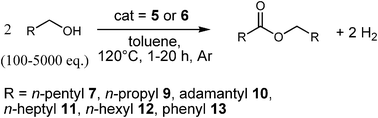
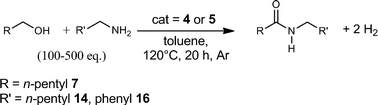
Direct coupling of alcohols to form esters and amides with evolution of H2 using in situ formed ruthenium catalysts
Martin H. G.
Prechtl†
*ab,
Kathrin
Wobser
b,
Nils
Theyssen
b,
Yehoshoa
Ben-David
c,
David
Milstein
c and
Walter
Leitner
*ab
aInstitut für Technische und Makromolekulare Chemie, Rheinisch-Westfälische Technische Hochschule Aachen, Worringer Weg 1, 52074 Aachen, Germany. E-mail: leitner@itmc.rwth-aachen.de; Web: www.itmc.rwth-aachen.de Fax: +49 241 80 22177; Tel: +49 241 80 26480
bMax-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim/Ruhr, Germany
cDepartment of Organic Chemistry, The Weizmann Institute of Science, Rehovot, 76100, Israel
First published on 10th July 2012
Abstract
A simple approach for the catalytic conversion of primary alcohols into their corresponding esters and amides, with evolution of H2 gas using in situ formed ruthenium PNP- and PNN-pincer catalysts, is presented. The evaluation showed conversions for the esterification with turnover numbers as high as 4300, and >400 for the amidation.
Catalytic hydrogenation and dehydrogenation reactions play a major role in industrial processes as well as in academic research. In recent years especially progress in the catalytic acceptorless dehydrogenation of primary alcohols is remarkable. In most cases ruthenium hydride or iridium complexes were used for the transformation of primary alcohols into aldehydes.1 Depending on the reaction conditions, tailor-made catalysts are also applicable for various tandem- or domino-reactions2 revealing high selectivity. In this respect the formation of esters,3 (aza)Wittig-,4Aldol-,4 and Knoevenagel-products is known.4 Most remarkably, in the presence of amines, amides5 are easily accessible. For this purpose today's most active catalyst is a ruthenium pincer complex [Ru(PNN)H(CO)] (1, PNN = {6-[(di-tert-butylphosphino)methyl]pyridin-2-ylmethyl}-diethylamine; Fig. 1) with a cooperative dearomatised pyridine pincer-ligand backbone. It is active for the direct-synthesis of esters starting from primary alcohols as well as amides if amines are present as well.5
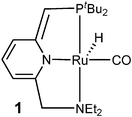 | ||
| Fig. 1 Complex 1 for the catalytic dehydrogenative coupling of primary alcohols to homoesters and amides. | ||
In other attempts it has been shown that certain ruthenium pincer-complexes are suitable to convert alcohols and amines into the corresponding coupled imines.6 Moreover, it has been shown that certain ruthenium and iridium pincer complexes are highly active for the production of hydrogen gas from isopropanol, for the direct conversion of ethanol into ethyl acetate and for the hydrogenation of (chiral) esters ruthenium and osmium pincer complexes were successfully applied.7 In the case of the osmium complexes, also selective hydrogenation of unsaturated fatty acid esters to the corresponding unsaturated alcohols was demonstrated, as well as the dehydrogenative coupling of aliphatic alcohols to form their esters. In general, ruthenium hydride complexes are often used for hydrogenation of a variety of compounds including ketones, aldehydes, alkynes and alkenes under hydrogen gas or under transfer hydrogenation conditions.1 And today it is widely accepted that in most of these transformations metal dihydrogen complexes are key intermediates.8
Based on previous results using the [Ru(PNN)H(CO)] catalyst 1 with a hemi-labile and cooperative pincer-backbone,3 now the simple in situ formation of ruthenium dehydrogenation catalysts (Scheme 1) for the transformation of primary alcohols into esters with evolution of two equivalents of dihydrogen gas is presented (Table 1). As depicted in Scheme 1, the treatment of the readily available ruthenium precursor [Ru(COD)(2-methylallyl)2] (2, COD = 1,5-cyclooctadiene) with either the hemi-labile PNN (3) or the stronger PNP (4) pincer ligand leads presumably to the in situ formation of the precatalysts [Ru(PNN)(2-methylallyl)2] 5 and [Ru(PNP)(2-methylallyl)2] 6. This stays in agreement with previous findings, where the exchange of COD in [Ru(COD)(2-methylallyl)2] 2 with chelating phosphine ligands has been described by different research groups.9 Thus, for the initial catalytic experiments we focussed first on the stronger coordinating PNP-ligand 4.
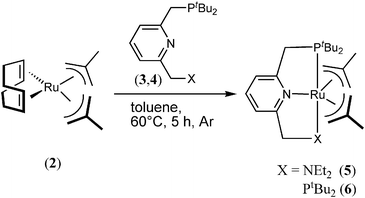 | ||
| Scheme 1 Protocol for the in situ formed precatalysts 5 and 6. | ||
| No | L | Cata [mol%] | ROH | t [h] | Conv. [%] | E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ab Ab |
|---|---|---|---|---|---|---|
a Reaction conditions: Ru (3)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L (3 or 4) = 1 L (3 or 4) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3, ROH: 1-hexanol 7, 1-butanol 9, 1-adamantylmethanol 10, 1-octanol 11, 1-heptanol 12, benzylalcohol 13, reflux in toluene under argon flow.
b Ratio: E 1.3, ROH: 1-hexanol 7, 1-butanol 9, 1-adamantylmethanol 10, 1-octanol 11, 1-heptanol 12, benzylalcohol 13, reflux in toluene under argon flow.
b Ratio: E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A = ester A = ester![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aldehyde. aldehyde.
|
||||||
| 1 | 4 | 1.0 | 7 | 15 | >99 | >98 |
| 2 | 4 | 0.1 | 7 | 20 | 33 | >98 |
| 3 | 4 | 1.0 | 9 | 20 | 90 | 84 |
| 4 | 4 | 1.0 | 10 | 20 | 68 | 82 |
| 5 | 4 | 1.0 | 11 | 20 | 85 | 86 |
| 6 | 4 | 0.2 | 12 | 20 | 62 | 97 |
| 7 | 3 | 1.0 | 7 | 1 | 97 | 98 |
| 8 | 3 | 0.05 | 7 | 20 | 98 | 99 |
| 9 | 3 | 0.02 | 7 | 20 | 86 | 99 |
| 10 | 3 | 0.1 | 9 | 20 | 99 | 99 |
| 11 | 3 | 0.05 | 9 | 20 | 83 | 99 |
| 12 | 3 | 0.1 | 10 | 20 | 68 | 82 |
| 13 | 3 | 1.0 | 13 | 20 | 80 | 96 |
| 14 | 3 | 0.05 | 11 | 20 | 97 | 99 |
Using a precatalyst loading of 1.0 mol%, a variety of aliphatic alcohols (7 and 9–13) show moderate to high conversions into the corresponding esters. For example, the treatment of 1-hexanol 7 with a mixture of 2 and PNP 4 (1.3 eq.), dissolved in toluene, gave >99% conversion into the corresponding homoester 8 within 15 h under reflux (entry 1). Using this protocol with other primary alcohols, moderate to good conversions and selectivities were obtained (Table 1, entries 3–6). Consistently, a lowering of the catalyst loading showed also decreasing conversions, i.e. 33% ester was formed for the treatment of 1-hexanol with the 0.1 mol% catalyst in 20 h (entry 2).
Encouraged by these results, consequently the PNN ligand 3 was tested, since its corresponding ruthenium complex 1 is known to show a superior activity for this kind of dehydrogenative couplings of primary alcohols.3 And indeed, the treatment of 7 with 2 and 3 (1.0 and 1.3 mol% respectively) gave 97% yield after 1 h using again toluene as a solvent (Table 1, entry 7). Lowering the catalyst loadings showed still very high conversion (98% after 20 h, entry 8) with just 0.05 mol% catalyst and still remarkable high conversions (86% after 20 h, entry 9) were obtained with 0.02 mol% catalyst. The conversion of 86% with 0.02 mol% of catalyst 5 is related to a turnover number (TON) of 4300 after 20 hours. Complex 1 gave otherwise a TON of ~1000 after six hours under similar conditions, and presumably with a higher substrate loading a similar high TON.3 However, the activity of the in situ formed species based on precatalyst 5 is remarkable in comparison to the one of complex 1. Other primary alcohols showed also moderate (68%) to very high conversions (99%) with catalyst loadings as low as 1.0 mol% to 0.05 mol% (Table 1; entries 10–14).
To further evaluate the potential of the protocol for the in situ formed catalytic systems, both systems were tested for the challenging dehydrogenative coupling of primary alcohols with primary amines (Table 2). This pioneering reaction has been published in 2007 using catalyst 1.5 And, indeed, treating a toluene solution with our in situ systems (2/3 or 2/4) results in the preferred formation of amides in the latter case (Table 2).
The addition of 100 eq. of 1-hexanol 7 and 100 eq. of 1-hexylamine 14 to a solution of Ru-2/PNP-4 in toluene resulted in a high substrate conversion, but relatively poor selectivity for the amide (amide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ester = 44
ester = 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56; Table 2: entry 1). Using the system Ru-2/PNN-3 for these substrates instead, hexanoic acid hexylamide 15 is formed with high conversions (98%) and much better selectivity (88%, entry 2). A lowering of the catalyst concentration to a value of 0.2 mol% still leads to the amide (82%; TON = 410) with almost unchanged selectivity (83%, entry 3). Similar results were obtained with 1-hexanol 7 and benzylamine 16 as substrates (conversion: 91%, 86% amide; entry 4). In comparison to previously reported in situ catalysts for the direct amidation of alcohols, we found here a quite active system which uses comparably low catalyst loadings without the need for the addition of base. Other direct amidation methods use higher metal precursor (2–10 mol%) and ligand loadings (2–10 mol%) and catalytic active species are only obtained in the presence of base (8–30 mol%).5b,c
56; Table 2: entry 1). Using the system Ru-2/PNN-3 for these substrates instead, hexanoic acid hexylamide 15 is formed with high conversions (98%) and much better selectivity (88%, entry 2). A lowering of the catalyst concentration to a value of 0.2 mol% still leads to the amide (82%; TON = 410) with almost unchanged selectivity (83%, entry 3). Similar results were obtained with 1-hexanol 7 and benzylamine 16 as substrates (conversion: 91%, 86% amide; entry 4). In comparison to previously reported in situ catalysts for the direct amidation of alcohols, we found here a quite active system which uses comparably low catalyst loadings without the need for the addition of base. Other direct amidation methods use higher metal precursor (2–10 mol%) and ligand loadings (2–10 mol%) and catalytic active species are only obtained in the presence of base (8–30 mol%).5b,c
Further experiments with 1-hexylamine 14 as the sole substrate support the previously proposed mechanism for this type of dehydrogenative coupling.3,5 Heating the amine in toluene in the presence of Ru-2/PNN-3 gave no products, neither the simple 1-hexylimine, nor one of the possible coupling products N-hexyl-hexanamidine or dihexyl amine (coupling under ammonia loss). This result indicates that the crucial reaction step is the formation of the aldehyde as the reactive intermediate,3,5 which then reacts with a primary amine (or primary alcohol) to give the hemi-amidale (or hemi-acetale) which is then dehydrogenated to the corresponding amide (or ester). The aldehyde intermediate was also identified by IR-techniques and trapped in an indirect Wittig-reaction in similar reactions.10 Moreover, it is known that ruthenium hydrides are capable of decarbonylating primary alcohols under mild conditions (room temperature),11a or at elevated temperature.11b This decarbonylation results in the formation of a ruthenium pincer complex carrying CO as a ligand. Such a complex might exhibit a similar reactivity to complex 1 for both dearomatisation/aromatisation of the cooperative pyridine backbone under dehydrogenation/hydrogenation conditions with formation of ruthenium hydride and trans-dihydride complexes.3,5,12 As previously discussed here, the catalytic activity of catalyst 5 is comparable with complex 1. And, this is probably related to a similar structure formed in situ under the applied conditions. However, the real structures of the in situ formed ruthenium catalysts remain unclear.
Conclusions
In conclusion, the easy applicable conversion of primary alcohols into their corresponding esters using in situ formed catalyst systems was demonstrated. Likewise, primary alcohols can be coupled with primary amines resulting in amide formation with high selectivity. This protocol allows use of a variety of substrates carrying OH– and NH2– functionalities as the presented catalysts are sensitive to these functional groups in hydrocarbons.Experimental section
General information
All reactions were carried out in flame-dried glassware under argon. All alcohols and amines were obtained from Sigma Aldrich or Acros and dried or deoxygenated prior to use. [Ru(COD)(2-methylallyl)2] 3 was used as received from Acros. Toluene was dried over magnesium anthracene. GC analyses were performed on a HP GC-MS/GC-EI SSQ7000 or on a HP 6890 GC System/HP Mass Selective Detector 5973.General procedure for the direct esterification
Experimental protocol for entry 1 (Table 1: esters): the reactions were performed under argon-flow in a dried 20 mL three-necked round bottom flask, equipped with a reflux condenser with an argon inlet/outlet, a second argon valve and a screw-capped adapter with a Teflon-coated septum. 10 mg (31.3 μmol) of [Ru(COD)(2-methylallyl)2] 2 were introduced followed by the addition of 1.3 equivalents of the PNP ligand 4 (16 mg, 40.7 μmol). Then, 5 mL of toluene were added via a syringe and the mixture was stirred at 60 °C for 5 hours followed by addition of 100 equivalents of 1-hexanol 7 (319 mg, 3.1 mmol) via a syringe through the Teflon-coated septum. Afterwards the reaction mixture was heated to 120 °C for 15 h. The sample was analysed by GC and GC-MS.General procedure for the direct amidation
Experimental protocol for entry 2 (Table 2: amides): the reactions were performed under argon-flow in a dried 20 mL three-necked round bottom flask, equipped with a reflux condenser with an argon inlet/outlet, a second argon valve and a screw-capped adapter with a Teflon-coated septum. 20 mg of [Ru(COD)(2-methylallyl)2] 2 (62.6 μmol) were introduced followed by the addition of 1.3 equivalents of the PNN 3 (26 mg, 81.4 μmol). Then, 5 mL of toluene were added via a syringe and the mixture was stirred at 60 °C for 5 hours followed by addition of 100 equivalents of 1-hexanol 7 (638 mg, 6.2 mmol) and 100 equivalents of 1-hexylamine 14 (626 mg, 6.2 mmol) via a syringe through the Teflon-coated septum. Afterwards the reaction mixture was heated to 120 °C for 20 h. The sample was analysed by GC and GC-MS.Acknowledgements
The authors would like to thank the Max-Planck-Society, the RWTH Aachen, the Fonds der Chemischen Industrie and the German-Israeli-Project Cooperation (DIP-G 7.1) for funding.Notes and references
- (a) P. J. Jessop and R. H. Morris, Coord. Chem. Rev., 1992, 121, 155 CrossRef CAS; (b) C. W. Jung and P. E. Garrou, Organometallics, 1982, 1, 658 CrossRef CAS; (c) D. Morton, D. J. Cole-Hamilton, I. D. Utuk, M. Paneque-Sosa and M. Lopez-Poveda, J. Chem. Soc., Dalton Trans., 1989, 489 RSC; (d) D. Morton and D. J. Cole-Hamilton, J. Chem. Soc., Chem. Commun., 1988, 1154 RSC; (e) L. A. Oro and M. A. Esteruelas, Chem. Rev., 1998, 98, 577 CrossRef; (f) Y. Lin and Y. Zhou, J. Organomet. Chem., 1990, 381, 135 CrossRef CAS; (g) S. Burling, M. K. Whittlesey and J. M. J. Williams, Adv. Synth. Catal., 2005, 347, 591 CrossRef CAS.
- (a) D. E. Fogg and E. N. dos Santos, Coord. Chem. Rev., 2004, 248, 2365 CrossRef CAS; (b) J.-C. Wasilke, S. J. Obrey, R. T. Baker and G. C. Bazan, Chem. Rev., 2005, 105, 1001 CrossRef CAS.
- J. Zhang, G. Leitus, Y. Ben-David and D. Milstein, J. Am. Chem. Soc., 2005, 127, 10840 CrossRef CAS.
- (a) M. G. Edwards and J. M. J. Williams, Angew. Chem., 2002, 114, 4934 ( Angew. Chem., Int. Ed. , 2002 , 41 , 4740 ) CrossRef; (b) M. G. Edwards, R. F. R. Jazzar, B. M. Paine, D. J. Shermer, M. K. Whittlesey, J. M. J. Williams and D. D. Edney, Chem. Commun., 2004, 90 RSC; (c) P. J. Black, M. G. Edwards and J. M. J. Williams, Eur. J. Org. Chem., 2006, 4367 CrossRef CAS; (d) G. Cami-Kobeci and J. M. J. Williams, Chem. Commun., 2004, 1072 RSC; (e) P. J. Black, G. Cami-Kobeci, M. G. Edwards, P. A. Slatford, M. K. Whittlesey and J. M. J. Williams, Org. Biomol. Chem., 2006, 4, 116 RSC.
- (a) C. Gunanathan, Y. Ben-David and D. Milstein, Science, 2007, 317, 790 CrossRef CAS; (b) L. U. Nordstrom, H. Vogt and R. Madsen, J. Am. Chem. Soc., 2008, 130, 17672 CrossRef CAS; (c) S. C. Ghosh, S. Muthaiah, Y. Zhang, X. Xu and S. H. Hong, Adv. Synth. Catal., 2009, 351, 2643 CrossRef CAS.
- B. Gnanaprakasam, J. Zhang and D. Milstein, Angew. Chem., 2010, 122, 1510 ( Angew. Chem., Int. Ed. , 2010 , 49 , 1468 ) CrossRef.
- (a) M. Nielsen, A. Kammer, D. Cozzula, H. Junge, S. Gladiali and M. Beller, Angew. Chem., 2011, 123, 9767 ( Angew. Chem., Int. Ed. , 2011 , 50 , 9593 ) CrossRef; (b) M. Nielsen, H. Junge, A. Kammer and M. Beller, Angew. Chem., 2012, 124, 5809 ( Angew. Chem., Int. Ed. , 2012 , 51 , 5711 ) Search PubMed; (c) D. Spasyuk, S. Smith and D. Gusev, Angew. Chem., 2012, 124, 2826 ( Angew. Chem., Int. Ed. , 2012 , 51 , 2772 ) CrossRef.
- (a) M. H. G. Prechtl, Y. Ben-David, D. Giunta, S. Busch, Y. Taniguchi, W. Wisniewski, H. Görls, R. J. Mynott, N. Theyssen, D. Milstein and W. Leitner, Chem.–Eur. J., 2007, 13, 1539 CrossRef CAS; (b) M. H. G. Prechtl, M. Holscher, Y. Ben-David, N. Theyssen, R. Loschen, D. Milstein and W. Leitner, Angew. Chem., 2007, 119, 2319 ( Angew. Chem., Int. Ed. , 2007 , 46 , 2269 ) CrossRef; (c) M. H. G. Prechtl, M. Holscher, Y. Ben-David, N. Theyssen, D. Milstein and W. Leitner, Eur. J. Inorg. Chem., 2008, 22, 3493 CrossRef.
- (a) J. P. Genet, S. Mallart, C. Pinel, S. Juge and J. A. Laffitte, Tetrahedron: Asymmetry, 1991, 2, 43 CrossRef CAS; (b) C. Six, K. Beck, A. Wegner and W. Leitner, Organometallics, 2000, 19, 4639 CrossRef CAS; (c) S. Busch and W. Leitner, Chem. Commun., 1999, 2305 RSC; (d) Y. Sun, H.-S. Chan, P. H. Dixneuf and Z. Xie, Organometallics, 2004, 23, 5864 CrossRef CAS.
- M. H. G. Prechtl, Dissertation, RWTH Aachen, 2007 Search PubMed.
- (a) L. S. Van Der Sluys, G. J. Kubas and K. G. Caulton, Organometallics, 1991, 10, 1033 CrossRef CAS; (b) F. M. A. Geilen, B. Engendahl, M. Hoelscher, J. Klankermayer and W. Leitner, J. Am. Chem. Soc., 2011, 133, 14349 CrossRef CAS.
- C. Gunanathan and D. Milstein, Acc. Chem. Res., 2011, 44, 588 CrossRef CAS.
Footnote |
| † Present address: Institut für Anorganische Chemie, Universität zu Köln, Greinstr. 6, 50939 Köln, Germany. E-mail: martin.prechtl@uni-koeln.de; http://catalysis.uni-koeln.de; Fax: +49 221 470 1788; Tel: +49 221 470 1981 |
| This journal is © The Royal Society of Chemistry 2012 |