condensation of aldehydes with 2-naphthol and 2-hydroxynaphthalene-1,4-dione (Table 2, entries 19–23). The aromatic aldehydes containing electron-donating as well as electron-withdrawing groups underwent the conversion. However, the reaction conducted with benzaldehyde and 4-chlorobenzaldehyde showed longer reaction times.
Table 3, compared our results (amount of catalyst, reaction conditions, time, yield) with results obtained by other groups. As can be seen, our method is simpler, more efficient, and the reaction completed within a short period of time.
| Entry | Catalyst | Amount of catalyst/mol% | Conditions | Time/h | Yield (%)a | Ref. |
|---|---|---|---|---|---|---|
| a Isolated yields. | ||||||
| 1 | p-TsOH | 10 | Neat/125 °C | 4 | 89 | 22c |
| 2 | Sulfamic acid | 10 | Neat/125 °C | 8 | 93 | 30 |
| 3 | K5CoW12O40·3H2O | 10 | Neat/125 °C | 2 | 91 | 41 |
| 4 | I2 | 20 | Neat/90 °C | 2.5 | 90 | 42 |
| 5 | LiBr | 15 | Neat/130 °C | 65 min | 82 | 43 |
| 6 | Montmorillonite K10 | 300 mg | Neat/120 °C | 3 | 75 | 44 |
| 7 | Amberlyst-15 | 10 mg | Neat/125 °C | 2 | 94 | 45 |
| 8 | Cellulose sulfuric acid | 80 mg | Neat/110–115 °C | 1.5 | 81 | 46 |
| 9 | Silica sulfuric acid | 80 mg | Neat/110–115 °C | 1.5 | 89 | 46 |
| 10 | Sulfuric acid | 10 | AcOH/110–115 °C | 1.5 | 91 | 46 |
| 11 | P(4-VPH)HSO4 | 2 | Neat/100 °C | 55 min | 94 | This work |
The recovery and reuse cycle of P(4-VPH)HSO4 was also studied. Hence, we decided to study the catalytic activity of recycled solid acid P(4-VPH)HSO4 for the synthesis of 14-(Phenyl)-14H-dibenzo[a,j]xanthene (Fig. 1). As shown in Fig. 1, the solid acid P(4-VPH)HSO4 can be recycled at least five times without significant decrease in catalytic activity, the yields ranged from 94% to 93%.
![The recycling of P(4-VPH)HSO4 in synthesis of 14-(phenyl)-14H-dibenzo[a,j]xanthene under solvent-free conditions.](/image/article/2012/CY/c2cy20276j/c2cy20276j-f1.gif) | ||
| Fig. 1 The recycling of P(4-VPH)HSO4 in synthesis of 14-(phenyl)-14H-dibenzo[a,j]xanthene under solvent-free conditions. | ||
Although the detailed mechanism of the above reaction was not fully established, the formation of dibenzoxanthenes could be explained by a reaction sequence similar to the literature reports40,45,47,48 (Scheme 2). We proposed that the reaction proceeded via a reaction sequence of condensation, addition, cyclization and dehydration. First, the condensation of aldehyde and 2-naphthol (A) or 2-hydroxynaphthalene-1,4-dione (B) gave the intermediate (C and D). The addition of 2-naphthol (A) or 2-hydroxynaphthalene-1,4-dione (B) to (C and D) leading to the formation of (E,F and G), which on intermolecular cyclization and dehydration gave rise to the desired xanthene derivatives.
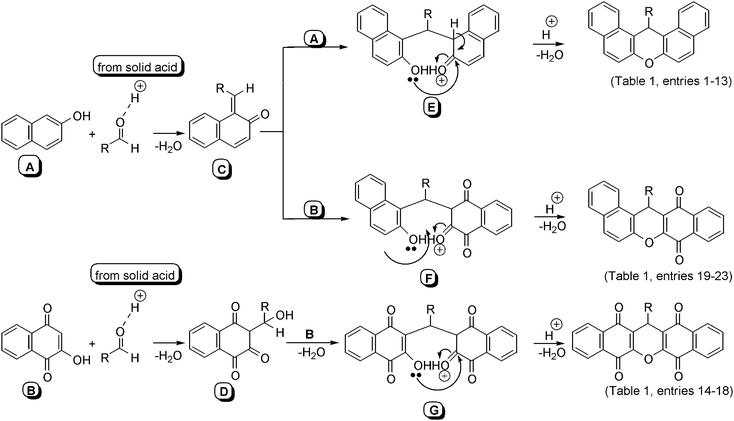 | ||
| Scheme 2 The plausible mechanism of the reaction. | ||
We have described a solid acid poly(4-VPH)HSO4 catalyzed, highly efficient, one-pot protocol for the synthesis of xanthene derivatives by the condensation of an aldehyde and 2-naphthol or 2-hydroxynaphthalene-1,4-dione or the mixture of 2-naphthol and 2-hydroxynaphthalene-1,4-dione under solvent-free conditions in excellent yields. The present methodology also has several other advantages such as: high reaction rates and excellent yields; no side reactions; ease of preparation and handling; cost efficiency and effective recovery; reusability of the catalyst; use of an inexpensive catalyst with lower loading; and a simple experimental procedure. Further work to explore this novel catalyst in other organic transformations is in progress.
Experimental section
General procedure for the synthesis of xanthene derivatives
A mixture of 2-naphthol (2 mmol) {2-hydroxynaphthalene-1,4-dione (2 mmol) or the mixture of 2-naphthol (1 mmol) and 2-hydroxynaphthalene-1,4-dione (1 mmol)}, aldehyde (1 mmol) and P(4-VPH)HSO4 (10 mg, ∼0.02 mmol) was heated at 100 °C under solvent-free conditions. The progress of the reaction was followed by TLC. After completion of the reaction, the reaction mixture was diluted with ethanol (10 mL) and stirred for 10 min in 80 °C. The solid (catalyst) were collected by filtration and the residue was kept at room temperature and the resulting crystalline product was collected by filtration. The product was found to be pure and no further purification was necessary.Representative spectral data
References
- (a) A. J. Fatiadi, Synthesis, 1987, 85–127 CrossRef CAS; (b) F. M. Menger and C. Lee, J. Org. Chem., 1979, 44, 3446–3448 CrossRef CAS.
- (a) K. Smith, Solid Supports and Catalysis in Organic Synthesis, Ellis Horwood and PTR Prentice Hall, New York, 1992 Search PubMed; (b) P. Laszlo, in Comprehensive Organic Synthesis, ed. B. M. Trost, New York, Pergamon, 1991, vol. 7, pp. 839–849 Search PubMed.
- D. Choudhary, S. Paul, R. Gupta and J. H. Clark, Green Chem., 2006, 8, 479–482 RSC.
- A. Ghorbani-choghamarani and S. Sardari, J. Sulfur Chem., 2011, 32, 63–69 CrossRef CAS.
- R. Dalpozzo, G. Bartoli, L. Sambri and P. Melchiorre, Chem. Rev., 2010, 110(6), 3501–3551 CrossRef CAS.
- A. K. Chakraborti, B. Singh, S. V. Chankeshwara and A. R. Patel, J. Org. Chem., 2009, 74(16), 5967–5974 CrossRef CAS.
- G. Sharma, R. Kumar and A. K. Chakraborti, Tetrahedron Lett., 2008, 49, 4272–4275 CrossRef CAS.
- B. Karimi and M. Khalkhali, J. Mol. Catal. A: Chem., 2005, 232, 113–117 CrossRef CAS.
- K. Niknam, D. Saberi and M. Nouri Sefat, Tetrahedron Lett., 2009, 50, 4058–4062 CrossRef CAS.
- K. Tanabe and W. F. Hölderich, Appl. Catal., A, 1999, 181, 399–434 CrossRef CAS.
- J. A. Melero, R. van Grieken and G. Morales, Chem. Rev., 2006, 106, 3790–3812 CrossRef CAS.
- (a) A. Akelah and D. C. Sherindton, Chem. Rev., 1981, 81, 557–587 CrossRef CAS; (b) D. C. Sherington and P. Hodge, Synthesis and Separation Using Functional Polymers, Wiley, Chichester, 1988 Search PubMed; (c) K. Takemoto, Y. Inaki and R. M. Ottenbrite, Functional Monomers and Polymers, Dekker, New York, 1987 Search PubMed; (d) S. V. Ley, I. R. Baxendale, R. N. Bream, P. S. Jackson, A. G. Leach, D. A. Longbottom, M. Nesi, J. S. Scott, R. I. Storer and S. J. Taylor, J. Chem. Soc., Perkin Trans. 1, 2000, 1, 3815–4195 RSC.
- V. Rao, P. V. Ashokan and M. H. Shridhar, Mater. Sci. Eng., A, 2000, 276, 266–268 CrossRef.
- T. Hideo, Chem. Abstr., 1981, 95, 80922b Search PubMed , Jpn. Tokkyo Koho JP 56005480, 1981.
- R. W. Lambert, J. A. Martin, J. H. Merrett, K. E. B. Parkes and G. J. Thomas, Chem. Abstr., 1997, 126, p212377y Search PubMed , PCT Int. Appl. WO 9706178, 1997.
- J. P. Poupelin, G. Saint-Rut, O. Foussard-Blanpin, G. Narcisse, G. Uchida-Ernouf and R. Lacroix, Eur. J. Med. Chem., 1978, 13, 67–71 CAS.
- (a) R. M. Ion, Prog. Catal., 1997, 2, 55–76 Search PubMed; (b) R. M. Ion, D. Frackowiak, A. Planner and K. Wiktorowicz, Acta Biochim. Pol., 1998, 45, 833–845 CAS.
- (a) G. Saint-Ruf, A. De and H. T. Hieu, Bull. Chim. Ther., 1972, 7, 83–86 Search PubMed; (b) G. Saint-Ruf, H. T. Hieu and J. P. Poupelin, Naturwissenschaften, 1975, 62, 584–585 CrossRef CAS.
- (a) S. M. Menchen, S. C. Benson, J. Y. L. Lam, W. Zhen, D. Sun, B. B. Rosenblum, S. H. Khan and M. Taing, Chem. Abstr., 2003, 139, p54287f Search PubMed, U.S. Patent, US6583168, 2003 Search PubMed (b) A. Banerjee and A. K. Mukherjee, Stain Technol., 1981, 56, 83–85 CAS; (c) G. A. Reynolds, S. A. Tuccio, O. G. Peterson and D. P. Specht, Chem. Abstr., 1971, 71, p81334c Search PubMed , Ger. Offen. DE2109040, 1971.
- (a) O. Sirkeeioglu, N. Talinli and A. Akar, J. Chem. Res., 1995, S, 502–506 Search PubMed; (b) M. Ahmad, T. A. King, Do-K. Ko, B. H. Cha and J. Lee, J. Phys. D: Appl. Phys., 2002, 35, 1473–1476 CrossRef CAS.
- C. G. Knight and T. Stephens, Biochem. J., 1989, 258, 683–689 CAS.
- (a) A. Bekaert, J. Andrieux and M. Plat, Tetrahedron Lett., 1992, 33, 2805–2806 CrossRef CAS; (b) R. J. Sarma and J. B. Baruah, Dyes Pigm., 2005, 64, 91–92 CrossRef CAS; (c) A. R. Khosropour, M. M. Khodaei and H. Moghannian, Synlett, 2005, 955–958 CrossRef CAS; (d) C. A. Buehler, D. E. Cooper and E. O. Scrudder, J. Org. Chem., 1943, 8, 316–319 CrossRef CAS.
- (a) R. Vazquez, M. C. de la Fuente, L. Castedo and D. Domínguez, Synlett, 1994, 433–434 CrossRef CAS; (b) H. Ishibashi, K. Takagaki, N. Imada and M. Ikeda, Synlett, 1994, 49–50 CrossRef CAS.
- (a) D. W. Knight and P. B. Little, Synlett, 1998, 1141–1143 CrossRef CAS; (b) D. W. Knight and P. B. Little, J. Chem. Soc., Perkin Trans. 1, 2001, 1, 1771–1777 RSC.
- A. Jha and J. Beal, Tetrahedron Lett., 2004, 45, 8999–9001 CrossRef CAS.
- C.-W. Kuo and J.-M. Fang, Synth. Commun., 2001, 31, 877–892 CrossRef CAS.
- P. Papini and R. Cimmarusti, Gazz. Chim. Ital., 1947, 77, 142–145 CAS.
- R. N. Sen and N. Sarkar, J. Am. Chem. Soc., 1925, 47, 1079–1091 CrossRef CAS.
- K. Ota and T. Kito, Bull. Chem. Soc. Jpn., 1976, 49, 1167–1168 CrossRef CAS.
- B. Rajitha, B. Sunil Kumar, Y. Thirupathi Reddy, Y. NarasimhaReddy and N. Sreenivasulu, Tetrahedron Lett., 2005, 46, 8691–8693 CrossRef CAS.
- F. Shirini and N. G. Khaligh, Phosphorus, Sulfur Silicon Relat. Elem., 2011, 186, 2156–2165 CrossRef CAS.
- N. G. Khaligh, J. Mol. Catal. A: Chem., 2011, 349, 63–70 CrossRef CAS.
- N. G. Khaligh and F. Shirini, J. Mol. Catal. A: Chem., 2011, 348, 20–29 CrossRef CAS.
- (a) N. G. Khaligh, Ultrason. Sonochem., 2012, 19, 736–739 CrossRef CAS; (b) N. G. Khaligh, Tetrahedron Lett., 2012, 53, 1637–1640 CrossRef CAS; (c) N. G. Khaligh, RSC Adv., 2012, 2, 3321–3327 RSC; (d) F. Shirini and N. G. Khaligh, Monatsh. Chem., 2011, 143, 631–635 CrossRef; (e) F. Shirini and N. G. Khaligh, J. Iran. Chem. Soc., 2012, 9, 495–502 CrossRef.
- M. Hong and C. Cai, J. Fluorine Chem., 2009, 130, 989–992 CrossRef CAS.
- R. Kumar, G. G. Nandi, R. Verma and M. S. Singh, Tetrahedron Lett., 2010, 51, 442–445 CrossRef CAS.
- A. K. Bhattacharya, K. C. Rana, M. Mujahid, I. Sehar and A. K. Saxena, Bioorg. Med. Chem. Lett., 2009, 19, 5590–5593 CrossRef CAS.
- A. Bazgir, Z. N. Tisseh, S. C. Azimi and P. Mirzaei, Dyes Pigm., 2008, 79, 273–275 CrossRef.
- A. Bazgir, Z. N. Tisseh and P. Mirzaei, Tetrahedron Lett., 2008, 49, 5165–5168 CrossRef CAS.
- L. Wu, J. Zhang, L. Fang, C. Yang and F. Yan, Dyes Pigm., 2010, 86, 93–96 CrossRef CAS.
- L. Nagarapu, S. Kantevari, V. C. Mahankhali and S. Apuri, Catal. Commun., 2007, 8, 1173–1177 CrossRef CAS.
- B. Das, B. Ravikanth, R. Ramu, K. Laxminarayana and B. Rao Vittal, J. Mol. Catal. A: Chem., 2006, 255, 74–77 CrossRef CAS.
- A. Saini, S. Kumar and J. S. Sandhu, Synlett., 2006, 1928–1932 CAS.
- M. Dabiri, S. C. Azimi and A. Bazgir, Chem. Pap., 2008, 62, 522–526 CrossRef CAS.
- S. Ko and C.-F. Yao, Tetrahedron Lett., 2006, 47, 8827–8829 CrossRef CAS.
- J. V. Madhav, V. T. Reddy, P. N. Reddy, M. N. Reddy, S. Kumar, P. A. Crooks and B. Rajitha, J. Mol. Catal. A: Chem., 2009, 304, 85–87 CrossRef.
- H. R. Shaterian, M. Ghashang and A. Hassankhani, Dyes Pigm., 2008, 76, 564–568 CrossRef CAS.
- L. Q. Wu, Y. F. Wu, C. G. Yang, L. M. Yang and L. J. Yang, J. Braz. Chem. Soc., 2010, 21, 941–945 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cy20276j |
| This journal is © The Royal Society of Chemistry 2012 |
