Photocatalytic conversion of CO2 and H2O to fuels by nanostructured Ce–TiO2/SBA-15 composites
Cunyu
Zhao
a,
Lianjun
Liu
a,
Qianyi
Zhang
a,
Jun
Wang
b and
Ying
Li
*a
aDepartment of Mechanical Engineering, University of Wisconsin-Milwaukee, Milwaukee, WI, 53211, USA. E-mail: liying@uwm.edu; Fax: +1 414-229-6958; Tel: +1 414-229-3716
bDepartment of Environmental Engineering Sciences, University of Florida, Gainesville, FL, 32611, USA
First published on 14th August 2012
Abstract
Cerium-doped titanium oxide (Ce–TiO2) nanoparticles were prepared by a simple sol–gel method. Ce-doping decreased the crystal size of TiO2, increased the catalyst surface area, and inhibited the growth of rutile TiO2 crystals. Ce–TiO2 nanoparticles were also dispersed on SBA-15, mesoporous silica with one-dimensional pores, forming a Ce–TiO2/SBA-15 nanocomposite. The nanocomposite materials were well characterized and tested as photocatalysts to convert CO2 and H2O to value-added fuels, mainly CO and CH4, under UV-vis illumination. Compared with pristine TiO2, TiO2 doped by 1 or 3% Ce improved the production of CO by four times. The reason may be due to the facilitated charge transfer induced by the doped Ce ions, the higher surface area of the catalyst, as well as the stabilization of anatase phase. However, too high a Ce concentration reduced the catalytic activity, likely due to the formation of recombination centers. Compared with unsupported Ce–TiO2, Ce–TiO2 supported on SBA-15 remarkably enhanced the CO2 reduction rate. Ce–TiO2/SBA-15 with a Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si ratio of 1
Si ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 demonstrated 8-fold enhancement in CO production and 115-fold enhancement in CH4 production. By contrast, amorphous silica as the substrate was much inferior to SBA-15. The findings in this work reveal a promising nanostructured catalyst material for solar fuel production using CO2 and H2O as the feedstock.
4 demonstrated 8-fold enhancement in CO production and 115-fold enhancement in CH4 production. By contrast, amorphous silica as the substrate was much inferior to SBA-15. The findings in this work reveal a promising nanostructured catalyst material for solar fuel production using CO2 and H2O as the feedstock.
1. Introduction
Photocatalytic reduction of CO2 using sunlight as the energy input is a promising way to reduce CO2 level in the atmosphere and in the meantime produce alternative fuels or building blocks for industrial chemicals. Semiconductor photocatalysts such as WO3, ZrO2, Ga2O3, and TiO21–4 have been studied for such applications, and among them, TiO2 has been considered the most appropriate photocatalyst due to its high photosensitivity, non-toxic nature, low cost, and easy availability.5–9 However, the photoefficiency of CO2 reduction on TiO2 is usually very low, mainly due to the fast recombination of photo-excited electron–hole (e–h) pairs and the wide band gap of TiO2 (3.2 eV for anatase) that does not allow the utilization of visible light.10,11To enhance the photoefficiency of TiO2 for CO2 photoreduction, several strategies have been reported in the literature by modifying the nanostructure of TiO2. The first strategy is to harvest visible light by tailoring the band gap of TiO2 or introducing impurity level with non-metal ion doping (e.g., I, N).12,13 The second strategy is to lower the activation energy and to facilitate electron trapping by depositing noble metal nanoparticles (e.g., Pt, Rh, Ag)14–17 on the TiO2 surface. Finally, pairing of transition metal oxides (e.g., CuO, Cu2O, Fe2O3)4,11,18,19 with TiO2 has been demonstrated to enhance CO2 reduction by facilitating the separation of electrons and holes, although the exact mechanism has not been well understood.
Rare earth element modified TiO2 has demonstrated enhanced photocatalytic oxidation ability than bare TiO2.7,20,21 Cerium (Ce) is one of the four most abundant rare earth elements, and composites of Ce–TiO2 have shown enhanced photocatalytic activity for water splitting and degradation of organic compounds.22–26 Ce-doping could result in smaller TiO2 nanocrystals and thus enhance catalytic activity.27 However, to the best of our knowledge, Ce-modified TiO2 has not been studied for the photocatalytic CO2 reduction with water.
Besides modifying the properties and nanostructures of TiO2 itself, many approaches have been used to immobilize TiO2 nanoparticles on mesoporous substrates such as molecular sieve 5 Å, amorphous silica, and SBA-15,4,28–30 and the direct benefits include larger surface area and better dispersion of the nano-sized catalysts. It has been reported that Cu–TiO2, Ru–TiO2, and TiO2 supported on amorphous silica showed higher CO2 photoreduction rates than the catalysts without supports.4,18,28 SBA-15, the well-known mesoporous silica with one-dimensional hexagonal pores, has been widely studied as a catalyst support and the ordered and uniform pore structures are normally advantageous over irregular pores in amorphous silica. Recently, a couple of studies have investigated TiO2 and Cu–TiO2 catalysts immobilized onto the SBA-15 matrix for photocatalytic CO2 reduction. The Si–OH group was found to enhance the interaction of CO2 and H2O on the catalyst surface based on the results of an IR study.31 Experimental results also demonstrated an enhancement of CO2 photoreduction to methanol on a 2%Cu–TiO2/SBA-15 sample compared with 2%Cu–TiO2, and the promotional effect was likely attributed to the synergistic effect between the metal species, TiO2 and the support.32 However, the observed enhancement benefited from SBA-15 in that work was only 20%, maybe because the loading of TiO2 was not optimized.
In this work, we aimed to utilize the co-benefits from Ce additives and SBA-15 as the substrate for TiO2 nanoparticle catalysts. Hence, Ce–TiO2/SBA-15 catalysts were synthesized and tested for CO2 photoreduction with water to produce solar fuels. To the best of our knowledge, this is a new nanocomposite material that has not been prepared before and has not been tested for CO2 photoreduction to fuels. Unsupported Ce–TiO2 and Ce–TiO2 loaded on amorphous silica was also prepared and tested as a comparison. Our results show more than 100-fold enhancement in CO2 photoreduction to CH4 compared with unsupported catalysts, demonstrating the superior photocatalytic activity of the prepared Ce–TiO2/SBA-15 nanocomposite materials.
2. Experimental
2.1 Catalyst preparation
The Ce–TiO2 nanoparticles were synthesized by a sol–gel method.7,33 In a typical synthesis process, 10 mL titanium butoxide (Ti(OBu)4, 99%) was ultrasonically dispersed in 40 mL absolute ethyl alcohol for 10 min (Solution A). A certain amount of Ce(NO3)3·6H2O (99.5%) was dissolved in 10 mL H2O, 10 mL absolute ethyl alcohol and 2 mL 62% nitric acid (Solution B). Then Solution A was added dropwise to Solution B (forming Solution C) with vigorous stirring for 3 h at room temperature. The obtained transparent sol was further aged for 6 h at room temperature, dried at 70 °C for 36 h and finally calcined in a muffle furnace at 500 °C for 2 h. Samples with molar ratios of Ce to Ti at 0.01, 0.03, 0.08 and 0.12 were prepared. For comparison, pristine TiO2 was also prepared without adding the Ce precursor.SBA-15 was prepared according to a well established procedure reported by Zhao et. al.34 4 g of Pluronic P123 (Aldrich) was dissolved in 125 g of 2 M HCl at 35 °C with stirring. Tetraethyl orthosilicate (TEOS, Aldrich) was then added into the solution after P123 was dissolved. The resultant solution was stirred for 20 h at 35 °C, after which the mixture was aged at 80 °C for 24 h in a sealed Teflon bottle. The solid product was recovered by filtration and air-dried at room-temperature overnight. SBA-15 was obtained by calcination of the solid product at 500 °C for 6 h.
Ce−TiO2 was loaded on SBA-15 by adding SBA-15 particles to Solution C in the sol−gel process of preparing Ce−TiO2 as previously described. The same aging, drying, and calcination procedure was applied. Ce−TiO2/SBA-15 composites with a molar ratio of Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti
Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si at 0.03
Si at 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.03
1, 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 0.03
2, and 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 were prepared. For comparison, Ce−TiO2 loaded on amorphous silica was also prepared following the same procedure, and the amorphous mesoporous silica particles were prepared according to the process reported in our previous study.4 The prepared samples are denoted xCe_yTi, xCe_yTi_zSi, and xCe_yTi_zaSi, where Ce represents cerium, Ti represents titania, Si represents SBA-15, aSi represents amorphous silica, and the numbers of x, y, and z indicate the molar ratio of Ce, Ti, and Si. For example, 0.03Ce_1Ti_4Si represents Ce−TiO2/SBA-15 sample with a Ce
4 were prepared. For comparison, Ce−TiO2 loaded on amorphous silica was also prepared following the same procedure, and the amorphous mesoporous silica particles were prepared according to the process reported in our previous study.4 The prepared samples are denoted xCe_yTi, xCe_yTi_zSi, and xCe_yTi_zaSi, where Ce represents cerium, Ti represents titania, Si represents SBA-15, aSi represents amorphous silica, and the numbers of x, y, and z indicate the molar ratio of Ce, Ti, and Si. For example, 0.03Ce_1Ti_4Si represents Ce−TiO2/SBA-15 sample with a Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti
Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si molar ratio at 0.03
Si molar ratio at 0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.
4.
2.2 Catalyst characterization
The Brunauer–Emmett–Teller (BET) specific surface area and pore size of the catalysts were measured by nitrogen adsorption–desorption isotherms using a Micrometrics ASAP 2020 Surface Area and Porosity Analyzer. The crystal structures of the powder catalysts were identified by X-ray diffraction (XRD) (Scintag XDS 2000) using Cu Kα irradiation at 45 kV and a diffracted beam monochromator operated at 40 mA in the 2θ range from 20° to 60° at a scan rate of 1° min−1. The fractional phase contents of anatase, brookite, and rutile TiO2 were calculated by the method reported in previous work.10 The Scherrer equation was applied to calculate the crystal size of TiO2. The UV-vis diffuse reflectance spectra were recorded by a UV-vis spectrophotometer (Ocean Optic) using BaSO4 as the background. Scanning electron microscopy (SEM) (Hitachi S4800) was used to obtain the catalysts surface morphology. The particle size and crystal lattice of TiO2 and the pore structure of SBA-15 were analyzed by transmission electron microscopy (TEM) (Hitachi H9000NAR) and high resolution TEM (HR-TEM). X-ray photoelectron spectroscopy (XPS) (Perkin-Elmer PHI 5100) was used to examine the valance states of Ce in the Ce/TiO2 samples before and after CO2 photoreduction with water vapor. A thin layer of powder samples were loaded on a silicon substrate and subject to XPS analysis. Subsequently, the same samples were exposed to photoillumination in the presence of CO2 and water vapor for 4 h and then subject to XPS analysis again.2.3 Measurement of photocatalytic activity
The photocatalytic activity of CO2 reduction with water vapor was investigated in an experimental system as shown in Fig. 1. Compressed CO2 (99.999%, Praxair) regulated by a mass flow controller (at a flow rate of 4 mL min−1) was passed through a deionized water bubbler to introduce CO2 and water vapor mixture (volume fraction of H2O ≈ 2.3%) into a photoreactor that has stainless steel walls and a quartz window. For each test, 200 mg powder catalyst was evenly dispersed on a glass–fiber filter and placed in the photoreactor facing the quartz window. A 450 W Xe lamp (Oriel) was used as the irradiation source with a light intensity around 400 mW cm−2 (UV-vis region), measured by a spectroradiometer (International Light Technologies ILT950). Circulated cooling water was applied to absorb the infrared portion of the Xe lamp irradiation. The photoreactor was operating at a continuous-flow mode and the effluent gas sample was analyzed every 30 min by a gas chromatograph (GC, Agilent 7890A) equipped with an automated gas sampling valve, a thermal conductivity detector (TCD) and flame ionization detector (FID).3. Results and discussion
3.1 Crystal structures of the catalysts
Fig. 2 shows the XRD patterns of pristine TiO2 and Ce–TiO2 nanoparticles with different Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti molar ratios. The calculated fractional phase contents and crystal sizes of TiO2 are summarized in Table 1. Pristine TiO2 exhibited prominent characteristic peaks for anatase (JCPDS No. 21-1272) and rutile (JCPDS No. 21-1276), as well as a very weak peak for brookite (JCPDS No. 29-1360). The unique diffraction peak at 30.80° (2θ) represents the brookite (121) plane, while all other major brookite diffraction peaks overlap with anatase peaks, for example, brookite (120) at 25.34° and brookite (111) at 25.69° overlap with anatase (101) at 25.28°.10,35–38 The pristine TiO2 had the highest rutile phase fraction, 53.7%. With the addition of 0.01% Ce, the fraction of rutile phase remarkably decreased to 4.8% with significant increases in anatase and brookite phases. Further increasing the Ce
Ti molar ratios. The calculated fractional phase contents and crystal sizes of TiO2 are summarized in Table 1. Pristine TiO2 exhibited prominent characteristic peaks for anatase (JCPDS No. 21-1272) and rutile (JCPDS No. 21-1276), as well as a very weak peak for brookite (JCPDS No. 29-1360). The unique diffraction peak at 30.80° (2θ) represents the brookite (121) plane, while all other major brookite diffraction peaks overlap with anatase peaks, for example, brookite (120) at 25.34° and brookite (111) at 25.69° overlap with anatase (101) at 25.28°.10,35–38 The pristine TiO2 had the highest rutile phase fraction, 53.7%. With the addition of 0.01% Ce, the fraction of rutile phase remarkably decreased to 4.8% with significant increases in anatase and brookite phases. Further increasing the Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti ratio to 0.03 and 0.08 resulted in the disappearance of the rutile TiO2. This result is in agreement with the literature reports that even a low concentration of ceria can stabilize the anatase phase and inhibit transformation of anatase to rutile.39,40 The stabilization mechanism was attributed to the preferential nucleation of ceria on the oxygen vacancies of anatase TiO2.39,40 In addition, no obvious peaks corresponding to cubic Ce or cerium oxides were observed in the XRD patterns of Ce–TiO2, probably due to the low concentration of Ce and the very small particle size of ceria, as well as its good dispersion on the TiO2 surface.41
Ti ratio to 0.03 and 0.08 resulted in the disappearance of the rutile TiO2. This result is in agreement with the literature reports that even a low concentration of ceria can stabilize the anatase phase and inhibit transformation of anatase to rutile.39,40 The stabilization mechanism was attributed to the preferential nucleation of ceria on the oxygen vacancies of anatase TiO2.39,40 In addition, no obvious peaks corresponding to cubic Ce or cerium oxides were observed in the XRD patterns of Ce–TiO2, probably due to the low concentration of Ce and the very small particle size of ceria, as well as its good dispersion on the TiO2 surface.41
 | ||
| Fig. 2 XRD patterns of pristine TiO2 and Ce–TiO2 samples with different Ce/Ti molar ratios: (a) pristine TiO2; (b) 0.01Ce_1Ti; (c) 0.03Ce_1Ti; and (d) 0.08Ce_1Ti. (A: Anatase; B: Brookite; R: Rutile). | ||
| Samples | Phase content (%) | Crystal size/nm | S BET/m2 g−1 | ||||
|---|---|---|---|---|---|---|---|
| A | B | R | A | B | R | ||
| TiO2 | 34.5 | 11.7 | 53.7 | 17 | 9.5 | 10.3 | 25.2 |
| 0.01Ce_1Ti | 66.3 | 28.9 | 4.8 | 7.6 | 7.2 | 9.9 | 139.9 |
| 0.03Ce_1Ti | 71.5 | 28.5 | 0 | 5.7 | 5.2 | — | 136.7 |
| 0.08Ce_1Ti | 100 | 0 | 0 | 3.6 | — | — | 179.0 |
| SBA-15 | — | — | — | — | — | — | 871.9 |
| 0.03Ce_1Ti_1Si | 100 | 0 | 0 | — | — | — | 334.2 |
| 0.03Ce_1Ti_2Si | 100 | 0 | 0 | — | — | — | 509.7 |
| 0.03Ce_1Ti_4Si | 100 | 0 | 0 | — | — | — | 443.3 |
| 0.03Ce_1Ti_4aSi | — | — | — | — | — | — | 374.4 |
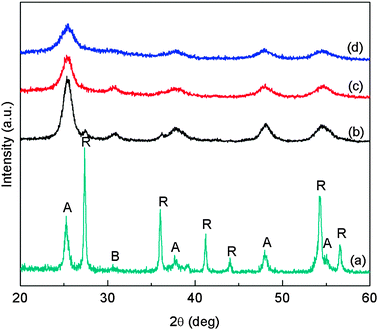
The diffraction peaks of Ce–TiO2 are broadened compared with pristine TiO2, suggesting smaller crystal size of TiO2 due to Ce addition. As summarized in Table 1, the average crystal sizes of pristine TiO2 were 17 nm for anatase and 9.5 nm for brookite, and they both decreased with the Ce concentration. It has been reported that the dopant in TiO2 favors the formation of smaller crystals.10,42 The smaller Ce–TiO2 size in this work is an indirect evidence that Ce was partially doped in TiO2 lattice. This also contributes to the reason that no Ce or CeO2 peaks were observed in the XRD patterns. Fig. 3 shows the XRD patterns of Ce–TiO2/SBA-15 samples. Only anatase TiO2 diffraction peaks were observed and the noisy background was due to the silica substrate.
 | ||
Fig. 3 XRD patterns of Ce–TiO2/SBA-15 samples with different Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si molar ratios: (a) 0.03Ce_1Ti_1Si; (b) 0.03Ce_1Ti_2Si; and (c) 0.03Ce_1Ti_4Si. Si molar ratios: (a) 0.03Ce_1Ti_1Si; (b) 0.03Ce_1Ti_2Si; and (c) 0.03Ce_1Ti_4Si. | ||
3.2 Textual properties and morphology of the catalysts
N2 adsorption–desorption was applied to explore the textual property of the prepared nanocomposite catalysts. As shown in Fig. 4a, the Ce–TiO2 samples showed typical type IV adsorption–desorption isotherms with a relatively wide H1-type hysteresis loop, an indicative of mesoporous structure. The mesopores may have resulted from the inter-space of aggregated nanoparticles. As shown in Fig. 4b, the Ce–TiO2 samples showed similar narrow BJH pore-size distribution with an average pore diameter in the range of 4–6 nm. Table 1 compares the BET specific surface areas of the different catalyst samples. The pristine TiO2 had the lowest surface area at 25.2 m2 g−1. The incorporation of a small concentration (1 or 3%) of Ce in TiO2 remarkably increased the surface area to approximately 140 m2 g−1. Further increasing the Ce concentration to 8% increased the surface area to 179 m2 g−1. The increased surface area is likely because of the smaller TiO2 particle size due to Ce doping, as evidenced in the crystal structure data (Table 1). | ||
| Fig. 4 N2 adsorption–desorption isotherms (a) and BJH pore size distributions (b) of TiO2 and Ce/TiO2 samples. | ||
Fig. 5 shows the N2 adsorption–desorption isotherms and BJH pore size distribution of SBA-15 and Ce–TiO2/SBA-15 samples. SBA-15 and Ce–TiO2/SBA-15 samples exhibited typical type IV adsorption–desorption isotherms (Fig. 5a), which are characteristic of mesoporous materials.30 For SBA-15, the P/P0 range of the H2-type hysteresis loop was from 0.55 to 0.80, while that of Ce–TiO2/SBA-15 was from 0.45 to 0.70. Typically, a hysteresis loop starts at a higher P/P0 for a material with a larger pore size.43 These isotherms suggest that Ce–TiO2/SBA-15 samples have smaller mesopores compared with that of SBA-15, very likely because some of the Ce–TiO2 nanoparticles were dispersed in the SBA-15 1-D channels or integrated into the SBA-15 framework forming chemical bonds. As shown in Fig. 5b, SBA-15 exhibits a mono-modal pore size distribution with a diameter of 5.8 nm. In contrast, 0.03Ce_1Ti_4Si and 0.03Ce_1Ti_2Si display a bi-modal pore size distribution. Besides the mode at 5.5 nm representing the SBA-15 pores, a new mode at around 4 nm appeared due to the existence of the Ce–TiO2 nanoparticles. When the TiO2 content further increased, i.e. for the 0.03Ce_1Ti_1Si sample, the 5.5 nm mode completely disappeared with only a single mode at around 3.8 nm. Similar shift in pore size distribution was reported by Zhang et al.30 that a smaller pore size was observed for a TiO2/SBA-15 composite with an increased TiO2 content. A possible reason is that the increased number of TiO2 nanoparticles may block the pores of SBA-15, and thus the pore size distribution shifts to the one that represents only TiO2 nanoparticles (single-mode and smaller size).
 | ||
| Fig. 5 N2 adsorption–desorption isotherms (a) and BJH pore size distributions (b) of SBA-15 and Ce–TiO2/SBA-15 samples. | ||
As shown in Table 1, the specific surface area of Ce–TiO2/SBA-15 was significantly increased compared with Ce–TiO2 due to the presence of SBA-15. SBA-15 has the highest surface area at 871.9 m2 g−1. It is interesting to find that the 0.03Ce_1Ti_4Si sample had a slightly smaller surface area than that of 0.03Ce_1Ti_2Si, whereas more SBA-15 was in the 0.03Ce_1Ti_4Si sample. It may be because that the Ce–TiO2 particles were better dispersed on the 0.03Ce_1Ti_4Si sample and partially blocked the pores of SBA-15, resulting in a smaller surface area.
Detailed morphological information of the Ce–TiO2/SBA-15 sample (0.03Ce_1Ti_4Si) was examined by SEM and TEM. The SEM images in Fig. 6 show micrometer size particles with irregular shapes as a mixture of rods and spheres, which are assigned to SBA-15 particles. The TiO2 nanoparticles are not discernible in the SEM images but are clearly shown in the TEM images in Fig. 7. Fig. 7a and b demonstrates the morphology of bare SBA-15 samples, where one-dimensional channels of ordered pores are clearly identified. The pore opening is around 5–7 nm, consistent with the pore size analysis data shown in Fig. 5. Fig. 7c demonstrates that for the Ce–TiO2/SBA-15 sample, the TiO2 nanoparticles are dispersed on the surface, at the edge, and possibly in the pores of the SBA-15 support. However, the fraction of TiO2 nanoparticles embedded in the pores of SBA-15 is difficult to measure. These embedded particles may block a portion of the pores, which explains why the measured specific surface area of the 0.03Ce_1Ti_4Si sample is smaller than that of the 0.03Ce_1Ti_2Si sample (see Table 1). Aggregates of TiO2 nanoparticles on the outer surface of SBA-15 were also identified, as shown in Fig. 7d. The TiO2 crystal size is around 5 nm, as shown in the HR-TEM image in Fig. 8e, which matches the calculated crystal size listed in Table 1 for 3%Ce–TiO2 sample. Clear lattice fringes of nanocrystal with an interplanar space of 0.350 nm were observed, which corresponds to the (101) plane of anatase TiO2.44 The lattice fringes of Ce or CeO2 were not seen under the observed projections, consistent with the XRD results that no Ce or CeO2 nanocrystals were formed.
 | ||
| Fig. 6 SEM images of the 0.03Ce_1Ti_4Si sample at lower (a) and higher (b) magnifications. | ||
 | ||
| Fig. 7 TEM and HRTEM images of SBA-15 (a,b) and 0.03Ce_1Ti_4Si (c,d,e) samples. | ||
 | ||
Fig. 8 UV-vis diffuse reflectance spectra of TiO2 and Ce–TiO2 with different Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti molar ratios (a) and Ce–TiO2/SBA-15 with different Ti Ti molar ratios (a) and Ce–TiO2/SBA-15 with different Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si molar ratios (b). Si molar ratios (b). | ||
3.3 UV-vis diffuse reflectance spectroscopy (DRS) analysis
Diffuse reflectance UV-vis spectra were recorded to explore the influence of Ce and SBA-15 addition on the optical property of TiO2. As shown in Fig. 8a, pristine TiO2 absorbs light at wavelength shorter than 400 nm, corresponding to a band gap of about 3.0 eV. Incorporation of Ce in TiO2 resulted in a red-shift in the absorption edge, extending to the visible light region in the range of 400–500 nm. The red-shift was enhanced at an increased Ce concentration, but the enhancement was not significant when the Ce concentration exceeded 3%. The DRS results in this work agree with the literature report that Ce doping in TiO2 could lead to formation of new energy levels within the band gap of TiO2 and thus may enable the photocatalytic activity under visible light.22–24Fig. 8b shows the UV-vis spectra of Ce–TiO2/SBA-15 samples. In contrast to the red-shift effect brought by the Ce doping, the incorporation of SBA-15 as the support resulted in a blue-shift of the absorption spectra, which is signified at a higher SBA-15 loading. The blue shift of the adsorption edge indicates the increasing of the forbidden band energy and thus a smaller semiconductor particle size, according to the quantum mechanics theory.45 The result in this study is in agreement with the literature that a blue shift of light absorption edge of TiO2/SBA-15 has been observed compared with pure anatase TiO2 or P25.46–48 The smaller TiO2 nanoparticle size in the presence of the SBA-15 matrix is likely due to the confinement effect of the mesopores in SBA-15 that inhibit the growth of TiO2 nanocrystals.46 This blue-shift in absorption spectra caused the inability of the Ce–TiO2/SBA-15 catalyst to respond to visible light excitation (λ > 400 nm), according to the result in this work, as opposed to the observed visible light activity for bare Ce–TiO2 catalysts reported in the literature.22–24 However, the absorption in the UV region (λ < 350 nm) was enhanced for all the Ce–TiO2/SBA-15 samples compared to Ce–TiO2 (Fig. 8b).
3.4 Oxidation states of doped cerium – XPS analysis
The results of XPS for the 0.03Ce_1Ti sample are shown in Fig. 9. The energy interval between approximately 882 eV and 903 eV was attributed by Ce 3d 5/2, while the Ce 3d 3/2 was at the 905 eV to 918 eV interval. To perform quantitative analysis, the background was first subtracted using Shirley approximation function in Augerscan software, and then the 3d 3/2 and 3d 5/2 peak structures were fitted. There were in total 10 peaks fitted for the XPS spectrum between 870 and 920 eV, where Ce4+ (CeO2) has six peaks (#5–10) for the total 3d lines due to strong hybridization of the oxygen 2p valence band with the Ce 4f orbital, while Ce3+ (Ce2O3) has four peaks (#1–4).49 Relative ratio/concentrations of Ce3+/Ce4+ were calculated based on the summation of the corresponding peak areas, which gives a Ce3+/Ce4+ ratio equal to 55.3%/44.7% before photoreaction and 49.5%/50.5% after photoreaction. The same analysis was done for the 0.08Ce_1Ti sample (XPS spectra not shown here), and the Ce3+/Ce4+ ratio was 51.8%/48.2% before photoreaction and 52.7%/47.2% after photoreaction. These results indicate that the Ce3+/Ce4+ ratios were similar for the different Ce/TiO2 samples and almost did not change during the CO2 photoreduction process. | ||
| Fig. 9 XPS spectra of Ce 3d of the 0.03Ce_1Ti sample before (a) and after (b) CO2 photocatalytic reduction with water vapor. | ||
3.5 Photocatalytic activity
A series of background tests were first conducted to prove that any carbon-containing compounds in the effluent gas measured by the GC indeed originated from CO2 through photocatalytic reactions. First, tests were conducted using CO2 and H2O vapor as the purging and reaction gas for the cases of (1) empty reactor and (2) blank glass–fiber filter in the reactor. No carbon-containing compounds were produced under UV-vis irradiation in each of the two cases. This demonstrates that the reactor and the glass–fiber filter were clean and that CO2 reduction cannot proceed without the photocatalyst. Second, pure helium (instead of CO2) and water vapor were used as the purging and reaction gases tested with the catalyst loaded in the reactor. Again, no carbon-containing compounds were produced by the catalyst under UV-vis irradiation. This verifies that the catalyst was clean (i.e. no interference from organic residues) and that any produced C-containing gases must be derived from CO2 in the reaction gases.In this study, CO and CH4 were identified as the main products of CO2 photoreduction with H2O on Ce–TiO2 and Ce–TiO2/SBA-15 nanocomposites. This was in line with our previous published work4,10,36 that CO and CH4 are the main products of CO2 photoreduction with water vapor, although other studies reported the generation of methanol, formaldehyde, etc., when TiO2 catalyst suspension in an aqueous phase was investigated.28,50Fig. 10 shows the production rates of CO and CH4 on selected photocatalysts as a function of photo-illumination time. For all catalysts, the production rates gradually decreased after reaching the maximum values, indicating a gradual deactivation of the catalytic performance. Similar trend in deactivation has been observed in our previous studies4,19 and in the literature reports;50,51 possible reasons are surface coverage of reaction intermediates or re-oxidation of the reaction products.50 To better compare the activities of the different photocatalysts, accumulative yields during the 4 h photo-illumination were calculated by integrating the production rate with time. The comparison results are presented as follows.
 | ||
| Fig. 10 Production rate of (a) CO and (b) CH4 over TiO2, 0.03Ce_1Ti, 0.03Ce_1Ti_4Si and 0.03Ce_1Ti_4aSi catalysts under UV-vis illumination. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti ratios. Pristine TiO2 exhibited very low photocatalytic activity, with a CO yield at 0.25 μmol g−1 and a CH4 yield at 0.05 μmol g−1. Ce-modification in TiO2 significantly influenced CO production. At 1% and 3% Ce concentration, the CO yield reached approximately 1.0 μmol g−1, four times as high as the pristine TiO2. However, further increasing the Ce concentration to 8% resulted in a lower CO yield, and at 12% Ce concentration, the CO yield was even lower than that of pristine TiO2. The CH4 yields of all the TiO2 and Ce–TiO2 samples remained in the same order and at least one magnitude lower than CO yields, indicating that CH4 yield was not affected by the Ce addition.
Ti ratios. Pristine TiO2 exhibited very low photocatalytic activity, with a CO yield at 0.25 μmol g−1 and a CH4 yield at 0.05 μmol g−1. Ce-modification in TiO2 significantly influenced CO production. At 1% and 3% Ce concentration, the CO yield reached approximately 1.0 μmol g−1, four times as high as the pristine TiO2. However, further increasing the Ce concentration to 8% resulted in a lower CO yield, and at 12% Ce concentration, the CO yield was even lower than that of pristine TiO2. The CH4 yields of all the TiO2 and Ce–TiO2 samples remained in the same order and at least one magnitude lower than CO yields, indicating that CH4 yield was not affected by the Ce addition.
 | ||
| Fig. 11 Product yields of CO and CH4 over TiO2 and Ce–TiO2 catalysts under UV-vis illumination for 4 h. | ||
The enhanced CO production due to low concentration Ce-modification on TiO2 can be attributed to several reasons. First, Ce-dopant decreased the crystal size of TiO2 and increased the surface area, as shown in Table 1, thus enhancing the catalytic activity. Second, Ce-dopant inhibited the transformation of anatase to rutile phase and promoted the growth of both anatase and brookite phases, as shown in Table 1. It is well known that anatase TiO2 is photocatalytically more active than rutile, but a mixture of the two phases can be advantageous. Studies by Gray and co-workers52,53 indicated that the enhanced photocatalytic activity of P25 (approximately 80% anatase and 20% rutile) is due to the presence of a nanostructured arrangement of interwoven anatase–rutile crystallites, unique interfacial trapping sites, as well as the electron transfer process across the interface from rutile to anatase. Similar mechanism may apply for the anatase–brookite mixture found in this work for the samples of 0.01Ce_1Ti and 0.03Ce_1Ti, considering the conduction band (CB) edge of brookite is slightly above that of anatase,54 making it possible for electrons to transfer from brookite to anatase. Both 0.01Ce_1Ti and 0.03Ce_1Ti samples showed similar phase composition (approximately 70% anatase and 30% brookite), similar surface area, and similarly high photocatalytic activity compared to other Ce/TiO2 samples (i.e., the 0.08Ce_1Ti sample that has pure anatase phase and the bare TiO2 that has a mixture of anatase–brookite–rutile with a dominating rutile phase). The above results indicate that a binary anatase–brookite with dominating anatase phase at low Ce concentration could promote CO2 photoreduction activity. This conclusion is in line with the literature reports55,56 that anatase–brookite mixtures are more active than pure anatase for photo-degradation of organic compounds, and with our recent research findings that an anatase–brookite mixture of TiO2 is active in CO2 photoreduction with water vapor.10,36
Another important reason for the enhanced activity may be related to the Ce3+/Ce4+ redox couple on TiO2 that facilitates electron–hole separation. It is well known that Ce4+ ions can trap photo-excited electrons at the CeO2/TiO2 interface through reaction (1).6,20,23,27 The Ce3+ ions can then react with gas-phase O2, if any (reaction (2)) to regenerate Ce4+,20,27 or react with CO2 (reaction (3)) in the case of this study, to form surface adsorbed CO2− that can be further reduced to CO (reaction (4)). The formation of CO2− species as CO2 reduction intermediates has been confirmed by our previous in situ DRIFTS study using Cu/TiO2 catalysts.19 Hence, the presence of Ce4+ enhanced electron trapping and transfer to CO2.
| e− + Ce4+ → Ce3+ | (1) |
| Ce3+ + O2 → Ce4+ + O2− | (2) |
| Ce3+ + CO2 → Ce4+ + CO2− | (3) |
| CO2− + e− → CO + O2− | (4) |
| Ce3+ + h+ → Ce4+ | (5) |
On the other hand, it is possible that Ce3+ ions can be directly oxidized back to Ce4+ by photo-excited holes, according to reaction (5),6 which may diminish the promoting effect of electron transfer as described above. However, the pre-existing Ce3+ ions on the TiO2, in the case of Ce–TiO2 samples prepared in this work, can provide abundant Ce3+ sites to promote reaction (3). Furthermore, the hole trapping effect of Ce3+ can also prevent holes from re-oxidizing the reaction product (i.e., CO) back to CO2. Therefore, the Ce3+/Ce4+ redox couples are advantageous over Ce4+ or Ce3+ alone to promote charge separation and enhance CO2 photoreduction. The XPS result that the Ce3+/Ce4+ ratio almost did not change during the photo-reaction was another evidence for the abovementioned Ce3+/Ce4+ redox reactions that remained at a dynamic equilibrium.
At a relatively high Ce loading (>8%), the CO production was not superior to pristine TiO2, probably because the higher concentrations of Ce species become recombination centers and thus lead to accelerated electron–hole recombination. Similar findings have been reported in the literature that Cu-modified TiO2 increased CO2 photoreduction at low Cu concentrations but too high a Cu concentration resulted in decreased catalytic activity due to formation of recombination centers.4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si ratio varied from 1 to 4. Compared with the bare Ce–TiO2 sample, dispersing the Ce–TiO2 nanoparticles on the SBA-15 matrix resulted in a significant enhancement in the yields of CO and CH4 production, as shown in Fig. 12. The enhancement seemed to be proportional to the loading of SBA-15 used. Among the three Ce–TiO2/SBA-15 samples tested, the sample with the highest SBA-15 loading, 0.03Ce_1Ti_4Si, showed the highest activity for both CO and CH4 production, reaching 7.5 and 7.9 μmol g−1, respectively. Compared with the 0.03Ce_1Ti sample, the 0.03Ce_1Ti_4Si sample demonstrated 8-fold enhancement in CO production and 115-fold enhancement in CH4 production.
Si ratio varied from 1 to 4. Compared with the bare Ce–TiO2 sample, dispersing the Ce–TiO2 nanoparticles on the SBA-15 matrix resulted in a significant enhancement in the yields of CO and CH4 production, as shown in Fig. 12. The enhancement seemed to be proportional to the loading of SBA-15 used. Among the three Ce–TiO2/SBA-15 samples tested, the sample with the highest SBA-15 loading, 0.03Ce_1Ti_4Si, showed the highest activity for both CO and CH4 production, reaching 7.5 and 7.9 μmol g−1, respectively. Compared with the 0.03Ce_1Ti sample, the 0.03Ce_1Ti_4Si sample demonstrated 8-fold enhancement in CO production and 115-fold enhancement in CH4 production.
 | ||
| Fig. 12 Product yields of CO and CH4 over Ce–TiO2/SBA-15 and Ce–TiO2/aSiO2 catalysts under UV-vis illumination for 4 h. | ||
This superior activity of SBA-15 supported Ce–TiO2 catalysts is believed to be mainly attributed to the higher surface area and better dispersion of the Ce–TiO2 nanoparticles, as verified by the BET and TEM analyses. Moreover, SBA-15 as the support may enhance the stability of the TiO2 anatase phase and prevent the grain growth of TiO2 nanocrystals.57 This mechanism was also supported by our XRD results that 100% anatase TiO2 was present in Ce–TiO2/SBA-15 samples and supported by our UV-vis DRS results that smaller TiO2 nanoparticles were present due to the SBA-15 confinement effect leading to a blue-shift in light absorption. In addition, mesoporous silica is also a well-known CO2 adsorbent that may increase the localized concentration of CO2 near the surface of TiO2, and thus improve CO2 adsorption on TiO2 and subsequent reduction. In a recent study by Yang et al.32 an enhanced photocatalytic CO2 reduction was observed on a 2%Cu–TiO2/SBA-15 sample (45 wt% TiO2 loading) compared with 2%Cu–TiO2; however, the enhancement due to the SBA-15 support was only 20%. In our work, the enhancement factor was much more significant on Ce–TiO2/SBA-15, suggesting the superior effect of Ce addition. Furthermore, the dramatic 115-fold enhancement in CH4 production observed in this study is very intriguing. Because eight protons are needed for generation of one CH4 molecule in comparison with two electrons for CO generation, the surface Si–OH groups or active OH sites due to the presence of SBA-15 may be more readily available for CO2 reduction and conversion to CH4. Investigations on this high CH4 selectivity as well as the potential synergies between the Ce species, TiO2 and the SBA-15 support are in our future research plan.
Another important finding in this study was that Ce–TiO2 dispersed on SBA-15 was much more active than that dispersed on amorphous mesoporous silica. As shown in Fig. 12, the CO yield on the amorphous silica supported sample, 0.03Ce_1Ti_4aSi was very small, even lower than the catalyst without silica support. The CH4 yield of 0.03Ce_1Ti_4aSi was 1.5 μmol g−1, higher than the catalyst without silica support but still much lower than the catalyst supported on SBA-15. The overall CO2 reduction rate to CO and CH4 was more than 10 times higher on 0.03Ce_1Ti_4Si than on 0.03Ce_1Ti_4aSi. Since the specific surface area of the 0.03Ce_1Ti_4Si sample was only slightly larger that of the 0.03Ce_1Ti_4aSi sample (Table 1), the much higher activity of the SBA-15 supported sample should result from other factors besides the surface area effect.
One possible reason is that ordered pores of SBA-15 are more accessible to TiO2 precursors during the preparation process, leading to a better dispersion of TiO2 nanoparticles. Amorphous silica with random arrays of pores and shapes in some cases are poor supports for functional agents because not all the volume of irregular pores is accessible to the incorporated species.58 The second possible reason could be the stronger interactions between the SBA-15 and the TiO2 nanoparticles confined inside the ordered pores, which favor the formation of interfacial active sites. Li et al.59 identified a particular Ti–O–Si bond at the interface of nano-TiO2 embedded in a mesoporous SiO2 through FTIR analysis, and they reported that this Ti–O–Si bond attributed to a stronger light absorption in the UV region compared with bare TiO2. Our UV-vis absorption results (Fig. 8b) also showed a similar stronger absorption for Ce–TiO2/SBA-15 samples at λ < 350 nm, suggesting a possible formation of a Ti–O–Si bond at the TiO2–SBA-15 interface. The third possible reason may be related to the larger CO2 adsorption capacity of SBA-1560 and easier diffusion of CO2 into the ordered pores compared with the amorphous silica. The enhanced local concentration of CO2 around the TiO2 nanoparticles could then promote the subsequent CO2 reduction reaction.
4. Conclusion
A novel Ce–TiO2/SBA-15 nanocomposite was synthesized for the first time in the literature and tested as a photocatalyst for converting CO2 and H2O to fuels such as CO and CH4 under photo-illumination. Modification of TiO2 with Ce significantly stabilized the TiO2 anatase phase and increased the specific surface area, which contributed to an improvement of CO production from CO2 reduction. Dispersing Ce–TiO2 nanoparticles on the mesoporous SBA-15 support further enhanced both CO and CH4 production. Particularly the CH4 production was enhanced by up to 115 times compared with unsupported Ce–TiO2. The superior catalytic activity may be related to the partially embedded Ce–TiO2 nanoparticles in the ordered 1-D pores in SBA-15 that form synergies between the different components of the catalysts and enhance the diffusion and adsorption of CO2. This mechanism also correlates well the results that using SBA-15 as the support led to more than 10 times higher activity in CO2 photoreduction than using amorphous silica as the support. Findings in this work demonstrate the feasibility of solar fuel production from CO2 and H2O using the prepared nanocomposite photocatalysts. On-going research is being dedicated to further improving the overall CO2 conversion rate and control of product selectivity.Acknowledgements
This work is supported by American Chemical Society – Petroleum Research Fund (ACS–PRF, Grant #50631-DNI10). The authors acknowledge Mr. Donald Robertson at the Physics Laboratory for High Resolution Transmission Electron Microscopy at UW-Milwaukee for his assistance in TEM and HRTEM analyses. The authors also thank Dr. Valentin Craciun at the Major Analytical Instrumentation Center (MAIC) at the University of Florida for his assistance in XPS analyses.References
- B. Aurian-Blajeni, M. Halmann and J. Manassen, Photoreduction of carbon dioxide and water into formaldehyde and methanol on semiconductor materials, Sol. Energy, 1980, 25(2), 165–170 CrossRef CAS.
- Y. Kohno, T. Tanaka, T. Funabiki and S. Yoshida, Photoreduction of CO2 with H2 over ZrO2. A study on interaction of hydrogen with photoexcited CO2, Phys. Chem. Chem. Phys., 2000, 2(11), 2635–2639 RSC.
- H. Tsuneoka, K. Teramura, T. Shishido and T. Tanaka, Adsorbed Species of CO2 and H2 on Ga2O3 for the Photocatalytic Reduction of CO2, J. Phys. Chem. C, 2010, 114(19), 8892–8898 CAS.
- Y. Li, W. N. Wang, Z. L. Zhan, M. H. Woo, C. Y. Wu and P. Biswas, Photocatalytic reduction of CO2 with H2O on mesoporous silica supported Cu/TiO2 catalysts, Appl. Catal., B, 2010, 100(1–2), 386–392 CrossRef CAS.
- O. K. Varghese, M. Paulose, T. J. LaTempa and C. A. Grimes, High-Rate Solar Photocatalytic Conversion of CO2 and Water Vapor to Hydrocarbon Fuels, Nano Lett., 2009, 9(2), 731–737 CrossRef CAS.
- H. M. Yang, K. Zhang, R. R. Shi and A. D. Tang, Sol–gel synthesis and photocatalytic activity of CeO2/TiO2 nanocomposites, J. Am. Ceram. Soc., 2007, 90(5), 1370–1374 CrossRef CAS.
- C. Wen, H. Deng, J. Y. Tian and J. M. Zhang, Photocatalytic activity enhancing for TiO2 photocatalyst by doping with La, Trans. Nonferrous Met. Soc. China, 2006, 16, S728–S731 CrossRef.
- C. S. Yuan, C. C. Lo, C. H. Hung and J. F. Wu, Photoreduction of carbon dioxide with H2 and H2O over TiO2 and ZrO2 in a circulated photocatalytic reactor, Sol. Energy Mater. Sol. Cells, 2007, 91(19), 1765–1774 CrossRef.
- A. Fujishima, X. Zhang and D. A. Tryk, TiO2 photocatalysis and related surface phenomena, Surf. Sci. Rep., 2008, 63(12), 515–582 CrossRef CAS.
- Q. Y. Zhang, Y. Li, E. A. Ackerman, M. Gajdardziska-Josifovska and H. L. Li, Visible light responsive iodine-doped TiO2 for photocatalytic reduction of CO2 to fuels, Appl. Catal., A, 2011, 400(1–2), 195–202 CrossRef CAS.
- I. H. Tseng, J. C. S. Wu and H. Y. Chou, Effects of sol–gel procedures on the photocatalysis of Cu/TiO2 in CO2 photoreduction, J. Catal., 2004, 221(2), 432–440 CrossRef CAS.
- W. Y. Su, Y. F. Zhang, Z. H. Li, L. Wu, X. X. Wang, J. Q. Li and X. Z. Fu, Multivalency iodine doped TiO2: Preparation, characterization, theoretical studies, and visible-light photocatalysis, Langmuir, 2008, 24(7), 3422–3428 CrossRef CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Visible-light photocatalysis in nitrogen-doped titanium oxides, Science, 2001, 293(5528), 269–271 CrossRef CAS.
- C. J. Wang, R. Thompson, J. Baltrus and C. Matranga, Visible Light Photoreduction of CO2 using CdSe/Pt/TiO2 Heterostructured Catalysts, J. Phys. Chem. Lett., 2010, 1(1), 48–53 CrossRef CAS.
- Y. Kohno, H. Hayashi, S. Takenaka, T. Tanaka, T. Funabiki and S. Yoshida, Photo-enhanced reduction of carbon dioxide with hydrogen over Rh/TiO2, J. Photochem. Photobiol., A, 1999, 126(1–3), 117–123 CrossRef CAS.
- K. Koci, K. Zatloukalova, L. Obalova, S. Krejcikova, Z. Lacny, L. Capek, A. Hospodkova and O. Solcova, Wavelength Effect on Photocatalytic Reduction of CO2 by Ag/TiO2 Catalyst, Chin. J. Catal., 2011, 32(5), 812–815 CrossRef CAS.
- C. Y. Zhao, A. Kroll, H. L. Zhao, Q. Y. Zhang and Y. Li, Ultrasonic spray pyrolysis synthesis of Ag/TiO2 nanocomposite photocatalysts for simultaneous H2 production and CO2 reduction, Int. J. Hydrogen Energy, 2012, 37(13), 9967–9976 CrossRef CAS.
- J. C. S. Wu, Photocatalytic Reduction of Greenhouse Gas CO2 to Fuel, Catal. Surv. Asia, 2009, 13(1), 30–40 CrossRef CAS.
- L. J. Liu, C. Y. Zhao and Y. Li, Spontaneous Dissociation of CO2 to CO on Defective Surface of Cu(I)/TiO2−x Nanoparticles at Room Temperature, J. Phys. Chem. C, 2012, 116(14), 7904–7912 CAS.
- Y. H. Xu and Z. X. Zeng, The preparation, characterization, and photocatalytic activities of Ce-TiO2/SiO2, J. Mol. Catal. A: Chem., 2008, 279(1), 77–81 CrossRef CAS.
- B. M. Reddy, A. Khan, P. Lakshmanan, M. Aouine, S. Loridant and J. C. Volta, Structural characterization of nanosized CeO2–SiO2, CeO2–TiO2, and CeO2–ZrO2 catalysts by XRD, Raman, and HREM techniques, J. Phys. Chem. B, 2005, 109(8), 3355–3363 CrossRef CAS.
- K. Ogura, M. Kawano, J. Yano and Y. Sakata, Visible-Light-Assisted Decomposition of H2O and Photomethanation of CO2 over CeO2–TiO2 Catalyst, J. Photochem. Photobiol., A, 1992, 66(1), 91–97 CrossRef CAS.
- J. M. Xie, D. L. Jiang, M. Chen, D. Li, J. J. Zhu, X. M. Lu and C. H. Yan, Preparation and characterization of monodisperse Ce-doped TiO2 microspheres with visible light photocatalytic activity, Colloids Surf., A, 2010, 372(1–3), 107–114 CrossRef CAS.
- G. S. Li, D. Q. Zhang and J. C. Yu, Thermally stable ordered mesoporous CeO2/TiO2 visible-light photocatalysts, Phys. Chem. Chem. Phys., 2009, 11(19), 3775–3782 RSC.
- X. J. Sun, H. Liu, J. H. Dong, J. Z. Wei and Y. Zhang, Preparation and Characterization of Ce/N-Codoped TiO2 Particles for Production of H2 by Photocatalytic Splitting Water Under Visible Light, Catal. Lett., 2010, 135(3–4), 219–225 CrossRef CAS.
- G. Magesh, B. Viswanathan, R. P. Viswanath and T. K. Varadarajan, Photocatalytic behavior of CeO2–TiO2 system for the degradation of methylene blue, Indian J. Chem., Sect. A: Inorg., Bio-Inorg., Phys., Theor. Anal. Chem., 2009, 48(4), 480–488 Search PubMed.
- F. Galindo, R. Gomez and M. Aguilar, Photodegradation of the herbicide 2,4-dichlorophenoxyacetic acid on nanocrystalline TiO2-CeO2 sol–gel catalysts, J. Mol. Catal. A: Chem., 2008, 281(1–2), 119–125 CrossRef CAS.
- N. Sasirekha, S. J. S. Basha and K. Shanthi, Photocatalytic performance of Ru doped anatase mounted on silica for reduction of carbon dioxide, Appl. Catal., B, 2006, 62(1–2), 169–180 CrossRef CAS.
- B. Srinivas, B. Shubhamangala, K. Lalitha, P. A. K. Reddy, V. D. Kumari, M. Subrahmanyam and B. R. Dema, Photocatalytic Reduction of CO2 over Cu-TiO2/Molecular Sieve 5A Composite, Photochem. Photobiol., 2011, 87(5), 995–1001 CrossRef CAS.
- S. C. Zhang, D. Jiang, T. Tang, J. H. Li, Y. Xu, W. L. Shen, J. Xu and F. Deng, TiO2/SBA-15 photocatalysts synthesized through the surface acidolysis of Ti(OnBu)4 on carboxyl-modified SBA-15, Catal. Today, 2010, 158(3–4), 329–335 CrossRef CAS.
- C. C. Yang, J. Vernimmen, V. Meynen, P. Cool and G. Mul, Mechanistic study of hydrocarbon formation in photocatalytic CO2 reduction over Ti-SBA-15, J. Catal., 2011, 284(1), 1–8 CrossRef CAS.
- H. C. Yang, H. Y. Lin, Y. S. Chien, J. C. S. Wu and H. H. Wu, Mesoporous TiO2/SBA-15, and Cu/TiO2/SBA-15 Composite Photocatalysts for Photoreduction of CO2 to Methanol, Catal. Lett., 2009, 131(3–4), 381–387 CrossRef CAS.
- L. Q. Jing, X. J. Sun, B. F. Xin, B. Q. Wang, W. M. Cai and H. G. Fu, The preparation and characterization of La doped TiO2 nanoparticles and their photocatalytic activity, J. Solid State Chem., 2004, 177(10), 3375–3382 CrossRef CAS.
- D. Y. Zhao, J. L. Feng, Q. S. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores, Science, 1998, 279(5350), 548–552 CrossRef CAS.
- L. J. Liu, H. L. Zhao, J. M. Andino and Y. Li, Photocatalytic CO2 reduction with H2O on TiO2 nanocrystals: Comparison with anatase, rutile, brookite polymorphs and exploration of surface chemistry, ACS Catal., 2012, 2, 1817–1828 CrossRef CAS.
- Q. Y. Zhang, T. T. Gao, J. M. Andino and Y. Li, Copper and Iodine Co-modified TiO2 Nanoparticles for Improved Activity of CO2 Photoreduction with Water Vapor, Appl. Catal., B, 2012, 123–124, 257–264 CrossRef CAS.
- T. A. Kandiel, A. Feldhoff, L. Robben, R. Dillert and D. W. Bahnemann, Tailored Titanium Dioxide Nanomaterials: Anatase Nanoparticles and Brookite Nanorods as Highly Active Photocatalysts, Chem. Mater., 2010, 22(6), 2050–2060 CrossRef CAS.
- A. Di Paola, G. Cufalo, M. Addamo, M. B. Ellardita, R. Campostrini, M. Ischia, R. Ceccato and L. Palmisano, Photocatalytic activity of nanocrystalline TiO2 (brookite, rutile and brookite-based) powders prepared by thermohydrolysis of TiCl4 in aqueous chloride solutions, Colloids and Surf., A, 2008, 317(1–3), 366–376 CrossRef CAS.
- J. Fang, X. Z. Bi, D. J. Si, Z. Q. Jiang and W. X. Huang, Spectroscopic studies of interfacial structures of CeO2–TiO2 mixed oxides, Appl. Surf. Sci., 2007, 253(22), 8952–8961 CrossRef CAS.
- T. Kidchob, L. Malfatti, D. Marongiu, S. Enzo and P. Innocenzi, An alternative sol–gel route for the preparation of thin films in CeO2–TiO2 binary system, Thin Solid Films, 2010, 518(6), 1653–1657 CrossRef CAS.
- Z. L. Liu, B. Guo, L. Hong and H. X. Jiang, Preparation and characterization of cerium oxide doped TiO2 nanoparticles, J. Phys. Chem. Solids, 2005, 66(1), 161–167 CrossRef CAS.
- X. L. Nie, S. P. Zhuo, G. Maeng and K. Sohlberg, Doping of TiO2 polymorphs for altered optical and photocatalytic properties, Int. J. Photoenergy, 2009, 2009, 294042 CrossRef.
- J. L. Blin and B. L. Su, Tailoring pore size of ordered mesoporous silicas using one or two organic auxiliaries as expanders, Langmuir, 2002, 18(13), 5303–5308 CrossRef CAS.
- Z. B. Wu, F. Dong, W. R. Zhao and S. Guo, Visible light induced electron transfer process over nitrogen doped TiO2 nanocrystals prepared by oxidation of titanium nitride, J. Hazard. Mater., 2008, 157(1), 57–63 CrossRef CAS.
- A. Henglein, Small-Particle Research – Physicochemical properties of extremely small colloidal metal and semiconductor particles, Chem. Rev., 1989, 89(8), 1861–1873 CrossRef CAS.
- D. R. Sahu, L. Y. Hong, S. C. Wang and J. L. Huang, Synthesis, analysis and characterization of ordered mesoporous TiO2/SBA-15 matrix: Effect of calcination temperature, Microporous Mesoporous Mater., 2009, 117(3), 640–649 CrossRef CAS.
- J. Yang, J. Zhang, L. W. Zhu, S. Y. Chen, Y. M. Zhang, Y. Tang, Y. L. Zhu and Y. W. Li, Synthesis of nano titania particles embedded in mesoporous SBA-15: Characterization and photocatalytic activity, J. Hazard. Mater., 2006, 137(2), 952–958 CrossRef CAS.
- H. G. Zhu, Z. W. Pan, B. Chen, B. Lee, S. M. Mahurin, S. H. Overbury and S. Dai, Synthesis of ordered mixed titania and silica mesostructured monoliths for gold catalysts, J. Phys. Chem. B, 2004, 108(52), 20038–20044 CrossRef CAS.
- J. P. Holgado, R. Alvarez and G. Munuera, Study of CeO2 XPS spectra by factor analysis: reduction of CeO2, Appl. Surf. Sci., 2000, 161(3–4), 301–315 CrossRef CAS.
- I. H. Tseng, W. C. Chang and J. C. S. Wu, Photoreduction of CO2 using sol–gel derived titania and titania-supported copper catalysts, Appl. Catal., B, 2002, 37(1), 37–48 CrossRef CAS.
- W. N. Wang, W. J. An, B. Ramalingam, S. Mukherjee, D. M. Niedzwiedzki, S. Gangopadhyay and P. Biswas, Size and Structure Matter: Enhanced CO2 Photoreduction Efficiency by Size-Resolved Ultrafine Pt Nanoparticles on TiO2 Single Crystals, J. Am. Chem. Soc., 2012, 134, 11276–11281 CrossRef CAS.
- D. C. Hurum, A. G. Agrios, S. E. Crist, K. A. Gray, T. Rajh and M. C. Thurnauer, Probing reaction mechanisms in mixed phase TiO2 by EPR, J. Electron Spectrosc. Relat. Phenom., 2006, 150(2–3), 155–163 CrossRef CAS.
- D. C. Hurum, A. G. Agrios, K. A. Gray, T. Rajh and M. C. Thurnauer, Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR, J. Phys. Chem. B, 2003, 107(19), 4545–4549 CrossRef CAS.
- A. Di Paola, M. Bellardita, R. Ceccato, L. Palmisano and F. Parrino, Highly Active Photocatalytic TiO2 Powders Obtained by Thermohydrolysis of TiCl4 in Water, J. Phys. Chem. C, 2009, 113(34), 15166–15174 CAS.
- T. Ozawa, M. Iwasaki, H. Tada, T. Akita, K. Tanaka and S. Ito, Low-temperature synthesis of anatase-brookite composite nanocrystals: the junction effect on photocatalytic activity, J. Colloid Interface Sci., 2005, 281(2), 510–513 CrossRef CAS.
- G. H. Tian, H. G. Fu, L. Q. Jing, B. F. Xin and K. Pan, Preparation and characterization of stable biphase TiO2 photocatalyst with high crystallinity, large surface area, and enhanced photoactivity, J. Phys. Chem. C, 2008, 112(8), 3083–3089 CAS.
- J. Yang, L. W. Zhu, J. Zhang, Y. M. Zhang and Y. Tang, Synthesis of nanosized TiO2/SiO2 catalysts by the ultrasonic microemulsion method and their photocatalytic activity, React. Kinet. Catal. Lett., 2007, 91(1), 21–28 CrossRef CAS.
- S. Choi, J. H. Drese and C. W. Jones, Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources, ChemSusChem, 2009, 2(9), 796–854 CrossRef CAS.
- Y. Z. Li and S. J. Kim, Synthesis and characterization of nano titania particles embedded in mesoporous silica with both high photocatalytic activity and adsorption capability, J. Phys. Chem. B, 2005, 109(25), 12309–12315 CrossRef CAS.
- Q. A. Wang, J. Z. Luo, Z. Y. Zhong and A. Borgna, CO2 capture by solid adsorbents and their applications: current status and new trends, Energy Environ. Sci., 2011, 4(1), 42–55 CAS.
| This journal is © The Royal Society of Chemistry 2012 |

