A novel approach to timberwood fragrances†
Jan
Schütz
* and
Werner
Bonrath
DSM Nutritional Products LTD., Process Research & Development, P.O. Box 2676, CH-4002 Basel, Switzerland. E-mail: jan.schuetz@dsm.com; Fax: +41 (0)61 815 8650; Tel: +41 (0)61 815 8119
First published on 20th June 2012
Abstract
Timberone (1-(2,2,6-trimethylcyclohexyl)hexan-3-one), a compound interesting the fragrance industry, was synthesized in three steps, starting from readily available citral. All reaction steps could be catalyzed by heterogeneous catalysts in good selectivity.
Timberone (1-(2,2,6-trimethylcyclohexyl)hexan-3-one) (1) has a woody, amberlike odor and is valued in the fragrance industry for manufacturing perfumes and cosmetics. Although various synthetic protocols are available for synthesis of timberone (1) or its alcohol equivalent 1-(2,2,6-trimethylcyclohexyl)hexan-3-ol (timberol®) (2), reports on industrially applicable syntheses remain scarce. Attractive processes from easily obtained and thus inexpensive starting materials, low cost, low waste and possibly heterogeneously catalyzed reactions which allow easy separation from substrates and catalysts in contrast to most homogeneously catalyzed reactions are desirable.1
Common precursors used in the synthesis of timberone (1) or timberol® (2) are 1-(2,6,6-trimethylcyclohex-1 or 2-enyl)hex-1-en-3-ones (ethyl ionones) (3). The preparation of ethyl-ionones (3) was described by several groups. In an aldol condensation citral (4) was reacted with 2-pentanone, applying homogeneous catalysts, followed by cyclization to ethyl-ionones (3) with Brønsted acids as catalysts (Fig. 1).2–5
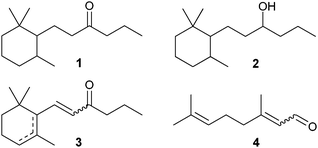 | ||
| Fig. 1 Compounds relevant for the synthesis of timberone (1). | ||
The synthesis of timberol® (2) (commercial name, origin: Dragoco, Holzminden, Germany) was studied in detail.6,7 The olfactory active trans-forms were synthesized, separated, and further applied in the fragrance industry.8,9 The commonly applied process starts from dehydrocyclocitral, followed by aldol condensation with 2-pentanone and homogeneously catalyzed hydrogenation. However, a direct synthesis of cyclocitral from citral (4) which would be industrially desirable is not reported.
Timberone (1) is less studied in the literature.6,8,10 The above described procedure for synthesis of timberol® delivers also timberone if the final hydrogenation is selective for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds.6,8 However, herein we describe a more direct pathway (3 steps) from citral (4) to timberone (1) applying heterogeneous catalysts in each step, turning this route to the most interesting one for timberone from an industrial point of view (Scheme 1).
C double bonds.6,8 However, herein we describe a more direct pathway (3 steps) from citral (4) to timberone (1) applying heterogeneous catalysts in each step, turning this route to the most interesting one for timberone from an industrial point of view (Scheme 1).
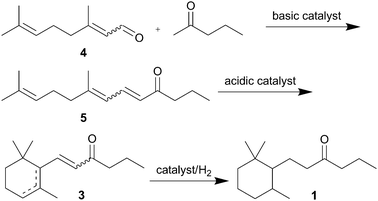 | ||
| Scheme 1 Studied synthesis of timberone (1). | ||
Industrial research and development is driven by the necessity to reduce waste, use less toxic reagents and solvents, and improve energy efficiency to achieve more sustainable processes. In the synthesis of timberone described herein heterogeneous catalysts can be applied in each step, avoiding waste formation and thus generating a more sustainable process than the ones reported in the literature. The three-step process needs less reagents and is more atom efficient than the published procedures for timberone.
The aldol condensation of citral (4) and 2-pentanone was studied with several basic catalysts. The use of solid bases is favoured because of the straightforward removal from the reaction mixture after the reaction and because of less waste formation as no neutralization is required. Homogeneously catalyzed reactions (aqueous NaOH, LiOH, Ba(OH)2, 16 mol%) resulted in selectivity between 83 and 95% (98% to full conversion) at 60 °C and up to 23 h reaction time. Several heterogeneous catalysts (20 wt%) were tested in the aldol condensation. Ambersp 900 OH, an anionic ion exchanger from Dow Chemicals, performed the best with 98% selectivity (97% conversion) at 60 °C and 23 h reaction time. Ca/Na on silica10 (22% and 23% respectively) required 80 °C reaction temperature for 4 h in the aldol condensation. Selectivity of 89% was achieved (99% conversion). If a catalyst with lower amounts of alkali or earthalkali metals on silica was chosen, the conversion dropped significantly (9% conversion with 10% alkali metals on silica).
The cyclization of 5 to ethyl-ionones (3) was catalyzed by acids. The acid catalyzed cyclization of 3 was investigated in the presence of liquid and solid Brønsted acids. However, application of liquid acids resulted in the highest selectivity (Table 1, entries 1–4) within short reaction times. Solid acids gave low selectivity because of the formation of various polymeric by-products (entries 5–10). Lowering of the reaction temperature did not improve the selectivity (entries 5 and 6). The ratio of the α- to β-isomer was usually in favour of the α-isomer. However, when sulfuric acid (entry 1), methanesulfonic acid (entry 3) or trifluoromethanesulfonic acid (entry 4) was applied the β-isomer was formed in larger amounts. Similar influences of the various acids on the α/β-ratio were also observed in the cyclization of 6,10-dimethylundeca-5,9-dien-2-one (pseudo-ionone) to 4-(2,6,6-trimethylcyclohex-1 or 2-enyl)but-3-en-2-one (α or β-ionone).11,12
| Entrya | Catalyst | t/h | T/°C | Select. (%) | Conv. (%) | Ratio | |
|---|---|---|---|---|---|---|---|
| α-3 | β-3 | ||||||
| a Reaction carried out in batch mode in heptane unless otherwise stated, using 20 wt% catalyst. b The reaction was carried out without solvent. c The product was distilled. d 15 wt% catalyst. | |||||||
| 1 | H2SO4 | 1.3 | −5 | 59c | 88c | 1 | 99 |
| 2b | H3PO4 | 0.8 | 22 | 59c | 95c | 86 | 14 |
| 3 | H3CSO3H | 0.8 | 10 | 88 | 99 | 32 | 68 |
| 4 | F3CSO3H | 1.0 | −15 | 67 | 97 | 8 | 92 |
| 5b | Amberlyst 15 | 4.0 | 80 | 8 | 99 | 81 | 19 |
| 6b | Amberlyst 15 | 8.0 | 60 | 12 | 50 | 68 | 32 |
| 7b | Deloxan ASP | 4.0 | 100 | 25 | 85 | 79 | 21 |
| 8b | Nafion NR 50 | 6.0 | 100 | 6 | 16 | 100 | 0 |
| 9 | H3O40PW12·nH2Od | 22.0 | 100 | 38 | 21 | 100 | 0 |
| 10 | H3O40PMo12·nH2Od | 22.0 | 100 | 16 | 87 | 100 | 0 |
Nonetheless, it does not play a role in the synthesis of timberone (1) if more α- or β-isomer is formed because of the subsequent hydrogenation of all C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds.
C double bonds.
The hydrogenation of ethyl-ionones (3) to timberone (1) was studied with palladium and platinum on several supports (standard conditions: 6.7 wt% catalyst in cyclohexane as solvent). Platinum (5%) on carbon was less suited (87% selectivity) compared to palladium (5%) on carbon (93% selectivity), when both reactions were run for 4 h to full conversion at 70 °C and 10 bar hydrogen pressure. Lowering the hydrogen pressure to 1 bar and the reaction temperature to 22 °C increased the reaction times for most catalysts to >20 h. However, 5% Pd on alumina proved to be an exception. This catalyst was the most active one, reaching full conversion after 1.5 h (92% selectivity) under the described reaction conditions. No significant differences could be observed if ethyl-α-ionone or ethyl-β-ionone were hydrogenated.
The aldol condensation between 2-pentanone and citral (4) in the presence of various homogeneous and heterogeneous catalysts was investigated. The highest yield obtained was 93%. Furthermore, the cyclization of 5 and subsequent hydrogenation were studied. We obtained almost quantitative conversion for the cyclization with phosphoric acid (56% yield after distillation) and methanesulfonic acid (87% yield). The hydrogenation in the last step was achieved nearly quantitatively with supported palladium or platinum catalysts (91% yield). The overall yield of timberone starting from citral was 74% with regard to the best reaction conditions for each step.
Notes and references
- W. Bonrath and T. Netscher, Appl. Catal., A, 2005, 280, 55 CrossRef CAS.
- E. Galera and A. Zabža, Bull. Acad. Pol. Sci., 1977, 25(8), 615 CAS.
- E. Klein and W. Rojahn (Dragoco), DE 2807584 A2, 1979 Search PubMed.
- H. Hibbert and L. T. Cannon, J. Am. Chem. Soc., 1924, 46, 119 CrossRef CAS.
- H. Köster, Chem. Ber., 1947, 80, 248 CrossRef.
- K.-H. Schulte-Elte, G. Ohloff, B. L. Müller, W. K. Giersch and G. Salvadori (Firmenich), EP 0118809 A2, 1984 Search PubMed.
- K.-H. Schulte-Elte (Firmenich), EP 0118817 A1, 1984 Search PubMed; T. Ohmoto, A. Shimada and T. Yamamoto (Takasago), EP 456932 A1, 1991 Search PubMed.
- K.-H. Schulte-Elte, W. Giersch, B. Winter, H. Pamingle and G. Ohloff, Helv. Chim. Acta, 1985, 68, 1961 CrossRef CAS.
- E. Brenna, G. Fronza, C. Fuganti, A. Righetti and S. Serra, Helv. Chim. Acta, 1999, 82, 1762 CrossRef CAS; D. Schatkowski (Symrise), EP 1749810 A1, 2007 Search PubMed.
- W. Bonrath and J. Schütz (DSM), WO 2008113545 A1, 2008 Search PubMed.
- W. Kimel and M. Montavon (F. Hoffmann-La Roche), DE 1109679, 1961 Search PubMed.
- G. Gerber, A. Luettin and C. Stritt (DSM), WO 2008055704 A1, 2008 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details and analytical data. See DOI: 10.1039/c2cy20241g |
| This journal is © The Royal Society of Chemistry 2012 |
