The Met80Ala point mutation enhances the peroxidase activity of immobilized cytochrome c†
Antonio
Ranieri
,
Fabrizio
Bernini
,
Carlo Augusto
Bortolotti
and
Elena
Castellini
*
Department of Chemistry, University of Modena and Reggio Emilia, Via Campi 183, I-41125 Modena, Italy. E-mail: elena.castellini@unimore.it; Fax: +39 059373543; Tel: +39 0592055113
First published on 9th July 2012
Abstract
The effects of replacement of the axial methionine 80 heme ligand with a non-coordinating alanine on the peroxidase activity of kaolinite-immobilized cytochrome c were investigated at different pH values. The catalytic activity of the adsorbed mutant was found to be remarkably higher than that of wild-type cytochrome c. The pH dependence of Vmax and KM values is discussed in terms of accessibility of the substrates to the metal center and surface charge of kaolinite. Our approach, based on the combined use of adsorption on kaolinite and protein engineering, endows this bioinorganic interface with remarkable catalytic properties.
The last few years witnessed an increasing interest in the peroxidase activity of cytochrome c, since it has been demonstrated that oxidation of cardiolipin, a mitochondrial specific phospholipid, by a non-native form of cytochrome c is an essential step in the activation of the mitochondrial stage of apoptosis.1–3 Another recent result is that unfolded cytochrome c bearing two histidines as axial ligands can act as a biocatalyst for the reduction of both H2O2 and O2.4,5 Moreover, exploitation of cytochromes in place of enzymes such as peroxidases to catalyze oxidative reactions is an attractive option for biotechnological applications.6 In fact, peroxidases, albeit extensively used in industrial and environmental catalysis,7–9 are scarcely stable.7 Conversely, cytochromes c are robust proteins in which the catalytic heme center is covalently bound to the protein chain and they can be immobilized on surfaces with no significant functionality loss.6,10 Most notably, cytochrome c can be easily engineered by site-directed mutagenesis.11,12 In particular, point mutations can be exploited to improve catalytic functions,11 such as the peroxidase activity, and to tune it toward a specific substrate.
Recent studies demonstrated that cytochrome c and its Lys-to-Ala variants can be strongly adsorbed onto kaolinite through electrostatic interactions.10 Cytochrome c immobilized onto kaolinite displays a peroxidase activity in the pH range 2–10.12 Therefore, the cytochrome-functionalized kaolinite, besides the advantages of heterogeneous catalysis, also extends the catalytic activity in a wider pH range compared to the cytochrome in solution.10,12
The peroxidase activity of cytochrome c adsorbed on kaolinite was ascribed to the weakening of the bond between the heme iron and the sulfur of the axial ligand methionine 80.12–15 Here, we study the peroxidase activity of the immobilized Met80Ala variant of yeast cytochrome c (M80A hereafter) in which the Met80 heme ligand is replaced with a noncoordinating Ala residue (Fig. 1), thereby deeply altering the coordinative properties of the heme center responsible for the catalytic activity.
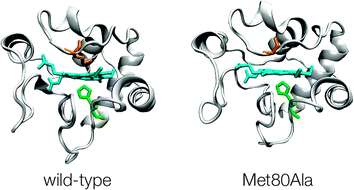 | ||
| Fig. 1 Three dimensional structure representation of (His, Met) ligated wild-type yeast cytochrome c (YCC) and (His, OH−) ligated M80A variant. PDB codes for YCC and M80A are 1YCC and 1FHB, respectively. | ||
As the pH plays a key role in modulating the electrostatic interaction between protein and kaolinite through the change of the surface charge,16 we have investigated the peroxidase activity of M80A immobilized onto kaolinite as a function of pH.
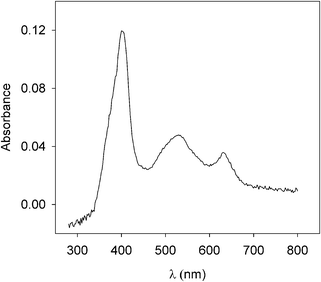
M80A was adsorbed on sodium exchanged kaolinite (Nak) at pH 3.5 (see S1 and S2, ESI†); the solid phase was washed three times with the buffer solution at pH 3.5 to remove the excess of free protein. This material constitutes the solid catalyst Nak-M80A. In order to characterize the adsorbed protein, Diffuse Reflectance (DR) UV-Vis spectra were recorded for ferric M80A in buffer solutions (S3) at pH 3.5 (S4), 7 (Fig. 2) and 9 (S4, ESI†). No significant changes were observed, with the absorption maxima falling in all cases at 400, 530 and 628 nm. The optical spectra, therefore, support the conclusion that the ferric heme iron is high-spin at all the investigated pH values. In particular, the 628 nm band is diagnostic of a ferric high-spin heme iron with a water molecule coordinated in the sixth position.12 In fact, M80A in solution shows a ferric low-spin heme iron at neutral and slightly acidic pH, characterized by absorption maxima in the optical spectrum at about 406, 538, 570 nm which indicate OH− binding to the iron.17 At acidic pH, however, a ferric high-spin heme iron with a water molecule coordinated to the metal center prevails. This form is characterized by absorption maxima in the optical spectrum at about 400, 530 and 620 nm.17 We therefore assign the axial ligand set (His, H2O) to M80A immobilized onto kaolinite in all the investigated pH range (2.5–9). It is worth noting that the formation of the high-spin form of the ferric heme iron in the adsorbed state invariably occurs at all the adsorption pH (data not shown); moreover, the high-low spin conversion is reversible and the desorbed form (detected in specific experiments made at high ionic strength, data not shown) restores the low-spin form at neutral/alkaline pH.
The peroxidase activity of Nak-M80A was determined through guaiacol (Gc) oxidation to tetraguaiacol (tetraGc) in the presence of H2O2, following the reaction:18
| 4Gc + 4H2O2 → tetraGc + 8H2O | (1) |
It is well known that cytochrome c is quickly degraded in the presence of high H2O2 concentrations.19 We found a similar behaviour also for M80A when H2O2 is added to the working solution containing the protein free or immobilized on kaolinite in the absence of guaiacol (S3 and S5, ESI†). Fig. S6a and b (ESI†) show the electronic spectra for solution and immobilized M80A in the presence of 12.2 mM H2O2 only, at different times. In both cases the spectra show the progressive disappearance of the M80A spectrum. In contrast, in the presence of excess guaiacol or as long as guaiacol is present in the reaction solution, the spectrum of solution and immobilized M80A remains unchanged. The UV-Vis and DR UV-Vis spectra of M80A in the presence of 12.2 mM H2O2 and 14 mM guaiacol are reported in Fig. S7a and b (ESI†), respectively. During the first 10 minutes the spectral features of M80A remain unaltered. This indicates that the ferryl group formed at the beginning of the reaction reacts faster with the substrate (guaiacol) instead of giving rise to intramolecular degradation. Accordingly, we have attributed the reported catalytic activity to the unaltered protein.
V
0 was measured as a function of H2O2 starting concentration at different pH values (Fig. 3 and S8, ESI†). The data of Fig. 3 were analyzed according to the Michaelis–Menten model (S9 and Fig. S9a–c, ESI†).20 As previously reported,12 the main steps of the complex mechanism include the H2O2 entrance in the M80A catalytic pocket to form compound I (cytc˙Fe+4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), probably through a reversible addition of H2O2 to the metal center (compound 0) and the subsequent oxidation of Gc by ferryl compounds I (cytc˙Fe+4
O), probably through a reversible addition of H2O2 to the metal center (compound 0) and the subsequent oxidation of Gc by ferryl compounds I (cytc˙Fe+4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and II (cytcFe+4
O) and II (cytcFe+4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).21 In such a way, the Michaelis constant KM (an index of the kinetic instability of the iron–H2O2 adduct) accounts for the affinity of H2O2 for the heme center while the maximum rate achieved by the system, Vmax, accounts for the reactivity of the ferryl compound (I or II) toward the Gc substrate. It is worth noting that the involvement of the ferryl heme group in the peroxidase activity of cytochrome c has been recently demonstrated through spectroscopic and kinetics studies.22,23
O).21 In such a way, the Michaelis constant KM (an index of the kinetic instability of the iron–H2O2 adduct) accounts for the affinity of H2O2 for the heme center while the maximum rate achieved by the system, Vmax, accounts for the reactivity of the ferryl compound (I or II) toward the Gc substrate. It is worth noting that the involvement of the ferryl heme group in the peroxidase activity of cytochrome c has been recently demonstrated through spectroscopic and kinetics studies.22,23
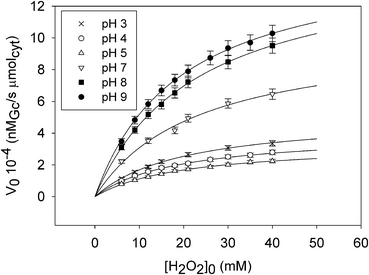 | ||
| Fig. 3 V 0 of reaction (1) catalyzed by Nak-M80A at 25 °C as a function of H2O2 starting concentration at selected pH values (pH = 3, 4, 5, 7, 8, 9) together with the curve of the data fitting performed with Sigma Plot v. 11.0 (Dundas Software LTD, Germany). | ||
To evaluate the role of protein immobilization, the catalytic activity of M80A was measured also for the protein in solution (Experimental in S10, ESI†). The initial reaction rate (V0), defined as the concentration of reagent Gc (in nanomolarity) consumed per second and per micromolarity of cytochrome c in solution (μMcyt), was measured as a function of H2O2 starting concentration at pH 3.5 and 7 and analysed through the Michaelis–Menten model (S11, ESI†). KM, Vmax and Vmax/KM values for solution and immobilized M80A at pH 3.5 and 7 are reported in Table 1.
| K M b/mM | V max a , b/nMGc s−1 μmolcyt−1 | V max b/nMGc s−1 μMcyt−1 | V max/KMb/s−1 μmolcyt−1 | ||
|---|---|---|---|---|---|
| a For calculation of Vmax in nMGc s−1 μmolcyt−1 see Experimental in S11 (ESI). b Associated errors: KM ± 7%, Vmax ± 5%, and Vmax/KM ± 12%. | |||||
| Ads | pH 3.5 | 19.9 | 4.75 × 104 | / | 2.4 × 10−3 |
| pH 7 | 18.3 | 9.30 × 104 | / | 5.1 × 10−3 | |
| Sol | pH 3.5 | 15.0 | 1.84 × 104 | 36.9 | 1.2 × 10−3 |
| pH 7 | 23.2 | 7.20 × 104 | 144 | 3.1 × 10−3 | |
The KM values are comparable, but at pH 3.5 KM for the solution protein is lower than that for the adsorbed species. In contrast, at pH 7, the lower KM value is shown by the immobilized protein. This behavior is most likely due to pH-dependent changes in iron coordination. In fact, M80A in solution undergoes the substitution of the OH− ligand with H2O at pH < 4.17 Instead, the immobilized protein retains a high-spin structure with a coordinated H2O in the entire pH range investigated (2.5–9). In such a way, the higher KM value observed at pH 7 for the solution protein can be confidently ascribed to the stronger coordination of OH− compared to H2O. At pH 3.5, the different behavior of the adsorbed and solution protein is reasonably due to the immobilization effect. Since the anionic kaolinite surface probably interacts with the lysine residues near the crevice exposing heme to the solvent,24 it is reasonable that the accessibility of H2O2 to the crevice and then to the heme is reduced. Concerning the Vmax values (Table 1), it is worth noting that in both cases Vmax is remarkably higher for the adsorbed protein with respect to that in solution. The reason for this result is not clear. Vmax describes the rate of the oxidation of the organic substrate (Gc) by the ferryl compound and therefore can be related to the affinity of the aromatic substrate for the catalytic pocket. The increased Vmax for the immobilized protein could be the consequence of adsorption-induced changes in hydrophilicity and dimension of the catalytic pocket once the ferryl compound is formed. Similar effects were reported for myoglobin, whose peroxidase activity was shown to be enhanced by an increase in hydrophobicity of the heme environment.25
The Vmax/KM ratio for the adsorbed protein is always remarkably higher than those of the solution protein both at pH 3.5 and 7. Immobilization therefore plays a critical role in the enhancement of the catalytic efficiency of M80A.
The plots of KM, Vmax and Vmax/KMvs. pH are shown in Fig. 4, 5 and 6, respectively, together with the corresponding data previously obtained for wild-type yeast cytochrome c (YCC) (Nak-YCC catalyst).12 Since the concentration of the protein on the surface is nearly the same for M80A and YCC (S2, ESI†), the ratio Vmax/KM can be used as a meaningful parameter to compare the catalytic efficiency of the two proteins.
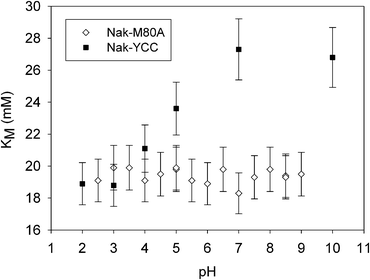 | ||
| Fig. 4 K M of reaction (1)vs. pH for the solid catalysts Nak-M80A and Nak-YCC at 25 °C. Nak-YCC data are from ref. 12. | ||
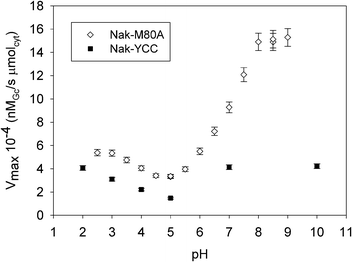 | ||
| Fig. 5 V max of reaction (1)vs. pH for the solid catalysts Nak-M80A and Nak-YCC at 25 °C. Nak-YCC data are from ref. 12. | ||
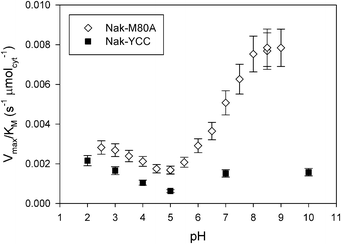 | ||
| Fig. 6 V max/KM ratio vs. pH for the solid catalysts Nak-M80A and Nak-YCC at 25 °C. Nak-YCC data are from ref. 12. | ||
At neutral and slightly alkaline pH values, KM for Nak-M80A is lower than that for Nak-YCC (Fig. 4); this can be ascribed to the effects of the different axial ligation of the heme iron (exogenous H2O for M80A and methionine sulfur for YCC26) which enhances the accessibility of the catalytic site to H2O2 in the case of the M80A, by increasing the dimensions of the heme pocket. Indeed, the calculated solvent-accessible surface area (SASA) were of 32 Å2 and 27 Å2 for M80A and YCC, respectively (S12, ESI†). Moreover, while in the case of Nak-YCC KM shows a clear pH dependence,12 the values of KM for Nak-M80A are pH-insensitive. This result is in line with those obtained by DR UV-Vis spectra, which indicate that the coordination sphere of the iron center remains unchanged over the entire investigated pH range (2.5–9).
It is worth noting that the mean value of KM is nearly the same as that obtained for Nak-YCC at acidic pH values. For Nak-YCC the decrease in KM from alkaline to acidic pH can be ascribed to replacement of the Met ligand with a water molecule26,27 which increases the kinetic stability of the iron–H2O2 adduct. The similarity of the KM values for Nak-M80A and Nak-YCC at acidic pH values is not surprising as the two species share the same axial ligation (His, H2O), therefore it is very likely that the structure of the iron–H2O2 adduct for both species is also very similar. A relevant finding of this work is that a simple mutation in the heme pocket is able to enhance the reactivity of the catalytic center toward H2O2 to give a KM value achievable with the wild-type cytochrome only under very acidic conditions.
It is known that cytochromes c in solution in the pH range 8–9 undergo a structural change (alkaline transition) which involves substitution of the axial ligand Met80 with a Lys residue, causing a dramatic decrease of the peroxidase activity.12,28,29 Studies of Silkstone et al.17 on the pH dependence of the heme axial coordination for M80A indicated that for this mutant the alkaline transition does not occur in solution. The data shown in Fig. 5 indicate that this is the case also for adsorbed M80A, at least up to pH 9. In fact, whilst the alkaline form of cytochrome c does not show any peroxidase activity,13Vmax for the Nak-M80A catalyst remarkably increases with increasing pH up to 9. This finding – which parallels that already obtained for the Nak-YCC catalyst12 – is extremely meaningful in view of the exploitation of Nak-M80A in heterogeneous catalysis, as it shows that the catalytic property of M80A can be maintained in a large pH range. Here, in particular, the peroxidase activity of the mutant is remarkably amplified with respect to the Nak-YCC catalyst, since the corresponding Vmax values at alkaline pH are about 4 times higher. Moreover, it is worth noting that the Vmax values for M80A are higher than those of the wild-type in the entire pH range. Probably, a larger hydrophobicity of the heme environment in M80A compared to YCC is the consequence of the substitution of a polar residue (Met) with a hydrophobic one (Ala). This would enhance the affinity of the catalytic pocket toward the organic Gc substrate. A similar effect has been proposed to explain the increase of peroxidase activity of engineered heme proteins in which a large increase in hydrophobicity of the catalytic pocket has been obtained by chemically modifying the heme group.25,30,31
The pH profiles of Vmax for Nak-YCC and Nak-M80A (Fig. 5) show a similar Vmax decrease between pH 3 and 5 with a minimum centered around pH 5. A further increase in pH induces only a modest increase in Vmax for Nak-YCC, but a dramatic one for Nak-M80A. As Vmax may be considered a sort of index of the population of catalytically active adsorbed cytochrome c present on the kaolinite surface,12 the data in Fig. 5 indicate that the fraction of the active form of M80A on the surface is a function of pH as for YCC. This behavior confirms that the charge of the adsorbing kaolinite surface drives the structural changes involving the activation of the peroxidase properties of cytochromes c.
The catalytic efficiency Vmax/KM of Nak-M80A is much higher than that of Nak-YCC in the whole pH range investigated (Fig. 6). In particular, replacement of methionine 80 with an alanine increases the catalytic efficiency by 5-fold at pH values higher than 8. This result indicates that Met to Ala substitution sensibly amplifies the peroxidase activity of immobilized cytochrome c.
To verify the stability of adsorbed M80A at different pH values, DR UV-Vis spectra have been recorded on the protein immobilized on kaolinite after various incubation times with solutions at pH 2.5 and 9. The spectra remain almost unchanged (S13, ESI†), indicating that M80A does not undergo denaturation in all the investigated pH range.
In order to evaluate how adsorption on kaolinite affects M80A stability, a comparative thermal denaturation study has been carried out by monitoring the Soret band intensity with time at increasing incubation temperatures (50 °C and 80 °C).
The time-dependence of the absorbance of the Soret band at 50 °C and 80 °C for the solution and immobilized protein is shown in Fig. 7. The data show that M80A is stable at 50 °C both in solution and in the adsorbed state. On the contrary, at 80 °C an irreversible thermal denaturation occurs in both cases. However, at 80 °C the adsorbed protein shows a slower absorbance decay suggesting an adsorption-induced increase in conformational stability of the protein at high temperature.
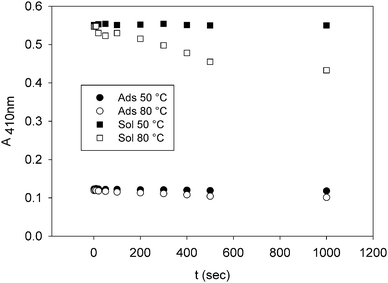 | ||
| Fig. 7 Absorbance of the Soret band (λ = 410 nm) of M80A vs. protein incubation time both in solution and in the adsorbed state at 50 °C and 80 °C, pH 7. | ||
The operational stability of M80A at 50 °C and 80 °C and pH 7 has been evaluated by measuring the time dependence of peroxidase activity both in solution and in the adsorbed state. The results parallel those obtained by spectrophotometric analysis: while at 50 °C the enzymatic activity remains almost unchanged (in the investigated time range), at 80 °C it decreases rapidly with time both in solution and in the adsorbed state, but quite faster in solution (S14, ESI†).
Conformational and operational stabilities have been ascertained also by means of a number of catalytic cycles performed by the same Nak-M80A sample. In Fig. 8 the peroxidase activity and the absorbance of the Soret band have been reported against the number of catalytic cycles. The plot shows that the catalytic activity and the conformation of the iron environment of the adsorbed protein do not change appreciably at least up to ten cycles.
![Peroxidase activity ([H2O2]0 = 12.2 mM) and absorbance of the Soret band (λ = 410 nm) of M80A vs. number of catalytic cycles in the adsorbed state at 25 °C, pH 7.](/image/article/2012/CY/c2cy20347b/c2cy20347b-f8.gif) | ||
| Fig. 8 Peroxidase activity ([H2O2]0 = 12.2 mM) and absorbance of the Soret band (λ = 410 nm) of M80A vs. number of catalytic cycles in the adsorbed state at 25 °C, pH 7. | ||
Our approach, based on the combined use of adsorption on kaolinite and protein engineering, endows this bioinorganic interface with remarkable catalytic properties. In particular, site-directed mutagenesis amplifies the peroxidase activity of cytochrome c immobilized on kaolinite acting both on KM and Vmax. Most notably, the peroxidase activity of M80A is almost three times higher than the wild-type one at pH 7 (2900 vs. 1100 nMGc (s μmolcyt mMH2O2)−1 at 12.2 mM H2O2), thus making this catalytic interface a suitable candidate for applications at mild, nearly physiological conditions. Moreover, since the contributions of KM and Vmax to the peroxidase activity could be tuned independently, there is much room for enhancement of enzymatic activity and selectivity through the rational design of further mutations.
We are grateful to Professor Marco Borsari and to Professor Marco Sola from the Department of Chemistry of the University of Modena and Reggio Emilia for helpful comments and discussions. This work was performed with the financial support of the University of Modena and Reggio Emilia.
Notes and references
- G. G. Borisenko, A. A. Kapralov, V. A. Tyurin, A. Maeda, D. A. Stoyanovsky and V. E. Kagan, Biochemistry, 2008, 47, 13699 CrossRef CAS.
- N. A. Belikova, Y. Y. Tyurina, G. Borisenko, V. Tyurin, A. K. Samhan Arias, N. Yanamala, P. G. Furtmuller, J. Klein-Seetharaman, C. Obinger and V. E. Kagan, J. Am. Chem. Soc., 2009, 131, 11288 CrossRef CAS.
- M. Huttemann, P. Pecina, M. Rainbolt, T. H. Sanderson, V. E. Kagan, L. Samavati, J. W. Doan and I. Lee, Mitochondrion, 2011, 11, 369 CrossRef.
- C. Tavagnacco, S. Monari, A. Ranieri, C. A. Bortolotti, S. Peressini and M. Borsari, Chem. Commun., 2011, 47, 11122 RSC.
- A. Ranieri, G. Battistuzzi, M. Borsari, C. A. Bortolotti, G. Di Rocco, S. Monari and M. Sola, Electrochem. Commun., 2012, 14, 29 CrossRef CAS.
- R. Vazquez-Duhalt, J. Mol. Catal. B: Enzym., 1999, 7, 241 CrossRef CAS.
- G. Battistuzzi, M. Bellei, C. A. Bortolotti and M. Sola, Arch. Biochem. Biophys., 2010, 500, 21 CrossRef CAS.
- K. Knutson, S. Kirzan and A. Ragauskas, Biotechnol. Lett., 2005, 27, 753 CrossRef CAS.
- H. Qayyum, K. Zoheb and Z. Banday, Water, Air, Soil Pollut., 2010, 212, 319 CrossRef.
- E. Castellini, A. Ranieri, D. A. Simari and G. Di Rocco, Langmuir, 2009, 25, 6849 CrossRef CAS.
- S. Casalini, G. Battistuzzi, M. Borsari, A. Ranieri and M. Sola, J. Am. Chem. Soc., 2008, 130, 15099 CrossRef.
- A. Ranieri, F. Bernini, C. A. Bortolotti, A. Bonifacio, V. Sergo and E. Castellini, Langmuir, 2011, 27, 10683 CrossRef CAS.
- R. E. M. Diederix, M. Ubbink and G. W. Canters, Biochemistry, 2002, 41, 13067 CrossRef CAS.
- S. Casalini, G. Battistuzzi, M. Borsari, C. A. Bortolotti, A. Ranieri and M. Sola, J. Phys. Chem. B, 2008, 112, 1555 CrossRef CAS.
- T. Ying, Z. H. Wang, Y. W. Lin, J. Xie, X. Tan and Z. X. Huang, Chem. Commun., 2009, 4512 RSC.
- E. Tombacz and M. Szekeres, Appl. Clay Sci., 2006, 34, 105 CrossRef CAS.
- G. G. Silkstone, C. E. Cooper, D. Svistunenko and M. T. Wilson, J. Am. Chem. Soc., 2005, 127, 92 CrossRef CAS.
- D. Mandelman, J. Jamal and T. L. Poulos, Biochemistry, 1998, 37, 17610 CrossRef CAS.
- A. Shakir, F. Humaira, P. Ram, N. Mohammad, R. Indusmita, Y. Savita and A. Faizan, Int. J. Biol. Macromol., 2010, 47, 109 CrossRef.
- A. Fersht, Structure and mechanism in protein science: a guide to enzyme catalysis and protein folding, W. H. Freeman and Company, New York, 1998 Search PubMed.
- H. B. Dunford, Coord. Chem. Rev., 2002, 233–234, 311 CrossRef CAS.
- C. Bischin, F. Deac, R. Silaghi-Dumitrescu, J. A. R. Worrall, B. S. Rajagopal, G. Damian and C. E. Cooper, Free Radical Res., 2011, 45, 439 CrossRef CAS.
- A. Lawrence, C. M. Jones, P. Wardman and M. J. Burkitt, J. Biol. Chem., 2003, 278, 29410 CrossRef CAS.
- G. Battistuzzi, M. Borsari, C. A. Bortolotti, G. Di Rocco, A. Ranieri and M. Sola, J. Phys. Chem. B, 2007, 111, 10281 CrossRef CAS.
- T. Hayashi, Y. Hitomi, T. Ando, T. Mizutani, Y. Hisaeda, S. Kitagawa and H. Ogoshi, J. Am. Chem. Soc., 1999, 121, 7747 CrossRef CAS.
- G. R. Moore and G. W. Pettigrew, Cytochromes c: Evolutionary, Structural and Physicochemical Aspect, Springer-Verlag, Berlin, 1990 Search PubMed.
- C. A. Bortolotti, G. Battistuzzi, M. Borsari, P. Facci, A. Ranieri and M. Sola, J. Am. Chem. Soc., 2006, 128, 5444 CrossRef CAS.
- G. Battistuzzi, M. Borsari, L. Loschi, A. Martinelli and M. Sola, Biochemistry, 1999, 38, 7900 CrossRef CAS.
- G. Battistuzzi, M. Borsari and M. Sola, Eur. J. Inorg. Chem., 2001, 2989 CrossRef CAS.
- E. Torres, A. Baeza and R. Vazquez-Duhalt, J. Mol. Catal. B: Enzym., 2002, 19–20, 437 CrossRef CAS.
- H. Y. Song, J. Z. Liu, L. P. Weng and L. N. Ji, J. Mol. Catal. B: Enzym., 2009, 57, 48 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: S1: Materials. S2: Preparation of the solid catalyst Nak-M80A. S3: Diffuse-Reflectance (DR) UV-Vis measurements. S4: DR UV-Vis spectra of M80A adsorbed on kaolinite as a function of pH, includes Fig. S4. S5: UV-Vis measurements in solution. S6: Time dependence of UV-Vis and DR UV-Vis spectra of M80A in solution and adsorbed on kaolinite at pH 7 in the presence of H2O2, includes Fig. S6a and b. S7: Time dependence of UV-Vis and DR UV-Vis spectra of M80A in solution and adsorbed on kaolinite at pH 7 in the presence of H2O2 and guaiacol, includes Fig. S7a and b. S8: Catalyst efficiency measurements of M80A adsorbed on kaolinite. S9: Michaelis–Menten model applied to M80A adsorbed on kaolinite, includes Fig. S9a–c. S10: Catalyst efficiency measurements of M80A in solution. S11: Michaelis–Menten model applied to M80A in solution, includes Fig. S11. S12: Solvent-accessible surface area (SASA) measurement. S13: DR UV-Vis spectra of adsorbed M80A at pH 2.5 and 9 as a function of time, includes Fig. S13. S14: Peroxidase activity of M80A adsorbed on kaolinite and in solution as a function of time at 50 °C and 80 °C, includes Fig. S14. References. See DOI: 10.1039/c2cy20347b |
| This journal is © The Royal Society of Chemistry 2012 |