Preparation of highly dispersed gold nanoparticles/mesoporous carbon nanofiber composites and their application toward detection of hydrazine
Huan
Wang
,
Xiangjie
Bo
,
Jian
Ju
and
Liping
Guo
*
Faculty of Chemistry, Northeast Normal University, 130024 Changchun, P. R. China. E-mail: guolp078@nenu.edu.cn; Fax: +86 0431 85099762; Tel: +86 0431 85099762
First published on 30th May 2012
Abstract
Novel mesoporous carbon nanofibers (M-CNF) were synthesized using a self-templating strategy and a solution growth process. A facile and controllable electrodeposition method was developed to directly attach gold nanoparticles (GNPs) on mesoporous carbon nanofibers (M-CNF-Au). The prepared composites were characterized by scanning electron microscopy (SEM), nitrogen adsorption–desorption, X-ray photoelectron spectra (XPS) and X-ray diffraction (XRD). Composite modified glass carbon electrodes (M-CNF-Au/GCE) were used for the detection of hydrazine. The results showed that the present electrode exhibited excellent electrocatalytic activity toward hydrazine and gave a wide linear range from 1.0 × 10−8 to 1.1 × 10−3 M, a very low detection limit of 8.1 nM, and a fast response time within 2 s. This indicates that M-CNF-Au is a promising material in electrocatalysis.
1. Introduction
Many advanced nanostructured carbon materials, including multi-walled carbon nanotubes (MWCNTs),1 single-walled carbon nanotubes (SWCNTs),2 graphene (GR)3 and ordered mesoporous carbons (OMCs)4 have attracted a great deal of attention, due to their superior electrocatalytic capability over traditional carbon materials.5–9 Besides the carbon nanomaterials mentioned above, recently, a new kind of carbon nanomaterial – porous carbon nanofibers (CNF) – has received much attention owing to their thermal and mechanical stability, large surface active groups, high surface-to-volume ratio and low ohmic resistance. Despite the excellent capability of CNF, it is difficult to precisely and simultaneously manipulate the pore texture and morphology, especially with regard to construction of 1D nanostructures with a mesoporous texture, which can greatly affect the electrocatalytic performance of CNF. Therefore, many studies have focused on producing CNF by carbonizing appropriate precursors within suitable templates,10,11 but the processes remain either complex or time consuming. To overcome this challenge, great efforts have been made to develop a facile template-free method for the preparation of CNF with modest pore size and large surface area. Recently, Li et al.12 reported a novel self-template method for the preparation of mesoporous carbon nanofibers (M-CNF) by using ethylene glycol (EG) as the carbon precursor and Zn(CH3COO)2 as the structural constructor and porogen. Despite such simple preparation and attractive characteristics such as 3D interconnected mesoporous structure and large surface area, there have been few studies on the electrocatalytic property and analytical applications of this M-CNF.Recently, gold nanoparticles (GNPs) have been extensively studied due to their unique properties such as nano-scaled dimension effect, good conductivity and biocompatibility. Taking account of the advantages of GNPs, combination of GNPs and nanomaterials to fabricate sensors has received much interest because of their intriguing properties and potential applications in chemical sensing. In the past few years, GNPs have been usually attached to OMCs, GR or SWCNTs13–15 to construct GNPs/nanomaterial composites for sensor applications, and found to show excellent electrocatalytic performance. However, no attention has paid to M-CNF which could be a good supporter for host–guest chemistry to obtain highly dispersed GNPs.
Hydrazine and its derivatives are widely used in chemical industry and pharmaceuticals, including as a precursor to blowing agents, fuel cell, heat stabilizers and rocket fuel. However, hydrazine is a neurotoxin, and hence produces mutagenic and carcinogenic effects causing damage to kidneys, lungs, liver, respiratory tract infection and long-term effects on the central nervous system.16 Due to the above reasons, it is highly desirable to fabricate a sensitive analytical tool for the effective detection of hydrazine. In the past, MnHCF/graphite–wax composite,17 ZnO/MWCNTs,18 rhodium19 and Ag–Ni20 modified electrodes have been reported for the determination of hydrazine. However, the electrochemical parameters such as the linear range and detection limit are not satisfactory. So, developing a novel nanocomposite for the sensitive determination of hydrazine is highly desirable.
Considering the potential advantage of high surface area and mesoporous structure of M-CNF and high electrocatalytic capability of GNPs, in this work, we present, for the first time, the synthesis of M-CNF-Au nanocomposites for hydrazine determination. Our findings demonstrate that such an M-CNF-Au/GCE shows remarkable electrocatalytic activity towards hydrazine and is a promising catalyst to catalyze oxidation of hydrazine.
2. Experimental
2.1. Reagents and apparatus
Hydrogen tetrachloroaurate(III) trihydrate (HAuCl4·3H2O, 99.9%) was purchased from Sigma-Aldrich. Hydrous hydrazine (N2H4·H2O, 80%) was obtained from Tianjin, China. The 0.1 M phosphate buffer solution (PBS pH 7.0), which was made up from NaH2PO4, Na2HPO4 and H3PO4, was employed as a supporting electrolyte. All other reagents used were of analytical grade and used as received without further purification.Electrochemical experiments were performed using a CHI 830b Electrochemical Analyzer (Chenhua Instruments, Shanghai). A glassy carbon electrode (GCE) was employed as the working electrode; an Ag/AgCl electrode and a Pt wire were used as the reference and auxiliary electrodes, respectively.
Scanning electron microscope (SEM) images were acquired using a Philips XL-30 ESEM operating at an accelerating potential difference of 3.0 kV. Nitrogen adsorption–desorption isotherms were performed on an ASAP 2020 (Micromeritics, USA). X-Ray photoelectron spectra (XPS) were collected using an ESCALAB-MKII 250 photoelectron spectrometer (VG Co.) with Al-Kα X-ray radiation as the X-ray source for excitation. The samples for XPS characterization were drop-cast on a Cu substrate. X-Ray diffraction (XRD) patterns were obtained on an X-ray D/max-2200vpc (Rigaku Corporation, Japan) instrument operated at 40 kV and 20 mA and using Cu-Kα radiation (λ = 0.15406 nm).
2.2. Synthesis of M-CNF
M-CNF was synthesized through a novel self-template strategy and a solution growth process,12 wherein EG was used as the carbon precursor, Zn(CH3COO)2 as the structural constructor and the porogen and the initially formed zinc glycolate as the built-in template. In a typical procedure, Zn(CH3COO)2 was mixed with EG and the mixture was stirred at 150 °C for 90 min, from which air was excluded by a liquid seal setup. The slurry was collected and calcined for 2 h at 600 °C in N2 and then immersed in dilute HCl solution to remove the ZnO. M-CNF was obtained after being washed with water and ethanol.2.3. Preparation of the modified electrode
A glassy carbon electrode (GCE) was polished before each experiment with 1, 0.3 and 0.05 μm alumina powder, respectively, and then rinsed thoroughly with double-distilled water between each polishing step. The cleaned GCE was dried with pure nitrogen and treated by dropping a suspension (5 μL) of the M-CNF in DMF (2 mg mL−1) on it and then dried under an IR lamp.The procedure for the deposition of GNPs is given below. Amperometric i–t experiments were performed in solution which was deaerated by nitrogen gas and a nitrogen atmosphere was kept over the solutions during the electrodeposition experiment. The M-CNF/GCE was immersed into a solution of 1 mM HAuCl4 in 0.5 M H2SO4 and an operating potential of 0.05 V and a deposition time of 500 s were applied.
3. Results and discussion
3.1. Characterization of M-CNF and M-CNF-Au
Typical SEM images of M-CNF and M-CNF-Au surfaces are shown in Fig. 1. As can be seen from Fig. 1A, the M-CNF exhibits a well-designed 1D fibrous morphology with a rough surface, which is expected to significantly facilitate electron transfer. Fig. 1B shows that GNPs are well fixed and uniformly dispersed on the M-CNF surface and no significant aggregation was observed. The EDX spectrum (inset of Fig. 1B) clearly shows the presence of Au element which further proves the successful deposition of GNPs on M-CNF. | ||
| Fig. 1 SEM images of (A) M-CNF and (B) GNPs on M-CNF surface. Inset: the EDX spectrum of M-CNF-Au. | ||
Fig. 2A presents the nitrogen adsorption–desorption isotherms of M-CNF. The obtained result is a type IV isotherm with a hysteresis loop,21,22 which corresponds to capillary condensation taking place in mesopores. The pore-size distribution of M-CNF is given in Fig. 2B, indicating a quite narrow pore size distribution centered at about 3.3 nm. More important, the BET surface area of this kind of M-CNF is up to 1860 m2 g−1 which is much higher than those of the porous CNF previously reported (10–1140 m2 g−1).23–27
 | ||
| Fig. 2 (A) Nitrogen adsorption–desorption isotherms of M-CNF. (B) The corresponding pore size distribution curve. | ||
The XPS spectrum of M-CNF-Au is shown in Fig. 3A. The Au 4f7/2 and Au 4f5/2 peaks appear at binding energies of 84.1 and 87.8 eV, respectively, confirming the formation of GNPs28 on M-CNF. Fig. 3B displays the XRD pattern of the M-CNF-Au in which the diffraction peaks located at 38.1 and 44.4° correspond to the (111) and (200) lattice planes29 of Au, which further demonstrates the existence of GNPs on M-CNF.
 | ||
| Fig. 3 (A) XPS spectra of the Au 4f regions of GNPs obtained on M-CNF-Au. (B) XRD patterns of M-CNF-Au. | ||
3.2. Electrochemical reactivity
The electron transfer capability of M-CNF-Au/GCE, M-CNF/GCE and GCE were investigated by electrochemical impedance spectroscopy (EIS, Fig. 4). In EIS, the diameter of the semicircle represents the interfacial electron transfer resistance, whereas the linear portion corresponds to the diffusion process. We used the Randles equivalence circuit model (inset of Fig. 4) to fit the impedance data. In the equivalent circuit, the resistance to charge transfer (Rct) and the diffusion impedance (W) are both in parallel with the interfacial capacitance (Cdl).30 According to the equivalent circuit, Rct of GCE (405 Ω) was reduced substantially as compared with that of the M-CNF/GCE (40 Ω) and M-CNF-Au/GCE (33 Ω). The low electron transfer resistance of the M-CNF-Au/GCE shows that the M-CNF containing GNPs forms high electron conduction pathways between the electrode and electrolyte.31 | ||
| Fig. 4 Nyquist plots for 5 mM K3Fe(CN)6/0.1 M KCl on GCE, M-CNF/GCE and M-CNF-Au/GCE. The frequency range is from 10 mHz to 10 kHz. Inset: Randles equivalent circuit. | ||
3.3. Electrocatalytic to hydrazine on M-CNF-Au/GCE
Fig. 5 shows typical CVs of different electrodes in the absence and presence of 2 mM hydrazine in PBS (pH 7.0). The bare GCE (Fig. 5A) presents a weak electrocatalytic oxidation current toward hydrazine. The M-CNF/GCE (Fig. 5B) makes the oxidation current of hydrazine larger than the bare GCE and shows a peak at a potential of 0.279 V. The M-CNF-Au/GCE exhibits a significantly lower overpotential (0.165 V) for the hydrazine oxidation as well as a 2.50-fold increase in the peak current compared with that of the M-CNF/GCE. The greatly improved electrocatalytic performance of M-CNF-Au/GCE might be due to the well-dispersed Au particles and 3D interconnected mesopores of M-CNF which can supply more surface-active sites for the adsorption of reactants and a lower resistance to the transport of electrolyte ions.32 | ||
| Fig. 5 CVs of (A) GCE (B) M-CNF/GCE and (C) M-CNF-Au/GCE in the absence (⋯) and presence (—) of 2 mM hydrazine in 0.1 M PBS (pH 7.0). Scan rate: 50 mV s−1. | ||
3.4. Effects of pH and scan rate on hydrazine oxidation
Fig. 6A shows the linear sweep voltammetry curves when the solution pH is varied from 6.0 to 10.0. The peak potential (EPa) of the electrocatalytic oxidation of hydrazine was shifted toward a less positive potential along with an increase in the pH of the PBS. Dependence of the peak potential with pH (Fig. 6B) shows the slope is −68 mV pH−1 which is close to the anticipated Nernstian value of −74 mV for a four-electron, five-proton process.33| N2H5+ → N2 + 4e + 5H+ |
Moreover, the current changes with the pH and the maximum current response is obtained at pH 8.0 (Fig. 6B). Considering the conditions of application, pH 7.0 was selected for the supporting electrolyte in all experiments.
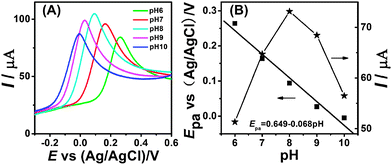 | ||
| Fig. 6 (A) Linear sweep voltammetry curves of M-CNF-Au/GCE in 0.1 M PBS containing 2 mM hydrazine at different pH values: 6.0; 7.0; 8.0; 9.0; 10.0. (B) Dependence of the peak potentials and peak currents with pH. | ||
The influence of scan rates on the anodic peak current was also studied, the peak potential shifted slightly toward positive direction with increasing scan rate (Fig. 7A). The plot of peak current vs. scan rates (Fig. 7B) showed the oxidation peak current of hydrazine increased linearly with v1/2 in the range of 10–300 mV s−1, showing a typical diffusion-controlled kinetics, which was the ideal case for quantitative applications.
 | ||
| Fig. 7 (A) CVs of 2 mM hydrazine on M-CNF-Au/GCE in 0.1 M PBS (pH 7.0) at scan rates of (a–s) 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 130, 150, 180, 210, 240, 270 and 300 mV s−1. (B) Dependence of the peak current with square root of the scan rate. | ||
3.5. Amperometric determination of hydrazine
As shown in Fig. 8, current–time curves illustrate the response of M-CNF-Au/GCE with each addition of hydrazine in stirred 0.1 M PBS (pH 7.0). As can be seen, the oxidation currents dramatically increase with each addition of hydrazine and reaches a steady-state level in less than 2 s. M-CNF-Au/GCE exhibits linear responses to hydrazine concentrations from 1.0 × 10−8 to 1.1 × 10−3 M (R = 0.999). A very low detection limit of 8.1 nM (S/N = 3) which is lower than previously reported literature values is obtained. The data obtained from Fig. 8 are summarized and compared with other results in Table 1.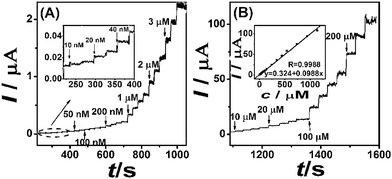 | ||
| Fig. 8 (A) (B) Current–time curves for M-CNF-Au/GCE with successive additions of different concentrations hydrazine in 0.1 M PBS (pH 7.0). Inset in (B): Calibration curves for hydrazine. Operating potential: 0.17 V. | ||
| Electrode material | Response time/s | Detection limit/μM | Linear range/μM | Ref. |
|---|---|---|---|---|
| a Multi-wall carbon nanotubes. b Carbon nanotubes. c Zirconium hexacyanoferrate. | ||||
| Hematoxylin-M-CNTa | <2 | 0.680 | 2.0–122.8 | 34 |
| Curcumin-M-CNT | <3 | 1.400 | 2.0–44.0 | 35 |
| Au/PPy/GCE | — | 0.200 | 1.0–500 | 36 |
| Nano-Au/porous-TiO2/GCE | <3 | 0.500 | 2.5–500 | 37 |
| ZnO nanonails | <5 | 0.200 | 0.1–1.2 | 38 |
| CNTb-wires-ZnO nanoflower | <3 | 0.180 | 0.6–250 | 39 |
| ZrHCFc/Au–PtNPs/NFs/GCE | — | 0.090 | 0.15–112.5 | 40 |
| M-CNF-Au/GCE | <2 | 0.008 | 0.01–1140 | This work |
The superior electrocatalytic performance of the M-CNF-Au/GCE towards hydrazine can be ascribed to the reasons listed below. Specifically, there are many 3D interconnected mesopores in M-CNF that result in a high specific surface area and a lower resistance to the transport of electrolyte ions. Moreover, the high specific surface area and unique nanostructure of M-CNF may make it a good supporting material to obtain a high dispersion of Au nanoparticles and thus enhanced catalytic activities of nanoparticle catalysts.41,42 All of the attractive structural properties of M-CNF-Au make it a promising electrode material for electro-catalysis applications.
3.6. Selectivity and reproducibility
The effect of various ions as potential interference ions, was studied on the determination of 1.0 × 10−4 M hydrazine in PBS. No interference was observed with common cations and anions (100-fold quantities of Na+, NO3−, Br−, I− and 300-fold quantities of K+, Cl−, Mg2+ and SO42−). Also the reproducibility of M-CNF-Au/GCE was examined by the current–time method for six repetitive measurements with additions of 1.0 × 10−4 M hydrazine. The relative standard deviation (RSD) of the current response was 1.13%, demonstrating high reproducibility of M-CNF-Au/GCE for the detection of hydrazine.4. Conclusions
By combining the advantages of unique nanostructure and large surface area of M-CNF combined with the electrocatalytic ability of Au nanoparticles to hydrazine oxidation, M-CNF-Au was successfully prepared as a hydrazine catalyst. Low detection limit, wide linear range and fast response time provide an opportunity for M-CNF-Au as a promising electrode material for the fabrication of an efficient amperometric sensor for hydrazine. Moreover, this nanocomposite suggests great potential applications in the design and construction of M-CNF based materials for electro-catalysis applications.Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (No. 21075014) and the Fundamental Research Funds for the Central Universities (No. 11SSXT144).Notes and references
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- S. Iijima and T. Ichihashi, Nature, 1993, 363, 603 CrossRef CAS.
- J. J. Wang, M. Y. Zhu, R. A. Outlaw, X. Zhao, D. M. Manos, B. C. Holloway and V. P. Mammana, Appl. Phys. Lett., 2004, 85, 1265 CrossRef CAS.
- R. Ryoo, S. H. Joo and S. Jun, J. Phys. Chem. B, 1999, 103, 7743 CrossRef CAS.
- Z. F. Li, J. H. Chen, W. Li, K. Chen, L. H. Nie and S. Z. Yao, J. Electroanal. Chem., 2007, 603, 59 CrossRef CAS.
- Y. H. Wu, X. Y. Mao, X. J. Cui and L. D. Zhu, Sens. Actuators, B, 2010, 145, 749 CrossRef.
- J. J. He, B. Fugetsu and S. Tanaka, J. Electroanal. Chem., 2010, 638, 46 CrossRef CAS.
- T. T. Baby, S. S. J. Aravind, T. Arockiadoss, R. B. Rakhi and S. Ramaprabhu, Sens. Actuators, B, 2010, 145, 71 CrossRef.
- M. Zhou, L. Shang, B. L. Li, L. J. Huang and S. J. Dong, Biosens. Bioelectron., 2008, 24, 442 CrossRef CAS.
- Y. Y. Liang, X. Y. Feng, L. J. Zhi, U. Kolb and K. Müllen, Chem. Commun., 2009, 809 RSC.
- H. J. Liu, X. M. Wang, W. J. Cui, Y. Q. Dou, D. Y. Zhao and Y. Y. Xia, J. Mater. Chem., 2010, 20, 4223 RSC.
- W. Li, F. Zhang, Y. Q. Dou, Z. X. Wu, H. J. Liu, X. F. Qian, D. Gu, Y. Y. Xia, B. Tu and D. Y. Zhao, Adv. Energy Mater., 2011, 1, 382 CrossRef CAS.
- Y. Z. Song, A. F. Zhu, H. Zhong, Y. Song, F. Y. Wu, W. L. Yang and H. Huang, Mater. Lett., 2011, 65, 3612 CrossRef CAS.
- Y. W. Hua, S. C. Hua, F. H. Li, Y. Y. Jiang, X. X. Bai, D. Li and L. Niu, Biosens. Bioelectron., 2011, 26, 4355 CrossRef.
- L. X. Wang, X. J. Bo, J. Bai, L. D. Zhu and L. P. Guo, Electroanalysis, 2010, 22, 2536 CrossRef CAS.
- W. B. Lu, R. Ning, X. Y. Qin, Y. W. Zhang, G. H. Chang, S. Liu, Y. L. Luo and X. P. Sun, J. Hazard. Mater., 2011, 197, 320 CrossRef CAS.
- D. Jayasri and S. Sriman Narayanan, J. Hazard. Mater., 2007, 144, 348 CrossRef CAS.
- B. Fang, C. H. Zhang, W. Zhang and G. F. Wang, Electrochim. Acta, 2009, 55, 178 CrossRef CAS.
- G. Z. Hu, Z. P. Zhou, Y. Guo, H. Q. Hou and S. J. Shao, Electrochem. Commun., 2010, 12, 422 CrossRef CAS.
- Q. F. Yi, L. Li, W. Q. Yu, X. P. Liu, Z. H. Zhou and H. D. Nie, Rare Met., 2010, 29, 26 CrossRef CAS.
- S. Brunauer, L. S. Deming, W. E. Deming and E. Teller, J. Am. Chem. Soc., 1940, 62, 1723 CrossRef CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- C. Kim, Y. I. Jeong, B. T. N. Ngoc, K. S. Yang, M. Kojima, Y. A. Kim, M. Endo and J. W. Lee, Small, 2007, 3, 91 CrossRef CAS.
- J. T. McCann, M. Marquez and Y. Xia, J. Am. Chem. Soc., 2006, 128, 1436 CrossRef CAS.
- E. J. Ra, T. H. Kim, W. J. Yu, K. H. An and Y. H. Lee, Chem. Commun., 2010, 46, 1320 RSC.
- K. Wang, W. Zhang, R. Phelan, M. A. Morris and J. D. Holmes, J. Am. Chem. Soc., 2007, 129, 13388 CrossRef CAS.
- L. Zhi, J. Wu, J. Li, M. Stepputat, U. Kolb and K. Müllen, Adv. Mater., 2005, 17, 1492 CrossRef CAS.
- T. F. Jaramillo, S. H. Baeck, B. R. Cuenya and E. W. McFarland, J. Am. Chem. Soc., 2003, 125, 7148 CrossRef CAS.
- M. H. Rashid, R. R. Bhattacharjee and T. K. Mandal, J. Phys. Chem. C, 2007, 111, 9684 CAS.
- Y. Liu, X. H. Qu, H. W. Guo, H. J. Chen, B. F. Liu and S. J. Dong, Biosens. Bioelectron., 2006, 21, 2195 CrossRef CAS.
- Y. Liu, M. K. Wang, F. Zhao, Z. H. Guo, H. J. Chen and S. J. Dong, J. Electroanal. Chem., 2005, 581, 1 CrossRef CAS.
- B. Z. Fang and L. Binder, J. Phys. Chem. B, 2006, 110, 7877 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods, Fundamentals and Applications, Wiley, New York, 2001 Search PubMed.
- H. R. Zare and N. Nasierzadeh, Electrochim. Acta, 2007, 52, 4153 CrossRef CAS.
- L. Zheng and J. F. Song, Sens. Actuators, B, 2009, 135, 650 CrossRef.
- J. Li and X. Q. Lin, Sens. Actuators, B, 2007, 126, 527 CrossRef.
- G. F. Wang, C. H. Zhang, X. P. He, Z. J. Li, X. J. Zhang, L. Wang and B. Fang, Electrochim. Acta, 2010, 55, 7204 CrossRef CAS.
- A. Umar, M. M. Rahman, S. H. Kim and Y. B. Hahn, Chem. Commun., 2008, 166 RSC.
- B. Fang, C. Zhang, W. Zhang and G. Wang, Electrochim. Acta, 2009, 55, 178 CrossRef CAS.
- M. B. Gholivand and A. Azadbakht, Electrochim. Acta, 2011, 56, 10044 CrossRef CAS.
- D. Mirabile Gattia, M. Vittori Antisari, L. Giorgi, R. Marazzi, E. Piscopiello, A. Montone, S. Bellitto, S. Licoccia and E. Traversa, J. Power Sources, 2009, 194, 243 CrossRef CAS.
- E. Antolini, Appl. Catal., B, 2009, 88, 1 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
