Selective hydrogenation of higher saturated carboxylic acids to alcohols using a ReOx–Pd/SiO2 catalyst†
Yasuyuki
Takeda
,
Yoshinao
Nakagawa
* and
Keiichi
Tomishige
*
Department of Applied Chemistry, School of Engineering, Tohoku University, 6-6-07, Aoba, Aramaki, Aoba-ku, Sendai, 980-8579, Japan. E-mail: yoshinao@erec.che.tohoku.ac.jp; tomi@erec.che.tohoku.ac.jp; Fax: +81 022-795-7215
First published on 31st July 2012
Abstract
Higher fatty alcohols were synthesized in high yield (>92%) by direct hydrogenation of fatty acids catalyzed by ReOx–Pd/SiO2. High catalytic performance of ReOx–Pd/SiO2 can be generated by the synergy between ReOx and Pd. Preferential adsorption of the carboxylic acid to alcohols prohibited the overreaction of alcohols to alkanes.
Much attention has been paid to biomass conversion to fuels and chemicals in terms of the substitution of fossil fuels such as petroleum on the basis that biomass has renewable and carbon-neutral characters.1–4 One of the biomass resources is triglyceride of fatty acids from vegetable oils. Fatty acids and fatty acid esters are given by the hydrolysis of triglyceride and transesterification with alcohols, respectively. Catalytic hydrogenation of fatty acids and their esters gives fatty alcohols, which are widely used in lubricants, surfactants, or plasticizers, cosmetics and biofuels.1 In addition, it has been known that various carboxylic acids can be produced by the fermentation of sugars, and these carboxylic acids will be regarded as platforms in the biomass refinery.5 Hydrogenation of these acids to alcohols may give useful products. Therefore, direct hydrogenation of carboxylic acids to alcohols is an important reaction. The side reactions include decarboxylation to alkanes (RCOOH → RH + CO2), consecutive hydrogenolysis of the produced alcohols to alkanes (RCH2OH + H2 → RCH3 + H2O), the esterification of the produced alcohols (RCOOH + RCH2OH → RCOOCH2R + H2O), and the hydrogenolysis of the C–C bond giving the degradation products. The hydrogenation of fatty acids over a copper-chromite catalyst, which is commercially used for hydrogenation of fatty acid esters to alcohols under severe conditions (2–30 MPa, 473–573 K),6–8 requires even severe conditions (20–50 MPa, 473–673 K).9 In addition, copper-chromite catalysts have a problem of the use of toxic chromium involving environmental and health hazards. Development of catalysts without using Cr and under milder reaction conditions has been attempted. Rhenium heptoxide (17–27 MPa, 423–538 K) produced C6–C18 fatty alcohols at 36–100% yield.10 Ruthenium–tin on alumina (8 MPa, 523 K) gave 80–85% yield of C12–C18 fatty alcohols.11 Pt–Re/TiO2 (2 MPa, 403 K) reported 61–90% selectivity to C10–C18 fatty alcohols at 79–83% conversion.12 The Ni/Al2O3 + In2O3 catalyst (1.76 MPa, 553 K) produced 1-octanol in 70% yield from octanoic acid hydrogenation under vapor phase.13 This indicated that most catalysts give 70–85% yield of alcohol, and the catalysts giving >90% yield need high hydrogen pressure and high temperature. This communication reports selective hydrogenation of higher fatty acids to alcohols with >92% yield at 413 K using ReOx–Pd/SiO2, where the synergy between ReOx species and Pd metal particles enhanced the activity and suppressed the consecutive hydrogenolysis of the produced alcohol by the preferential adsorption of the carboxylic acid substrate.
The M/SiO2 (M = Ir, Rh, Ru, Pt and Pd) catalysts were prepared by impregnating SiO2 (BET surface area 535 m2 g−1) with an aqueous solution of H2IrCl6, RhCl3·3H2O, RuCl3·nH2O, H2PtCl6·6H2O and PdCl2. The M–ReOx/SiO2 (M = Ir, Rh, Ru, Pt and Pd) were prepared by impregnating M/SiO2 after the drying procedure with an aqueous solution of NH4ReO4. Catalysts were calcined in air at 773 K for 3 h after drying. The loading amount of Ir, Rh, Ru, Pt and Pd was 1 wt%. The activity test was performed in a stainless steel autoclave with an inserted glass vessel. Products were analyzed by GC. Yields and selectivities were calculated on carbon basis. Details of methods for catalyst preparation and activity test are described in the ESI.†
Hydrogenation of stearic acid (STA) was conducted over modified or unmodified ReOx/SiO2 catalysts with noble metal (Fig. 1). ReOx/SiO2 catalyzed the hydrogenation of STA, this property agreed well with that reported previously.12,14–16 The modification effect of ReOx/SiO2 with noble metals was strongly dependent on the metal elements. The addition of Ru, Rh, Pd, and Pt enhanced the catalytic activity of hydrogenation, and the additive effect of Ir was very small. The addition of Rh did not improve the selectivity of hydrogenation to 1-octadecanol (C18OH), and the addition of Ru decreased the selectivity significantly. In contrast, the addition of Pd and Pt improved the selectivity. Totally simultaneous promotion in terms of the catalytic activity and selectivity is realized by the addition of Pd, therefore, further investigation focused on ReOx–Pd/SiO2 catalysts. The hydrogenation of Re and Pd was optimized in terms of TOF based on total Re, and determined to be Re = 14 wt% and Re/Pd = 8 wt% in molar ratio (Fig. S2).† We investigated the supports for ReOx–Pd catalysts using SiO2, ZrO2, Al2O3, CeO2, TiO2 and carbon (Fig. S1).† SiO2 showed the highest activity and selectivity to C18OH, and we used SiO2 as a support.
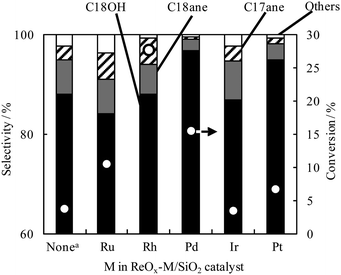 | ||
| Fig. 1 STA hydrogenation over ReOx–M/SiO2 (Re/M = 4, M: 1 wt%). Reaction conditions: 5 wt% STA solution in 1,4-dioxane 20 g, catalyst 0.1 g, H2 pressure 8 MPa, temperature 413 K, reaction time 4 h. STA: stearic acid, C18OH: 1-octadecanol, C18ane: n-octadecane, C17ane: n-heptadecane, Others: methane, ethane and CO2. aReOx/SiO2, Re: 7.2 wt%. | ||
Table 1 lists the results of hydrogenation of various saturated C6–C18 fatty acids over optimized ReOx–Pd/SiO2. Regarding all these substrates, high yields (>92%) of fatty alcohols were achieved. High alcohol yield could be achieved even when using lower amounts of catalyst (entry 8), and the TON based on Re reached near 300. The amount of eluted metal in the reaction solution was analyzed by inductively-coupled plasma atomic emission spectrometry (ICP-AES). It was verified that the leaching of Re and Pd on ReOx–Pd/SiO2 was negligible.
| Entry | Substrate (n: carbon number) | Time/h | Yield/% | TONa | ||
|---|---|---|---|---|---|---|
| Alcohol | Alkanes | Others | ||||
| Reaction conditions: Cn−1H2n−1COOH 1 g, 1,4-dioxane 19 g, ReOx–Pd/SiO2 (Re/Pd = 8, 1 wt% Pd) 0.1 g, H2 pressure 8 MPa, temperature 413 K. Alcohol: CnH2n+1OH, alkanes: CnH2n+2 + Cn−1H2n, others: methane, ethane, CO2 and ester.a TON = alcohol (mol)/Re (mol).b Catalyst weight 0.015 g, temperature 433 K. | ||||||
| 1 | Stearic acid (18) | 16 | 93.8 | 5.6 | 0.6 | 43 |
| 2 | Palmitic acid (16) | 16 | 92.5 | 6.8 | 0.6 | 48 |
| 3 | Myristic acid (14) | 16 | 95.5 | 4.2 | 0.3 | 56 |
| 4 | Lauric acid (12) | 16 | 95.0 | 4.8 | 0.2 | 63 |
| 5 | Capric acid (10) | 16 | 96.6 | 3.1 | 0.3 | 75 |
| 6 | Octanoic acid (8) | 16 | 98.3 | 1.6 | 0.1 | 94 |
| 7 | Hexanoic acid (6) | 24 | 98.7 | 0.3 | 1.0 | 115 |
| 8 | Stearic acid (18)b | 96 | 93.9 | 5.4 | 0.7 | 287 |
In the STA hydrogenation, by-products are n-octadecane (C18ane) and n-heptadecane (C17ane), which are thought to be formed by the consecutive hydrogenolysis of the C18OH and decarboxylation of STA, respectively. In the case of ReOx–Pd/SiO2, the selectivity to C18ane was much higher than that to C17ane, therefore, the main side reaction is the hydrogenolysis of C18OH. Therefore, in order to evaluate the contribution of the consecutive hydrogenolysis of fatty alcohol, the reaction test of C16OH and STA + C16OH was carried out and the results are listed in Table 2. The reaction of C16OH proceeded to give C16ane mainly, and the C–C cracking reaction also proceeded to give C15ane and others with rather low selectivity. The rate of the C16OH reaction (Table 2, entry 2) was 1/4 of that of the STA reaction (Table 2, entry 1). In contrast, the consumption of C16OH did not proceed in the reaction of STA + C16OH at all (Table 2, entry 3), and this result suggests that hydrogenolysis of alcohols in the liquid phase did not proceed on ReOx–Pd/SiO2 at all in the presence of carboxylic acid. It is interpreted that the adsorption of the carboxylic acid is more favorable than that of the alcohol. This interpretation is also supported by the decrease in both the activity and selectivity to C18OH in the hydrogenation of methyl stearate (Table 2, entry 4). Hydrogenation of glyceryl tristearate gave even poorer result (1% C18OH yield). Another important point is that a significant amount of C18ane was formed in the hydrogenation of STA. One possible explanation is that C18ane is formed by the hydrogenolysis of the adsorbed C18OH species before the release of the adsorbed C18OH species such as alkoxide from the catalyst surface. Considering that Pd/SiO2 has no activity in hydrogenation of STA under the present reaction conditions, and ReOx/SiO2 shows some activity, the active site in ReOx–Pd/SiO2 can be ReOx species. In order to investigate the effect of Pd addition, we tested the activity of the physical mixture of Pd/SiO2 and ReOx/SiO2, and the result is also described in Table 2, entry 5. The activity of ReOx/SiO2 + Pd/SiO2 was little higher than that of ReOx/SiO2, and Pd did not promote the hydrogenation reaction so significantly. This tendency indicates that the direct interaction between ReOx and Pd is essential for the high activity. We conducted temperature-programmed reduction (TPR) of unreduced ReOx/SiO2 and ReOx–M/SiO2 with H2 (Fig. 2 and Table 3). All catalysts with noble metal were more easily reduced than ReOx/SiO2. However, the correlation between the activity and the valence of Re is not clear. One possible explanation is that the hydrogen species activated on noble metal is supplied to the ReOx site adsorbing substrate, and this step affects the reaction rate. High selectivity of ReOx–Pd/SiO2 may be related to low hydrogenolysis activity of Pd metal. Ru, Rh–ReOx and Ir–ReOx catalysts have been known to show C–O hydrogenolysis activity,17–19 which may lower the C18OH selectivity in this system. Further investigation is necessary for the elucidation of the active site and the reaction mechanism.
| Entry | Substrate | STA | C16OH | |||||
|---|---|---|---|---|---|---|---|---|
| Conv./% | Selectivity/% | Conv./% | Selectivity /% | |||||
| C18OH | C18ane | C17ane | Others | C16ane | ||||
| Reaction conditions: 5 wt% substrate solution of 1,4-dioxane 20 g, ReOx–Pd/SiO2 (Re/Pd = 8, 1 wt% Pd) 0.1 g, initial H2 pressure 8 MPa, temperature 413 K, reaction time 4 h.a STA 1 g + C16OH 0.25 g + 1,4-dioxane 18.75 g. STA: stearic acid, C18OH: 1-octadecanol, C18ane: n-octadecane, C17ane: n-heptadecane, C16OH: 1-hexadecanol, C16ane: n-hexadecane, others: methane, ethane and CO2.b Selectivity of methyl stearate hydrogenation was calculated with the exclusion of C1 products originated from methanol in the ester.c Physical mixture of 0.1 g ReOx/SiO2 (14 wt%) + 0.1 g Pd/SiO2 (1 wt%, Re/Pd = 8) was used as a catalyst. | ||||||||
| 1 | STA | 45.3 | 96.6 | 2.6 | 0.4 | 0.3 | — | — |
| 2 | C16OH | — | — | — | — | — | 12.2 | 91.8 |
| 3 | STA + C16OHa | 43.1 | 96.8 | 2.5 | 0.4 | 0.3 | 0.0 | 100 |
| 4 | Methyl stearateb | 28.6 | 89.7 | 6.7 | 0.6 | 3.0 | — | — |
| 5 | STAc | 23.0 | 96.2 | 2.8 | 0.6 | 0.4 | — | — |
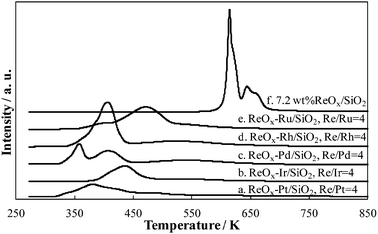 | ||
| Fig. 2 TPR profiles of ReOx/SiO2 and ReOx–M/SiO2. | ||
| Entry | M | M amount/mmol g cat−1 | Re amount/mmol g cat−1 | Consumption peak | Valence of Rea | STA conversion rate based on Reb/h−1 | |
|---|---|---|---|---|---|---|---|
| Temperature range/K | H2 consumption/mmol g cat−1 | ||||||
| a Calculated valence of Re after the peak. Ru, Rh and Ir were assumed to be reduced simultaneously during the peak. Pd and Pt were assumed to be reduced at room temperature. b Based on the data in Fig. 1. | |||||||
| 1 | None | — | 0.39 | 563–704 | 1.0 | 2.0 | 0.8 |
| 2 | Ru | 0.099 | 0.40 | 333–525 | 0.77 | 3.9 | 2.3 |
| 3 | Rh | 0.097 | 0.39 | 322–450 | 0.82 | 3.5 | 6.2 |
| 4 | Pd | 0.094 | 0.38 | 320–455 | 0.48 | 4.5 | 3.6 |
| 5 | Ir | 0.052 | 0.21 | 351–502 | 0.39 | 4.2 | 1.5 |
| 6 | Pt | 0.051 | 0.21 | 319–465 | 0.41 | 3.0 | 2.8 |
A part of this research is funded by the Cabinet Office, Government of Japan through its “Funding Program for Next Generation World-leading Researchers”.
Notes and references
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411 CrossRef CAS.
- P. Gallezot, Green Chem., 2007, 9, 295 RSC.
- M. Schlaf, Dalton Trans., 2006, 4645 RSC.
- J. N. Chheda, G. W. Huder and J. A. Dumesic, Angew. Chem., Int. Ed., 2007, 46, 7164 CrossRef CAS.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539 RSC.
- H. Adkins and K. Folkers, J. Am. Chem. Soc., 1931, 35, 1095 CrossRef.
- U. R. Kreutzer, J. Am. Oil Chem. Soc., 1984, 61, 343 CrossRef CAS.
- R. D. Rieke, D. S. Thakur, B. D. Roberts and G. T. White, J. Am. Oil Chem. Soc., 1997, 74, 333 CrossRef CAS.
- J. Pohl, F. J. Carduck and G. Goebel, US Pat., 4935556, 1990 Search PubMed.
- H. S. Broadbent, G. C. Campbell, W. J. Bartley and J. H. Johnson, J. Org. Chem., 1959, 24, 1847 CrossRef CAS.
- M. Toba, S.-I. Niwa, F. Mizukami, Z. Koppány, L. Guczi, K.-Y. Cheah and T.-S. Tang, Appl. Catal., A, 1999, 189, 243 CrossRef CAS.
- H. G. Manyar, C. Paun, R. Pilus, D. W. Rooney, J. M. Thompson and C. Hardacre, Chem. Commun., 2010, 46, 3279 RSC.
- G. Onyestyák, S. Harnos and D. Kalló, Catal. Commun., 2011, 16, 184 CrossRef.
- H. S. Broadbent and T. G. Selin, J. Org. Chem., 1962, 28, 2343 CrossRef.
- H. S. Broadbent and W. J. Bartley, J. Org. Chem., 1962, 28, 2345 CrossRef.
- H. S. Broadbent and D. W. Seegmiller, J. Org. Chem., 1962, 28, 2347 CrossRef.
- I. Furikado, T. Miyazawa, S. Koso, A. Shimao, K. Kunimori and K. Tomishige, Green Chem., 2007, 9, 582 RSC.
- S. Koso, I. Furikado, A. Shimao, T. Miyazawa, K. Kunimori and K. Tomishige, Chem. Commun., 2009, 2035 RSC.
- Y. Nakagawa, Y. Shinmi, S. Koso and K. Tomishige, J. Catal., 2010, 272, 191 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and results. See DOI: 10.1039/c2cy20302b |
| This journal is © The Royal Society of Chemistry 2012 |
