A facile preparation of palladium nanoparticles supported on magnetite/s-graphene and their catalytic application in Suzuki–Miyaura reaction†
Jiefeng
Hu
,
Yuping
Wang
,
Min
Han
,
Yaoming
Zhou
,
Xiaoqing
Jiang
* and
Peipei
Sun
*
Jiangsu Key Laboratory of New Power Batteries, College of Chemistry and Materials Science, Nanjing Normal University, 122 Ninghai Road, Nanjing 210097, PR China. E-mail: jiangxiaoqing@njnu.edu.cn; xiaoqing_j@yahoo.com.cn; sunpeipei@njnu.edu.cn; Fax: +86 25 83598280; Tel: +86 25 83598280
First published on 13th June 2012
Abstract
Fe3O4 and Pd nanoparticles were assembled on sulfonated graphene (s-G) by an easy chemical approach and characterized by transmission electron microscopy, X-ray diffraction and energy dispersive X-ray spectroscopy. The resulting material could be dispersed homogeneously in water or water/ethanol and further used as an excellent semi-heterogeneous catalyst for the Suzuki–Miyaura cross-coupling reaction in an environmentally friendly solvent under ligand-free ambient conditions. The high heterogeneous catalytic activity appears to be due to the small size of Pd nanoparticles and homogeneous distribution of the nanoparticles on the Fe3O4/s-G matrix. In addition, the catalytic activity did not deteriorate even after repeated applications, which may be due to the easy and efficient magnetic separation of the catalyst and the high dispersion and stability of the catalyst in an aqueous solution.
1. Introduction
Nanoscale palladium particles have drawn particular attention due to their catalytic and electronic properties.1,2 Pd nanoparticles used as heterogeneous or semi-heterogeneous catalysts for C–C coupling reactions, such as Mizoroki–Heck, Suzuki–Miyaura and Sonogashira reactions, have been described in the literature.3–13 Since the heterogeneous catalysis reaction occurs entirely on the surface of the metal particles, a high dispersion or size control is required to improve the catalytic activity and reduce the consumption of the catalysts. Unfortunately, aggregation of naked nanoparticles often prohibits tailoring of particle size. For this reason, many attempts have been made to immobilize or stabilize Pd nanoparticles in a wide variety of supports, such as polymers,6,7 organic–inorganic hybrid materials8 and glass-polymer composites,9 as well as on common inorganic substrates, such as carbon,10 silica,11 zeolites12 or carbon nanotubes (CNTs).13Graphene, a single layer of sp2 carbon atoms bonded in a hexagonal lattice, has produced an explosion of interest for both theoretical studies and applications due to its extraordinary properties, such as a large specific surface area,14 high thermal and electrical conductivity,15 and high corrosion resistance. Especially in the new kind of composite materials, graphene has opened a new avenue as a substrate to host metal nanoparticles.16–18 Several graphene-based palladium nanoparticles as catalysts with high activity and selectivity for carbon–carbon bond forming reactions have been reported.19–23 It is speculated that the interaction between graphene and aromatic compounds may make the reactants easily accessible to the active sites of noble metal nanoparticles and therefore to accelerate the chemical conversion. However, these graphene-based catalysts, although with remarkably high activity, were also reported to dramatically lose their catalytic activity in recycling experiments after being recovered by commonly used separation methods, such as filtration or centrifugation.19
In recent years, magnetic separation has emerged as a robust, highly efficient and fast separation tool with many advantages compared with product/catalyst isolation by means of other chemical or physical procedures, such as liquid–liquid extraction, chromatography, distillation, filtration or centrifugation. On the other hand, magnetic nanoparticles have attracted increasing interest in the material and colloid science communities in recent years. Several researchers have combined iron oxides with activated carbon fiber,24 CNT25 and graphene.26,27 In organic synthesis, Fe3O4-supported catalysts can be separated from the reaction medium by an external permanent magnet,28–31 which circumvents time-consuming and laborious separation steps, keeps the catalytic activity of the catalyst and allows for practical continuous catalysis.
In the current work, we report a novel and easy approach to homogeneously immobilize Fe3O4 and Pd nanoparticles on sulfonated graphene (s-G). The catalyst is designed with an aim to combine the excellent supporting property of graphene to effectively immobilize and stabilize Fe3O4 and Pd nanoparticles with the magnetic property of the Fe3O4 nanoparticles for easy catalyst separation and therefore to improve their reusability. The obtained Pd/Fe3O4/s-G composite remained soluble in water, but could be easily separated from reaction solutions by an external permanent magnet. More importantly, this new composite was shown to act as an efficient semi-heterogeneous catalyst for the Suzuki–Miyaura cross-coupling reaction in aqueous solution without the need of any ligand or surfactant under aerobic condition and could be efficiently reused whilst keeping the inherent catalytic activity. To the best of our knowledge, this is the first report on applications of magnetically separable and recyclable graphene-based semi-heterogeneous Pd nanoparticles to catalyze carbon–carbon bond forming reactions.
2. Experimental
2.1 General
Transmission electron microscopy (TEM) measurements were carried out on a Hitachi-7650 transmission electron microscope operated at an accelerated voltage of 80 kV and high-resolution TEM (HRTEM) on JEOL-2100 at an accelerated voltage of 200 kV. X-ray diffraction (XRD) measurements were carried out using a D/max 2500 VL/PC X-ray diffractometer with graphite-monochromatized Cu-Kα radiation. Energy dispersive X-ray spectroscopy (EDX) measurements were carried out on JSM-5610LV/NORAN-VANTAGE. Gas chromatography (GC) determinations were carried out on an Agilent 4890D instrument equipped with a capillary column (Crosslinked 5% PH ME Siloxane) (15 m × 0.53 mm i. d. × 1.5 μm film thickness). The GC parameters were as follows: initial temperature, 110 °C; initial time, 1 min; temperature ramp, 20 °C min−1; final temperature, 260 °C; final time, 13.5 min; injector port temperature, 270 °C; detector (FID) temperature, 280 °C; and injection volume, 0.2 μL. The Pd content was determined by means of inductively coupled plasma atomic emission spectroscopy (ICP-AES) on Thermo Elemental IRIS Intrepid II. Graphite powder, sodium dodecyl sulfate (SDS), potassium persulfate, 4-aminobenzene sulfonic acid, sodium borohydride, 30% H2O2 and potassium permanganate were purchased from Sinopharm Chemical Reagent Co. Ltd. Palladium acetate (Pd(OAc)2) was bought from Aladdin Reagent Company. These reagents were used as received.2.2 Preparation of partly sulfonated graphene (s-G).
Partly sulfonated and water soluble graphene were synthesized by a method similar to that reported by Samulski et al.32 Graphite oxide prepared from natural graphite powder by the modified Hummer’s method was used as the starting material.33 Typically, graphite oxide (1 mg mL−1) was exfoliated by sonication in water for 1.5 h. 600 mg sodium borohydride in 15 mL water was added into 75 mL of the exfoliated graphite oxide dispersion after its pH was adjusted to 9–10 with 5 wt% sodium carbonate solution. The mixture was then kept at 80 °C for 1 h under constant stirring. After centrifuging and rinsing with water several times, the partially reduced graphene was obtained and could be easily redispersed in 75 mL water. Then 52 mg 4-aminobenzene sulfonic acid and 26 mg sodium nitrite in 10 mL water and 0.5 mL 1 M HCl solution was added under constant stirring for 2 h in an ice bath. The obtained product named s-G was rinsed several times with distilled water.2.3 Preparation of Pd/Fe3O4/s-G and Pd/s-G.
The prepared s-G was first subjected to dialysis to remove residual salts until the pH reached 6.2. An aqua solution of FeSO4·7H2O (26.4 mg) and FeCl3·6H2O (51.3 mg) with a mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in 5 mL distilled water was prepared and then was added to a s-G solution (25 mg s-G in 25 mL water) with constant stirring under nitrogen. After several minutes, ammonia solution (0.3 mL, 25%) was added. Then the mix was kept under constant stirring at 90 °C for 4 h. The product named as Fe3O4/s-G was rinsed several times with distilled water. The obtained Fe3O4/s-G or 25 mg pure s-G in 10 ml water was mixed with a solution of 590 mg SDS in 20 mL water. After sonication of the mixture for several minutes, Pd(OAc)2 (12 mg) was added and then the mixture was refluxed for 5 h. The obtained composite, designated as Pd/Fe3O4/s-G or Pd/s-G, was then rinsed several times with distilled water to remove excess surfactant. Large-scale synthesis (up to 8 times the above scale) can be carried out by enlarging the amount of reagents in each step accordingly.
2 in 5 mL distilled water was prepared and then was added to a s-G solution (25 mg s-G in 25 mL water) with constant stirring under nitrogen. After several minutes, ammonia solution (0.3 mL, 25%) was added. Then the mix was kept under constant stirring at 90 °C for 4 h. The product named as Fe3O4/s-G was rinsed several times with distilled water. The obtained Fe3O4/s-G or 25 mg pure s-G in 10 ml water was mixed with a solution of 590 mg SDS in 20 mL water. After sonication of the mixture for several minutes, Pd(OAc)2 (12 mg) was added and then the mixture was refluxed for 5 h. The obtained composite, designated as Pd/Fe3O4/s-G or Pd/s-G, was then rinsed several times with distilled water to remove excess surfactant. Large-scale synthesis (up to 8 times the above scale) can be carried out by enlarging the amount of reagents in each step accordingly.
2.4 Activity test of the catalysts for Suzuki–Miyaura reaction.
In a typical experiment, 1.0 mmol of aryl halide was dissolved in a mixture of 7 mL water/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). To this were added the aryl boronic acid (1.2 mmol) and potassium carbonate (414 mg, 3.0 mmol). Catalyst (Pd/Fe3O4/s-G or Pd/s-G) containing certain amount of Pd (0.000033–0.012 mmol) (for a Pd/Fe3O4/s-G catalyst containing 8.33 wt% Pd, corresponding to 0.042–15.3 mg of the catalyst) was added to the mixture. If the total amount of the catalyst used was less than 1.0 mg, a water/ethanol dispersion of the catalyst with a concentration less than 1.0 mg mL−1 was first prepared and then a small amount of the dispersion was taken and used as the catalyst. The mixture was stirred under predetermined temperature and time. For the time-dependant experiments, reaction samples were taken at regular intervals and monitored by GC. After completion of the reaction, the catalyst was recovered by magnetic separation for Pd/Fe3O4/s-G or centrifugation for Pd/s-G and subsequently washed with ethyl acetate, followed by ethanol and water. The reaction mixture was diluted with 10 mL H2O and extracted with ethyl acetate (3 × 10 mL). The organic layers were combined, dried over anhydrous Na2SO4 and filtered. The filtrate was concentrated by vacuum. The pure products were obtained by thin layer chromatography.
1). To this were added the aryl boronic acid (1.2 mmol) and potassium carbonate (414 mg, 3.0 mmol). Catalyst (Pd/Fe3O4/s-G or Pd/s-G) containing certain amount of Pd (0.000033–0.012 mmol) (for a Pd/Fe3O4/s-G catalyst containing 8.33 wt% Pd, corresponding to 0.042–15.3 mg of the catalyst) was added to the mixture. If the total amount of the catalyst used was less than 1.0 mg, a water/ethanol dispersion of the catalyst with a concentration less than 1.0 mg mL−1 was first prepared and then a small amount of the dispersion was taken and used as the catalyst. The mixture was stirred under predetermined temperature and time. For the time-dependant experiments, reaction samples were taken at regular intervals and monitored by GC. After completion of the reaction, the catalyst was recovered by magnetic separation for Pd/Fe3O4/s-G or centrifugation for Pd/s-G and subsequently washed with ethyl acetate, followed by ethanol and water. The reaction mixture was diluted with 10 mL H2O and extracted with ethyl acetate (3 × 10 mL). The organic layers were combined, dried over anhydrous Na2SO4 and filtered. The filtrate was concentrated by vacuum. The pure products were obtained by thin layer chromatography.
3. Results and discussion
3.1 Structural characteristics
Fig. 1a shows a TEM image of a single s-G sheet. It appears transparent and is folded over the edge. The TEM image of Pd/s-G in Fig. 1b shows that densely spherical Pd (4–5 nm) were formed and homogeneously distributed on the s-G surface. In Fig. 1c, the TEM image of Fe3O4/s-G shows that Fe3O4 nanoparticles were dispersed on the s-G matrix with a particle size of 7–15 nm, larger than Pd nanoparticles. Fig. 1d shows a TEM image of the Pd/Fe3O4/s-G composite. The particles in Fig. 1d appear to be bigger than those in Fig. 1b and c and have a rough surface and irregular shapes, which remind us that they might be made up of Fe3O4 particles with Pd nanospheres grown on their surfaces. In order to get more detailed structural information, HRTEM analysis of the Pd/Fe3O4/s-G composite was carried out. As seen in the HRTEM image (Fig. 2), the Pd nanoparticles are distinguishable with the difference in their contrast compared to those of Fe3O4. The dark areas are Pd particles of 2–5 nm and some have agglomerated into bigger ones. Further evidence comes from the image in Fig. 2b, which demonstrates the (111) planes of the Pd component with a d-spacing of 0.23 nm in a small dark area,31,34and the (220) planes of the Fe3O4 component with a d-spacing of 0.28 nm in the bright base area.31 The Pd on Fe3O4 structured catalyst has been reported by Amali et al. as Pd@Fe3O4 nanoparticles,30 where Pd nanoparticles were loaded on Fe3O4 nanoparticles functionalized by branched polyethylenimine. Jang et al. also reported a kind of Pd–Fe3O4 heterodimer nanocrystals as a catalyst for a Suzuki–Miyaura coupling reaction.31 However, to the best of our knowledge, the Pd/Fe3O4/graphene structured catalyst has never been reported before. More importantly, the catalytic activity of Pd/Fe3O4/s-G prepared in this work, with the s-G as a soluble support, was demonstrated to be improved greatly compared to those Pd/Fe3O4 catalysts without the soluble support and the details will be described later.30,31 | ||
| Fig. 1 TEM images of (a) s-G sheet, (b) Pd/s-G, (c) Fe3O4/s-G, (d) Pd/Fe3O4/s-G. | ||
 | ||
| Fig. 2 A HRTEM image of Pd/Fe3O4/s-G. Panel (b) is the magnification of the square region in panel (a). | ||
In the synthesis of Pd/s-G or Pd/Fe3O4/s-G, SDS was used as both the surfactant and the reducing agent, since on heating SDS may decompose to 1-dodecanol and then reduce the Pd(II) to Pd(0).20,35 In addition, GO may also act as the reductant of Pd(OAc)2, which was recently reported by Chen et al.36
The palladium content in Pd/s-G and Pd/Fe3O4/s-G was determined by means of ICP-AES and amounted to 11.7 wt% and 8.33 wt%, respectively. The 11.7 wt% should be the highest loading amount among those Pd/graphene or Pd/graphene oxide composites reported in the literature.19–23 The decrease of Pd content in Pd/Fe3O4/s-G is understandable considering the weight of Fe3O4, and even so the palladium content in Pd/Fe3O4/s-G is still higher than those reported for Pd/graphene catalysts so far.19–23
The X-ray diffraction (XRD) pattern of Pd/Fe3O4/s-G in Fig. 3a is also evidence for the existence of crystal structures of zero-valent Pd and Fe3O4. The position and relative intensity of diffraction peaks in Fig. 3a are in accordance with those of Fe3O4 and metal Pd. The peaks at 30.1, 35.3, 43.2, 53.6, 57.3, 62.7 and 74.3° are ascribed to (220), (311), (400), (422), (511), (440) and (533) reflections of Fe3O4.31 Those observed at 40.0, 46.3 and 67.7° can be well-indexed as the (111), (200) and (220) diffractions of crystalline Pd (0).31 It is noted that the Pd (111) peak has the highest intensity and therefore the Pd (111) plane should be the predominant crystal facet, which also coincides with the HRTEM observations. The broad peak at around 2θ = 23.0° in the XRD pattern should be related to the disordered and randomly arranged graphene flakes in the samples. The characteristic peak of Fe (111) at 20.4° is almost masked by this broad peak.
 | ||
| Fig. 3 (a) The XRD pattern and (b) EDX spectrum of Pd/Fe3O4/s-G. | ||
Further evidence comes from the EDX spectra shown in Fig. 3b. The loading amount of Pd of the Pd/Fe3O4/s-G catalyst is estimated to be 4.73 atom% and the content of Fe is 14.36 atom% from EDX analysis. The carbon signal arises from graphene, and oxygen and sulfur signals arise from the sulfonate groups and the residual dodecanoate or other oxygen-containing groups on graphene.
3.2 Catalytic test of Pd/Fe3O4/s-G for Suzuki–Miyaura coupling reaction
The catalytic activity of Pd/Fe3O4/s-G to C–C cross coupling was studied with the Suzuki–Miyaura cross-coupling reaction, which is one of the most investigated synthesis protocols in modern chemistry and in industrial applications.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume ratio). This mixed solvent could dissolve the reactants much better and was also an economical and environmentally friendly system. According to the GC results, as the above reaction was carried out in the mixed solvent, no diphenyl was detected and p-methyldiphenyl was the only product, indicating that the homocoupling of reactant was eliminated completely. In addition, the reaction time was also shortened greatly. Therefore, all of the following Suzuki–Miyaura cross-coupling reactions for testing catalytic activity were carried out in H2O/EtOH (1
1 in volume ratio). This mixed solvent could dissolve the reactants much better and was also an economical and environmentally friendly system. According to the GC results, as the above reaction was carried out in the mixed solvent, no diphenyl was detected and p-methyldiphenyl was the only product, indicating that the homocoupling of reactant was eliminated completely. In addition, the reaction time was also shortened greatly. Therefore, all of the following Suzuki–Miyaura cross-coupling reactions for testing catalytic activity were carried out in H2O/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In addition, the experiment showed that the presence of surfactant and ligand, such as SDS, tetra-n-butylammonium bromide and triphenylphosphine, did not evidently assist in increasing the yield, so all reactions were carried out in the absence of any surfactant or ligand in this work to simplify the reaction conditions.
1). In addition, the experiment showed that the presence of surfactant and ligand, such as SDS, tetra-n-butylammonium bromide and triphenylphosphine, did not evidently assist in increasing the yield, so all reactions were carried out in the absence of any surfactant or ligand in this work to simplify the reaction conditions.
| Catalyst | FeSO4·7H2O | FeCl3·6H2O | Fe3O4 loading | Pd loading |
|---|---|---|---|---|
| (mg) | (mg) | (wt%) | (wt%) | |
| a All catalysts were prepared on 25 mg s-G. The loading amounts of Fe3O4 and Pd on catalysts were measured by ICP-AES after digestion of samples in aqua regia and HClO4. b The data in parentheses are the Fe3O4 and Pd contents of the same catalyst after the 10th run of catalytic reaction. | ||||
| Pd/Fe3O4/s-G1 | 7.2 | 14.0 | — | — |
| Pd/Fe3O4/s-G2 | 12.0 | 23.3 | 17.2 | 8.52 |
| Pd/Fe3O4/s-G3 | 16.8 | 32.7 | 18.8 | 7.12 |
| Pd/Fe3O4/s-G4 | 21.0 | 40.8 | 27.8 | 9.02 |
| Pd/Fe3O4/s-G5 | 26.4 | 51.3 | 30.5 (30.4)b | 8.33 (7.28)b |
| Pd/Fe3O4/s-G6 | 31.2 | 60.7 | 33.7 | 7.46 |
| Pd/Fe3O4/s-G7 | 43.2 | 84.0 | 37.8 | 6.29 |
The catalytic activity of various Pd/Fe3O4/s-G catalysts in Table 1 was further investigated using the Suzuki–Miyaura reaction of 1-bromo-4-methylbenzene and phenyl boronic acid in a mixture of H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1
EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 80 °C, as shown in Scheme 1 by adding various catalysts containing 1.2 mol% Pd.
1) at 80 °C, as shown in Scheme 1 by adding various catalysts containing 1.2 mol% Pd.
 | ||
| Scheme 1 The Suzuki–Miyaura cross-coupling reaction. | ||
Fig. 4a illustrates the catalytic activity of these catalysts (yields were measured by GC). For each catalyst in Table 1, the Suzuki–Miyaura reaction could be completed after 30 min with a yield of product beyond 90%. While all catalysts demonstrated high activity toward Suzuki–Miyaura coupling, as shown in Fig. 4a, the magnetic separation ability was found to be apparently weakened as the Fe3O4 loadings was less than 17.3 wt%. The Pd/Fe3O4/s-G1 was difficult to separate by magnetic separation because of a long separation time. For other catalysts the separation time was less than 5 min. Fig. 4b shows the photographs for the magnetic separation of Pd/Fe3O4/s-G5 from water/ethanol phase by an external magnet after only 20 s. The experimental results demonstrate that the loading amount of Fe3O4 mainly influences the magnetic separation ability, but apparently does not affect the catalytic activity of the catalyst. In this work, the Pd/Fe3O4/s-G catalyst was usually prepared under the same fabrication conditions as for Pd/Fe3O4/s-G5 in Table 1. Thus, prepared catalyst could be readily separated (in 20 s) and recovered from the reaction mixture simply by using an external magnet (Fig. 4b) after completion of the coupling reaction. This allowed an easy investigation of the stability and reusability of the catalyst, as described later.
 | ||
| Fig. 4 (a) The catalytic activity of Pd/Fe3O4/s-G catalysts with different Fe3O4 contents for the Suzuki–Miyaura cross-coupling reaction in Scheme 1. Yields were measured by GC. (b) Photographs showing the magnetic separation of Pd/Fe3O4/s-G catalyst from water/ethanol phase by an external magnet after 20 s. | ||
 | ||
| Fig. 5 The effect of concentration of Pd/Fe3O4/s-G on the yield of the Suzuki–Miyaura cross coupling reaction in Scheme 1. Yields were measured by GC. | ||
For catalyst Pd/Fe3O4/s-G, as the Pd amount was decreased to 0.15 mol%, the reaction was completed after 30 min with a yield of 97% of product. With the same amount of Pd (0.15 mol%), Pd/s-G also demonstrated high activity toward the Suzuki–Miyaura coupling reaction. However, under the same Pd loading, Pd/C exhibits only 51% yield after 30 min. In Fig. 5, the curve of Pd/Fe3O4/s-G (0.15 mol%) coincides well with that of Pd/s-G (0.15 mol%), which demonstrates that the immobilizing of magnetic Fe3O4 on s-G for imparting magnetic separation capability for easy recovery and recycling of the catalyst does not reduce the catalytic activity any further.
The maximum enhancement in activity of Pd/Fe3O4/s-G was subsequently explored by lowering the Pd amount. Further decreasing the Pd concentration to 0.038 mol% still gave a yield of 90% after 30 min. Even when 0.014 mol% loading was used, it still worked effectively, giving a yield of 95% after 3 h. By further decreasing the amount of Pd to 0.0075 mol%, the reaction could also be completed after 4 h, affording a yield of 90% of product. With the lowest loading of 0.0033 mol% of Pd/Fe3O4/s-G, the reaction was only partly completed after 4 h, giving a yield of 78%. Extending the reaction time could not lead to an increase of yield. The experimental results demonstrate the remarkable catalytic activity of Pd/Fe3O4/s-G with a turnover number (TON) of 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and turnover frequency (TOF) of 6000 h−1 (0.0033 mol% Pd/Fe3O4/s-G, 78% yield at 80 °C for 4 h). The catalytic activity of Pd/Fe3O4/s-G is much higher than those for the same Suzuki–Miyaura reaction catalyzed by the Pd/Fe3O4 heterodimer nanocrystal catalyst (1.0 mol% Pd, 71% yield under reflux for 24 h)31 or the Pd@Fe3O4 catalyst (90% yield at 40–65 °C for 18 h, TON = 11.0, TOF = 0.61).30 The enhanced catalytic activity compared to these heterogeneous Pd/Fe3O4 catalysts should be related to the high dispersion of Pd/Fe3O4/s-G, which is due to using the soluble s-G as the support and then converting the catalyst to a semi-heterogeneous one. The catalytic activity of Pd/Fe3O4/s-G is much higher than the commercial available Pd/C, as shown in Fig. 5, and comparable or even higher than those for Pd/graphene catalysts.19–23
000 and turnover frequency (TOF) of 6000 h−1 (0.0033 mol% Pd/Fe3O4/s-G, 78% yield at 80 °C for 4 h). The catalytic activity of Pd/Fe3O4/s-G is much higher than those for the same Suzuki–Miyaura reaction catalyzed by the Pd/Fe3O4 heterodimer nanocrystal catalyst (1.0 mol% Pd, 71% yield under reflux for 24 h)31 or the Pd@Fe3O4 catalyst (90% yield at 40–65 °C for 18 h, TON = 11.0, TOF = 0.61).30 The enhanced catalytic activity compared to these heterogeneous Pd/Fe3O4 catalysts should be related to the high dispersion of Pd/Fe3O4/s-G, which is due to using the soluble s-G as the support and then converting the catalyst to a semi-heterogeneous one. The catalytic activity of Pd/Fe3O4/s-G is much higher than the commercial available Pd/C, as shown in Fig. 5, and comparable or even higher than those for Pd/graphene catalysts.19–23
| Run | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
a Reaction conditions: 1-bromo-4-methylbenzene (1.0 mmol), phenylboronic acid (1.2 mmol), potassium carbonate (3.0 mmol), 7 mL water/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume ratio), Pd/Fe3O4/s-G catalyst containing 0.012 mmol Pd, at 80 °C for 45 min.
b Isolated yields.
c Reaction time was extended to 2 h. 1 in volume ratio), Pd/Fe3O4/s-G catalyst containing 0.012 mmol Pd, at 80 °C for 45 min.
b Isolated yields.
c Reaction time was extended to 2 h.
|
||||||||||
| Yieldb (%) | 95 | 96 | 95 | 95 | 95 | 90 | 86 | 89 | 73 | 84c |
The recyclability of Pd/Fe3O4/s-G has been improved greatly compared to those Pd/graphene catalysts reported by Scheuermann et al.19 In their work, a massive decrease in catalytic activity was found even in the second run, which was ascribed to the ineffective catalyst separation method of filtration or centrifugation. We notice the excellent recyclability for Pd/graphene catalysts recently reported by Siamaki et al., where the catalyst separation was carried out by decantation instead of filtration or centrifugation.23 Compared to decantation, the magnetic separation should be faster and more efficient, especially for those catalysts with high dispersion. It is noteworthy that the Pd/Fe3O4/s-G catalyst even after 10 runs of application was still soluble and did not aggregate in 2 h in water/ethanol mixture and could be re-dispersed easily even after precipitation. Although having good solubility or dispersity, after each run the Pd/Fe3O4/s-G could be separated efficiently and quickly from the reaction mixture by using an external permanent magnet.
To further understand the catalytic mechanism, the Pd and Fe3O4 contents in the Pd/Fe3O4/s-G catalyst after 10 runs were also measured by ICP-AES and amounted to 7.28% and 30.4%, respectively. Compared with the fresh catalyst, the Fe3O4 content remained almost unchanged, while the Pd content decreased slightly. In order to investigate the leaching of Pd from the Pd/Fe3O4/s-G catalyst, the Pd concentration in the product solution after one reaction followed by magnetic separation was also measured and the ICP-AES data revealed that the Pd concentration was 69 ppb. This value is much lower than that reported by Siamaki et al. for Pd/graphene catalyst (300 ppb),23 indicating that less Pd was leached out from the catalyst in this work, which may be owing to the fact that the Pd nanoparticles were mainly immobilized on the surface of Fe3O4. It has been reported that hardly any Pd leached out from the Pd/Fe3O4 heterodimer nanocrystal catalyst.31 Therefore, it might imply that the Pd nanoparticles supported on Fe3O4/s-G may be more stable than those supported on graphene. Although the Pd leaching was suppressed in this work, the small amount of leached palladium still argues against the complete heterogeneous catalysis. To confirm that the catalytic activity originated from the supported Pd nanoparticles and not from the leached Pd, a controlled experiment was performed by carrying out the Suzuki–Miyaura reaction in Scheme 1 in the presence of the supernatant after one reaction, which was obtained after careful removal of the solid catalyst at the same reaction temperature. After 12 h of reaction, only a trace amount of the cross coupling product could be identified. In order to further understand the catalytic mechanism we performed a three-phase test. This test, developed by Rebek et al.40,41 and Davies et al.,42 is often used to detect the presence of catalytically active homogeneous metal species, in which one of the substrate is anchored on a solid different from the catalyst and can only react if a soluble catalytic palladium source is present. Thus, 4-bromobenzoic acid was linked to polystyrene resin (Merrifield Resin, 1% DVB) by the esterification. The modified resin was first employed to react with phenylboronic acid under the same reaction conditions as the general. After hydrolyzing the reacted resin, no cross-coupling product, biphenyl-4-carboxylic acid, was separated. However, according to those results of Davies and Crudden et al.,42,43 it is critical in the three-phase test to add soluble aryl halide to the reaction mixture in addition to the supported aryl halide. Thus, the modified resin, together with the soluble 1-bromo-4-methylbenzene was employed to react with phenylboronic acid in the presence of Pd/Fe3O4/s-G catalyst under 80 °C for 20 h. The mole ratio of phenylboronic acid/soluble 1-bromo-4-methylbenzene is 2.2. The soluble fraction was monitored for the presence of p-methyldiphenyl, which indicates the existence of an active catalyst. The polystyrene resin supported reagent was then separated from the solution by filtration. The filtrate was analyzed and about 90% of p-methyldiphenyl was obtained from the coupling of two soluble reagents. Then, the solid (polystyrene resin-bound reagent) was treated with an ethanol/H2O solution of NaOH at 90 °C for 48 h. The solution was filtered and the filtrate was acidified and extracted with ether. Then, the ether layer was analyzed and 4-bromobenzoic acid was obtained, together with trace 4-phenylbenzoic acid. This result, combined with the result above, suggests that a heterogeneous catalytic mechanism may mainly exist in this Pd/Fe3O4/s-G-catalyzed Suzuki–Miyaura reaction. The reaction proceeds near the surfaces of the palladium nanoparticles. The high catalytic activity is supposed to result from the small size of Pd nanoparticles and the homogeneous distribution of the nanoparticles on the highly soluble Fe3O4/s-G, which provide enough reaction sites for heterogeneous catalysis.
The leaching of Pd may lead to the reduction of Pd content of the Pd/Fe3O4/s-G after multiple runs. However, this should not be the main reason for the decrease of catalytic activity for Pd/Fe3O4/s-G after 5 applications, since the Pd content of the Pd/Fe3O4/s-G even after 10th run was still as high as 7.28 wt%. Therefore the TEM measurements of Pd/Fe3O4/s-G after multiple applications of catalytic reaction were carried out. Fig. 6a and b show the TEM image of Pd/Fe3O4/s-G catalyst after the 5th and 10th runs of recycling experiments, respectively. Slight agglomeration and accumulation of the Pd/Fe3O4 nanoparticles on the surface of s-G can be observed in Fig. 6a, whereas in Fig. 6b, enhanced agglomeration and accumulation and a number of larger particles with a diameter beyond 50 nm can be clearly seen. Therefore, the deactivation of the Pd/Fe3O4/s-G catalyst may be related to the aggregation of Pd/Fe3O4 nanoparticles, which leads to the decrease in the surface area and therefore saturation of the reaction sites.
 | ||
| Fig. 6 TEM images of Pd/Fe3O4/s-G (a) after 5 runs, (b) after 10 runs of recycling experiments. | ||
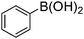
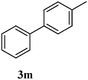
| Entry | Aryl halide | Boronic acid | Product | Yield (%)b |
|---|---|---|---|---|
a Reaction conditions: aryl bromide (1.0 mmol), arylboronic acid (1.2 mmol), potassium carbonate (3.0 mmol), Pd/Fe3O4/s-G catalyst containing 0.003 mmol Pd, in 7 mL H2O/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 80 °C for 45 min.
b Isolated yields. 1) at 80 °C for 45 min.
b Isolated yields.
|
||||
| 1 |

|

|

|
88 |
| 2 |

|

|

|
95 |
| 3 |

|

|
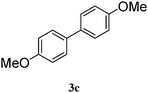
|
93 |
| 4 |

|

|

|
95 |
| 5 |

|
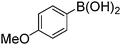
|
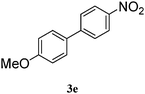
|
79 |
| 6 |

|

|

|
86 |
| 7 |

|

|
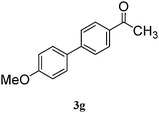
|
87 |
| 8 |

|
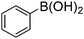
|

|
93 |
| 9 |

|

|

|
95 |
| 10 |

|

|
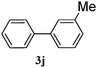
|
95 |
| 11 |

|
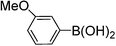
|

|
93 |
| 12 |

|

|

|
94 |
| 13 |

|
|
|
95 |
4. Conclusion
We have fabricated a Pd/Fe3O4/s-G catalyst by reduction of palladium acetate to Pd (0) on the water soluble s-G decorated with magnetic Fe3O4 nanoparticles using an easy chemical method. The obtained Pd/Fe3O4/s-G catalyst could be dispersed homogeneously in water or water/ethanol and further used as an excellent semi-heterogeneous catalyst for the Suzuki–Miyaura cross-coupling reaction in an environmentally friendly solvent under ligand-free ambient conditions. The catalyst can be handled easily as it is very stable in air and can be easily removed from the reaction mixture by magnetic separation. In addition, it can be recycled 8 times with minimal loss of activity. The high catalytic activity is thought to result from the small size of Pd nanoparticles and the homogeneous distribution of the nanoparticles on Fe3O4/s-G matrix; and the excellent recyclability should be due to the stable immobilization of Fe3O4 on s-G, which imparts fast and efficient magnetic separation capability to the catalyst. The high dispersion of s-G must also have rendered great assistance to the catalyst to preserve excellent catalytic activity during multiple runs of recycling experiments.Acknowledgements
This work is supported by the National Natural Science Foundation of China (20773066 and 20972068) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.References
- K. Esumi, R. Isono and T. Yoshimura, Langmuir, 2004, 20, 237–243 CrossRef CAS.
- A. K. Manocchi, N. E. Horelik, B. Lee and H. Yi, Langmuir, 2010, 26, 3670–3677 CrossRef CAS.
- M. Moreno-Manas and R. Pleixats, Acc. Chem. Res., 2003, 36, 638–643 CrossRef CAS.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef CAS.
- Y. G. Li, P. Zhou, Z. H. Dai, Z. X. Hu, P. P. Sun and J. C. Bao, New J. Chem., 2006, 30, 832–837 RSC.
- R. Narayanan and M. A. El-Sayed, J. Am. Chem. Soc., 2003, 125, 8340–8347 CrossRef CAS.
- B. J. Gallon, R. W. Kojima, R. B. Kaner and P. L. Diaconescu, Angew. Chem., Int. Ed., 2007, 46, 7251–7254 CrossRef CAS.
- S. Niembro, A. Shafir, A. Vallribera and R. Alibes, Org. Lett., 2008, 10, 3215–3218 CrossRef CAS.
- K. Mennecke, R. Cecilia, T. N. Glasnov, S. Gruhl, C. Vogt, A. Feldhoff, M. A. L. Vargas, C. O. Kappe, U. Kunz and A. Kirschning, Adv. Synth. Catal., 2008, 350, 717–730 CrossRef CAS.
- T. Tagata and M. Nishida, J. Org. Chem., 2003, 68, 9412–9415 CrossRef CAS.
- R. B. Bedford, U. G. Singh, R. I. Walton, R. T. Williams and S. A. Davis, Chem. Mater., 2005, 17, 701–707 CrossRef CAS.
- L. Djakovitch and K. Koehler, J. Am. Chem. Soc., 2001, 123, 5990–5999 CrossRef CAS.
- X. C. Chen, Y. Q. Hou, H. Wang, Y. Gao and J. H. He, J. Phys. Chem. C, 2008, 112, 8172–8176 CrossRef CAS.
- M. D. Stoller, S. J. Park, Y. W. Zhu, J. H. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS.
- A. A. Balandin, S. Ghosh, W. Z. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902–907 CrossRef CAS.
- H. M. A. Hassan, V. Abdelsayed, A. E. R. S. Khder, K. M. AbouZeid, J. Terner, M. S. El-Shall, S. I. Al-Resayes and A. A. El-Azhary, J. Mater. Chem., 2009, 19, 3832–3837 RSC.
- R. Muszynski, B. Seger and P. V. Kamat, J. Phys. Chem. C, 2008, 112, 5263–5266 CrossRef CAS.
- X. Z. Zhou, X. Huang, X. Y. Qi, S. X. Wu, C. Xue, F. Y. C. Boey, Q. Y. Yan, P. Chen and H. Zhang, J. Phys. Chem. C, 2009, 113, 10842–10846 CrossRef CAS.
- G. M. Scheuermann, L. Rumi, P. Steurer, W. Bannwarth and R. Mulhaupt, J. Am. Chem. Soc., 2009, 131, 8262–8270 CrossRef CAS.
- Y. Li, X. B. Fan, J. J. Qi, J. Y. Ji, S. L. Wang, G. L. Zhang and F. B. Zhang, Nano Res., 2010, 3, 429–437 CrossRef CAS.
- A. Mastalir, Z. Kiraly, A. Patzko, I. Dekany and P. L'Argentiere, Carbon, 2008, 46, 1631–1637 CrossRef CAS.
- N. Li, Z. Y. Wang, K. K. Zhao, Z. J. Shi, S. K. Xu and Z. N. Gu, J. Nanosci. Nanotechnol., 2010, 10, 6748–6751 CrossRef CAS.
- A. R. Siamaki, A. E. R. S. Khder, V. Abdelsayed, M. S. El-Shall and B. F. Gupton, J. Catal., 2011, 279, 1–11 CrossRef CAS.
- S. J. Zhang, X. Y. Li and J. P. Chen, Carbon, 2010, 48, 60–67 CrossRef CAS.
- D. Yang, F. Yang, J. H. Hu, J. Long, C. C. Wang, D. L. Fu and Q. X. Ni, Chem. Commun., 2009, 4447–4449 RSC.
- X. Y. Yang, X. Y. Zhang, Y. F. Ma, Y. Huang, Y. S. Wang and Y. S. Chen, J. Mater. Chem., 2009, 19, 2710–2714 RSC.
- H. P. Cong, J. J. He, Y. Lu and S. H. Yu, Small, 2009, 6, 169–173.
- A. G. Hu, G. T. Yee and W. B. Lin, J. Am. Chem. Soc., 2005, 127, 12486–12487 CrossRef CAS.
- M. Kawamura and K. Sato, Chem. Commun., 2007, 3404–3405 RSC.
- A. J. Amali and R. K. Rana, Green Chem., 2009, 11, 1781–1786 RSC.
- Y. Jang, J. Chung, S. Kim, S. W. Jun, B. H. Kim, D. W. Lee, B. M. Kim and T. Hyeon, Phys. Chem. Chem. Phys., 2011, 13, 2512–2516 RSC.
- Y. C. Si and E. T. Samulski, Nano Lett., 2008, 8, 1679–1682 CrossRef CAS.
- N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Chem. Mater., 1999, 11, 771–778 CrossRef CAS.
- H. Li, Z. H. Zhu, H. X. Li, P. Li and X. G. Zhou, J. Colloid Interface Sci., 2010, 349, 613–619 CrossRef CAS.
- N. Karousis, G.-E. Tsotsou, F. Evangelista, P. Rudolf, N. Ragoussis and N. Tagmatarchis, J. Phys. Chem. C, 2008, 112, 13463–13469 CrossRef CAS.
- X. Chen, G. Wu, J. Chen, X. Chen, Z. Xie and X. Wang, J. Am. Chem. Soc., 2011, 133, 3693–3695 CrossRef CAS.
- R. B. Bedford, M. E. Blake, C. P. Butt and D. Holder, Chem. Commun., 2003, 466–467 RSC.
- G. Zou, Z. Y. Wang, J. R. Zhu, J. Tang and M. Y. He, J. Mol. Catal. A: Chem., 2003, 206, 193–198 CrossRef CAS.
- G. A. Molander and B. Biolatto, Org. Lett., 2002, 4, 1867–1870 CrossRef CAS.
- J. Rebek and F. Gavina, J. Am. Chem. Soc., 1974, 96, 7112–7114 CrossRef CAS.
- J. Rebek, D. Brown and S. Zimmerman, J. Am. Chem. Soc., 1975, 97, 454–455 CrossRef CAS.
- I. W. Davies, L. Matty, D. L. Hughes and P. J. Reider, J. Am. Chem. Soc., 2001, 123, 10139–10140 CrossRef CAS.
- C. M. Crudden, K. McEleney, S. L. MacQuarrie, A. Blanc, M. Sateesh and J. D. Webb, Pure Appl. Chem., 2007, 79, 247–260 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cy20263h |
| This journal is © The Royal Society of Chemistry 2012 |