Synthesis, characterization, and the ethylene (co-)polymerization behaviour of half-titanocene dichloride 2-aryliminoquinolin-8-olates†
Received
16th April 2012
, Accepted 21st May 2012
First published on 22nd May 2012
Abstract
A series of half-titanocene dichloride 2-aryliminoquinolin-8-olates, CpTi LCl2 (C1–C6: Cp as C5H5, L as 2-(1-(o-benzhydrylarylimino)methyl)quinolin-8-olates or 2-(1-(o-benzhydrylarylimino)ethyl)quinolin-8-olates), was synthesized and fully characterized; the molecular structures of representative complexes C2 and C4 are reported. Upon activation with modified methylaluminoxane (MMAO), the title complexes exhibited good activities for ethylene polymerization (up to 1.56 × 106 g mol−1 (Ti) h−1). The C4/MMAO system was further investigated for the co-polymerization of ethylene with 1-hexene, 1-octene, or isoprene.
Introduction
Metallocene complexes were used as model pre-catalysts in understanding the mechanism of Ziegler–Natta type catalysis in the 1950s.1 Further dramatic developments in the 1980s were initiated following the discovery that highly active catalytic metallocene systems were accessible upon activation with the co-catalyst methylaluminoxane (MAO).2,3 Subsequent studies revealed how metallocene pre-catalysts could be finely tuned, characteristically producing polyolefins with narrow molecular weight distributions and desirable microstructures.4–7 Moreover, bridged half-metallocene pre-catalysts (constrained geometry catalysts, CGCs), were developed for industrial processes by the Dow and Exxon Chemical companies.8–13 Problems associated with CGCs still remain, in-particular, there are only a limited number of alternative (and useful) ligand types available, and synthetic problems relating to both yield and cost still persist. To overcome the shortfall in available ligand sets, non-bridged half-metallocene pre-catalysts were designed, and included various modifications of the anionic ligands. Both high activity and precise control over co-polymerization ability were accomplished.14–17 The anionic ligands deployed could be classified as mono-dentate such as aryloxy18,19 or ketimide,20 bi-dentate such as acetamidinato,21–24 iminophenoxy,25,26 pyridinylalkyloxy,27 and N-substituted (iminomethyl) pyrrolides,28,29 as well as multi-dentate such as β-ketoimine.30,31 More recently, titanium-based catalytic systems such as the FI catalysts employing iminophenolates32–38 and PI catalysts containing iminopyrrolide have been developed.39–42 The non-bridged half-metallocene pre-catalysts showed high activities for co-polymerizations,17–19,43–46 and we recently reported half-titanocene chloride complexes bearing anionic multidentate ligands, which exhibited both good stability and activity.47–51 The ligand family 2-(1-(arylimino)alkyl)quinolin-8-olates were used in preparing aluminium pre-catalysts for the ring-opening polymerization of ε-caprolactone,52 and were also used to prepare trichlorotitanium,53 half-titanocene chloride,54,55 and zirconium complexes,56 which acted as highly active pre-catalysts for ethylene homo-polymerization and co-polymerization. Within the catalytic system comprising half-titanocene dichloride 2-(1-(arylimino)alkyl)quinolin-8-olates,54,55 the longer the side-chain in the ligand, the higher the catalytic activity observed, i.e. pre-catalysts using the 2-(1-(arylimino)propyl)quinolin-8-olate ligand set54 exhibited higher activities than did those bearing 2-(1-(arylimino)ethyl)quinolin-8-olates.55 Given this, it would be interesting to examine the different catalytic behavior of half-titanocene pre-catalysts bearing 2-aldiminoquinolin-8-olates versus 2-ketiminoquinolin-8-olates. Furthermore, bulky substituents at the arylimino group of ligands derived from such aldimine/ketimine systems are very likely to influence the catalytic activities of the resultant complexes. Thus, bulky ligands derived from 2-(1-(o-benzhydrylarylimino)alkyl)quinolin-8-ol were prepared via the condensation reaction of o-benzhydrylanilines with either 8-hydroxyquinoline-2-carbaldehyde or 1-(8-hydroxy-quinolin-2-yl)ethanone. On further reaction with CpTiCl3, the title half-titanocene complexes were prepared. Catalytic screening revealed good activities for ethylene homo-polymerization and co-polymerization with 1-hexene, 1-octene or isoprene. Herein, the synthesis and characterization of the title complexes as well as their catalytic behavior are reported.
Results and discussion
Synthesis and characterization
The stoichiometric reaction of CpTiCl3 with the corresponding potassium 2-aryliminoquinolin-8-olate in toluene, afforded the (η5-cyclopentadienyl)titanium dichloride 2-(1-(o-benzhydrylaryl-imino)alkyl)quinolin-8-olates (C1–C6; Scheme 1) in good yields (55–80%).
 |
| | Scheme 1 Synthesis of titanium complexes C1–C6. | |
Successful complexation resulting in the formation of Ti–O bonds could be followed by either FT-IR or 1H NMR spectroscopy; all complexes possessed a lack of bands at about 3410 cm−1 in their IR spectra and the peak at ca. 8.07 ppm in the 1H NMR spectra of the parent ligands. Furthermore, in the 13C NMR spectra there was a 3–5 ppm downfield shift of the resonances associated with the CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N functionality in complexes C1–C3 when compared to those observed for the parent ligands L1–L3 (164–165 ppm), whilst there was a 1–3 ppm downfield shift for CCH3
N functionality in complexes C1–C3 when compared to those observed for the parent ligands L1–L3 (164–165 ppm), whilst there was a 1–3 ppm downfield shift for CCH3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the complexes C4–C6cf. to the ligands L4–L6 (168–169 ppm). Elemental analytical data were consistent with complexes of molecular formula CpTiLCl2. Representative molecular structures were confirmed for complexes C2 and C4via single crystal X-ray diffraction.
N in the complexes C4–C6cf. to the ligands L4–L6 (168–169 ppm). Elemental analytical data were consistent with complexes of molecular formula CpTiLCl2. Representative molecular structures were confirmed for complexes C2 and C4via single crystal X-ray diffraction.
X-ray crystallographic studies
As shown in Fig. 1, complex C2 contains one chelating ligand, together with a Cp ring and two chlorides, all of which form a pseudo octahedral geometry at the titanium centre. The distance between the centroid of the Cp ring and the titanium atom is 2.075 Å, whilst the Ti1–C bond lengths are slightly different with a range of 2.371 Å to 2.401 Å (ΔTi–C = 0.03 Å); the carbons (C44, C45, C46, C47, and C48) are coplanar. The tridentate N⁁N⁁O ligand coordinated with titanium in a meridianal manner, and the two chlorides located in trans-form with the Cl1–Ti1–Cl2 as 151.92(7)°. The Ti–Cl bond lengths are slightly different at around 2.43 Å, whilst the Ti1–O1 bond length is 1.959 Å, consistent with observations for analogous complexes.54,55 Selected bonds and angles are tabulated in Table 1.
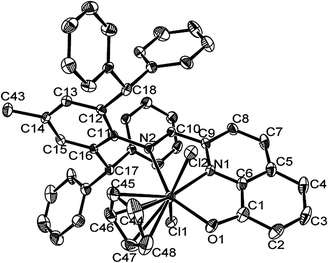 |
| | Fig. 1 ORTEP drawing of complex C2 with thermal ellipsoids at 30% probability level. Hydrogen atoms have been omitted for clarity. | |
Table 1 Selected bond lengths (Å) and angles (°) for C2 and C4
| |
C2
|
C4
|
| Bond lengths (Å) |
|
|
| Ti1–O1 |
1.959(4) |
1.948(4) |
| Ti1–N1 |
2.131(5) |
2.127(5) |
| Ti1–N2 |
2.372(5) |
2.402(5) |
| Ti1–Cl1 |
2.4398(19) |
2.4528(19) |
| Ti1–Cl2 |
2.4331(19) |
2.4518(18) |
| Ti1–Cpcent |
2.075 |
2.078 |
| Bond angles (°) |
|
|
| O1–Ti1–N1 |
77.45(18) |
77.47(19) |
| O1–Ti1–N2 |
146.62(17) |
146.09(18) |
| N1–Ti1–N2 |
69.17(16) |
68.62(17) |
| O1–Ti1–Cl1 |
87.85(13) |
88.99(14) |
| N1–Ti1–Cl1 |
75.70(13) |
75.26(14) |
| N2–Ti1–Cl1 |
84.28(12) |
83.04(12) |
| O1–Ti1–Cl2 |
87.74(14) |
86.16(14) |
| N1–Ti1–Cl2 |
76.27(13) |
76.52(14) |
| N2–Ti1–Cl2 |
84.28(12) |
85.60(12) |
| Cl1–Ti1–Cl2 |
151.92(7) |
151.75(7) |
| Cpcent–Ti1–N1 |
177.08 |
177.29 |
| Cpcent–Ti1–N2 |
107.98 |
108.67 |
| Cpcent–Ti1–O1 |
105.40 |
105.23 |
| Cpcent–Ti1–Cl1 |
103.60 |
104.59 |
| Cpcent–Ti1–Cl2 |
104.31 |
103.53 |
There are only slight structural differences between C2 and complex C4 (Fig. 2). The dihedral angle defined by the two chelating rings ΘTi1–O1–C1–C6–N1 and ΘTi1–N1–C9–C10–N2 is 2.98° for C4, slightly larger than the corresponding dihedral angle in C2 (1.75°).
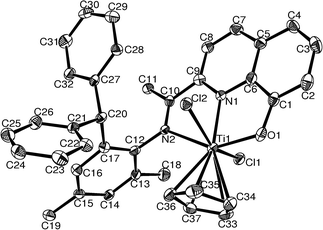 |
| | Fig. 2 ORTEP drawing of the molecular structure of C4 (ellipsoids enclose 30% electronic density; H atoms are omitted for clarity). | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at a temperature of 60 °C over 30 min at 10 atm, the activity was observed to be 1.9 × 105 g mol−1 (Ti) h−1 in the presence of MAO, and 6.1 × 105 g mol−1 (Ti) h−1 when using MMAO. Given these results, further investigations were conducted using MMAO as co-catalyst.
1 at a temperature of 60 °C over 30 min at 10 atm, the activity was observed to be 1.9 × 105 g mol−1 (Ti) h−1 in the presence of MAO, and 6.1 × 105 g mol−1 (Ti) h−1 when using MMAO. Given these results, further investigations were conducted using MMAO as co-catalyst.
To find the most suitable Al/Ti molar ratio, trials with varying Al/Ti molar ratios, from 2000 to 7000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, were conducted at 60 °C over 30 min (Runs 2–7, Table 2), which indicated that the highest activity (9.4 × 105 g mol−1 (Ti) h−1) was achieved at an Al/Ti ratio of 5000
1, were conducted at 60 °C over 30 min (Runs 2–7, Table 2), which indicated that the highest activity (9.4 × 105 g mol−1 (Ti) h−1) was achieved at an Al/Ti ratio of 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. GPC measurements showed that the obtained polyethylenes had lower molecular weights on increasing the molar ratio of Al/Ti (Fig. 3), consistent with the explanation that a higher Al/Ti molar ratio enhances chain transfer to aluminium, i e. termination.57,58
1. GPC measurements showed that the obtained polyethylenes had lower molecular weights on increasing the molar ratio of Al/Ti (Fig. 3), consistent with the explanation that a higher Al/Ti molar ratio enhances chain transfer to aluminium, i e. termination.57,58
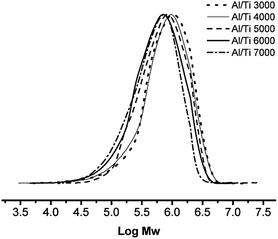 |
| | Fig. 3 GPC profiles of PEs obtained from Runs 3–7 in Table 2. | |
| Run |
Co-cat |
t/min |
Al/Ti |
T/°C |
Polym/g |
Actb |
T
m
c/°C |
M
w
d
,
e
|
M
w/Mne |
|
Conditions: 1 μmol complex C1, toluene (total volume 100 mL), 10 atm.
105 g mol−1 (Ti) h−1.
Determined by DSC.
104 g mol−1.
Determined by GPC.
|
| 1 |
MAO |
30 |
3000 |
60 |
0.095 |
1.90 |
134.7 |
74.1 |
6.17 |
| 2 |
MMAO |
30 |
3000 |
60 |
0.305 |
6.10 |
135.3 |
74.7 |
2.82 |
| 3 |
MMAO |
30 |
2000 |
60 |
0.230 |
4.60 |
135.4 |
76.2 |
3.27 |
| 4 |
MMAO |
30 |
4000 |
60 |
0.270 |
5.40 |
134.3 |
74.9 |
2.94 |
| 5 |
MMAO |
30 |
5000 |
60 |
0.470 |
9.40 |
135.0 |
70.1 |
1.93 |
| 6 |
MMAO |
30 |
6000 |
60 |
0.397 |
7.94 |
135.1 |
61.6 |
2.84 |
| 7 |
MMAO |
30 |
7000 |
60 |
0.282 |
5.64 |
130.2 |
58.9 |
2.06 |
| 8 |
MMAO |
20 |
5000 |
60 |
0.181 |
5.43 |
130.0 |
58.1 |
3.09 |
| 9 |
MMAO |
10 |
5000 |
60 |
0.124 |
7.44 |
129.5 |
57.8 |
2.38 |
| 10 |
MMAO |
30 |
5000 |
50 |
0.196 |
3.92 |
135.0 |
78.8 |
2.68 |
| 11 |
MMAO |
30 |
5000 |
70 |
0.346 |
6.92 |
133.9 |
65.7 |
2.10 |
| 12 |
MMAO |
30 |
5000 |
80 |
0.220 |
4.40 |
134.2 |
63.2 |
2.54 |
With regard to the reaction temperature (runs 5, 10–12, Table 2), the optimum temperature was found to be 60 °C, for which the activity was 9.4 × 105 g mol−1 (Ti) h−1. The higher the reaction temperature, the lower the concentration of ethylene in solution, and therefore the lower the activity observed. The molecular weights of the obtained polyethylenes decreased on elevating the temperature (Fig. 4); i.e., faster chain terminations at elevated temperatures. These trends are consistent with those observed for related titanium complexes55 and other half-titanocene pre-catalysts.48
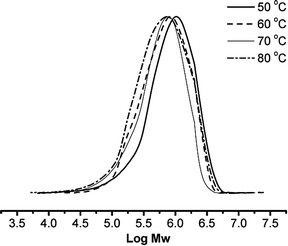 |
| | Fig. 4 GPC profiles of PEs obtained from Runs 5 and 10–12 in Table 2. | |
Over different reaction periods of 10, 20, and 30 min (Runs 5, 8, and 9, Table 2), the highest activity was observed at 30 min, indicative of a rather stable species for this catalytic system; meanwhile the slightly lower activity value for 20 min (Run 8) indicated the induction period required to form active species in the current catalytic system. As indicated by their GPC curves, some polyethylene of lower molecular weight was formed in a short reaction time (Fig. 5). On prolonging the reaction time, the obtained polyethylene possessed higher molecular weight and narrower molecular distribution. When compared to the analogous half-titanocene dichloride 2-(1-(arylimino)ethyl)quinolin-8-olates,55 lower activity was observed, and higher molecular weights and narrower molecular distributions for the polyethylenes were obtained. It is thought that the bulky ligand present prevented the coordination of ethylene and protected the active species in this catalytic system.
 |
| | Fig. 5 GPC profiles of PEs obtained from Runs 5, 8 and 9 in Table 2. | |
Using the optimum conditions of Al/Ti ratio of 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 60 °C under 10 atm over 30 min, all the pre-catalysts C1–C6 were screened for ethylene polymerization (Table 3). The bulkier the ligand used, the lower the activity observed, thus C1 (R1 = Me, R2 = Ph2CH) > C2 (R1 = R2 = Ph2CH), C4 (R1 = Me, R2 = Ph2CH) > C5 (R1 = R2 = Ph2CH), consistent with bulkier ortho-substituents at the arylimino group preventing access of ethylene to the active centre. For the same ortho-substituent at the arylimino group, pre-catalysts possessing a para-isopropyl substituent at the arylimino showed higher activity than did analogues possessing a para-methyl substituent at the arylimino group, i e.C3 (R3 = i-Pr, R1 = R2 = Ph2CH) > C2 (R3 = Me, R1 = R2 = Ph2CH) and C6 (R3 = i-Pr, R1 = R2 = Ph2CH) > C5 (R3 = Me, R1 = R2 = Ph2CH), indicating the better activity was caused by the better soluble para-isopropyl group. In addition, better catalytic activities were observed for the ketimine systems versus the aldimines, i e.C4 (R = Me) > C1 (R = H), C5 (R = Me) > C2 (R = H), C6 (R = Me) > C3 (R = H). These were consistent with our previous observations for half-titanocene dichloride 2-aryliminoquinolin-8-olates, where higher activities were achieved by 2-(1-(arylimino)propyl)quinolin-8-olates54 over 2-(1-(arylimino)ethyl)quinolin-8-olates.55 Besides the better solubility of the complexes bearing ligands with longer alkyl groups, the longer chain alkyl substituents can also be considered better electron-donors capable of stabilizing electron-deficient titanium complexes.59,60
1 at 60 °C under 10 atm over 30 min, all the pre-catalysts C1–C6 were screened for ethylene polymerization (Table 3). The bulkier the ligand used, the lower the activity observed, thus C1 (R1 = Me, R2 = Ph2CH) > C2 (R1 = R2 = Ph2CH), C4 (R1 = Me, R2 = Ph2CH) > C5 (R1 = R2 = Ph2CH), consistent with bulkier ortho-substituents at the arylimino group preventing access of ethylene to the active centre. For the same ortho-substituent at the arylimino group, pre-catalysts possessing a para-isopropyl substituent at the arylimino showed higher activity than did analogues possessing a para-methyl substituent at the arylimino group, i e.C3 (R3 = i-Pr, R1 = R2 = Ph2CH) > C2 (R3 = Me, R1 = R2 = Ph2CH) and C6 (R3 = i-Pr, R1 = R2 = Ph2CH) > C5 (R3 = Me, R1 = R2 = Ph2CH), indicating the better activity was caused by the better soluble para-isopropyl group. In addition, better catalytic activities were observed for the ketimine systems versus the aldimines, i e.C4 (R = Me) > C1 (R = H), C5 (R = Me) > C2 (R = H), C6 (R = Me) > C3 (R = H). These were consistent with our previous observations for half-titanocene dichloride 2-aryliminoquinolin-8-olates, where higher activities were achieved by 2-(1-(arylimino)propyl)quinolin-8-olates54 over 2-(1-(arylimino)ethyl)quinolin-8-olates.55 Besides the better solubility of the complexes bearing ligands with longer alkyl groups, the longer chain alkyl substituents can also be considered better electron-donors capable of stabilizing electron-deficient titanium complexes.59,60
| Run |
Cat |
Polym/g |
Actb |
T
m
c/°C |
M
w
d
,
e
|
M
w/Mne |
|
Conditions: 1 μmol complexes, toluene (total volume 100 mL), Al/Ti = 5000, 30 min, 60 °C, 10 atm.
105 g mol−1 (Ti) h−1.
Determined by DSC.
104 g mol−1.
Determined by GPC.
|
| 1 |
C1
|
0.470 |
9.40 |
135.0 |
70.1 |
1.93 |
| 2 |
C2
|
0.430 |
8.60 |
122.2 |
44.5 |
3.68 |
| 3 |
C3
|
0.463 |
9.26 |
130.3 |
38.8 |
3.03 |
| 4 |
C4
|
0.780 |
15.6 |
135.0 |
65.4 |
2.00 |
| 5 |
C5
|
0.585 |
11.7 |
130.8 |
62.5 |
3.26 |
| 6 |
C6
|
0.612 |
12.2 |
125.9 |
49.7 |
2.44 |
Ethylene co-polymerization
The co-polymerization of ethylene with 1-hexene was investigated using the pre-catalyst C4 and employing the optimum conditions determined for ethylene homo-polymerization, i.e. Al/Ti ratio at 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 60 °C over 30 min under 10 atm of ethylene, whilst at the same time changing the concentration of 1-hexene (Table 4). A positive effect of the co-monomer was observed, leading to better activities for the co-polymerization than for ethylene homo-polymerization. However, the catalytic activities decreased with increasing co-monomer concentration (runs 1–3, Table 4), consistent with the observations for half-titanocene pre-catalysts featuring amidate48 or imino-indolate ligands.51 GPC analysis showed that the molecular weights of the obtained polymers decreased with increasing concentration of 1-hexene, suggesting possibly that chain termination occurred during the insertion of co-monomer. According to DSC analysis, the melting points of the obtained polymers were lower, indicating more branching on increasing the concentration of 1-hexene. Similar catalytic tendencies were also observed for the ethylene/1-octene co-polymerization (Table 4), though the activities for 1-octene were slightly lower than those observed for 1-hexene.
1 at 60 °C over 30 min under 10 atm of ethylene, whilst at the same time changing the concentration of 1-hexene (Table 4). A positive effect of the co-monomer was observed, leading to better activities for the co-polymerization than for ethylene homo-polymerization. However, the catalytic activities decreased with increasing co-monomer concentration (runs 1–3, Table 4), consistent with the observations for half-titanocene pre-catalysts featuring amidate48 or imino-indolate ligands.51 GPC analysis showed that the molecular weights of the obtained polymers decreased with increasing concentration of 1-hexene, suggesting possibly that chain termination occurred during the insertion of co-monomer. According to DSC analysis, the melting points of the obtained polymers were lower, indicating more branching on increasing the concentration of 1-hexene. Similar catalytic tendencies were also observed for the ethylene/1-octene co-polymerization (Table 4), though the activities for 1-octene were slightly lower than those observed for 1-hexene.
Table 4 Ethylene/1-olefin co-polymerization by the C4/MMAO systema
| Run |
Monomer |
Polym/g |
Actb |
T
m
c/°C |
M
w
d
,
e
|
M
w/Mne |
|
Conditions: 1 μmol complex C4, toluene (total volume 100 mL), Al/Ti = 5000, 30 min, 60 °C, 10 atm.
105 g mol−1 (Ti) h−1.
Determined by DSC.
104 g mol−1.
Determined by GPC.
|
| 1 |
0.4 M C6= |
0.568 |
11.4 |
125.3 |
60.0 |
2.32 |
| 2 |
0.7 M C6= |
0.43 |
8.60 |
116.4 |
45.5 |
2.79 |
| 3 |
1 M C6= |
0.23 |
4.60 |
103.2 |
18.9 |
2.65 |
| 4 |
0.4 M C8= |
0.308 |
6.16 |
122.8 |
50.0 |
3.49 |
| 5 |
0.7 M C8= |
0.256 |
5.12 |
116.3 |
44.9 |
3.25 |
| 6 |
1 M C8= |
0.177 |
3.54 |
101.2 |
11.3 |
11.9 |
The 13C NMR spectrum (Fig. 6) of the ethylene/1-hexene co-polymer indicated a 3.39 mol% incorporation of 1-hexene in the resultant polymer (Run 2, Table 4). The 1-hexene incorporation ability herein was lower than other similar half-titanocenes,54,55 indicating that the bulky ligand probably prevented the insertion of the 1-hexene monomer. The 13C NMR spectrum was assigned according to the literature.61 Meanwhile the 13C NMR spectrum (Fig. 7) of the ethylene/1-octene co-polymer indicated a 3.22 mol% incorporation of 1-octene (Run 5, Table 4).
Moreover, co-polymerization of ethylene with isoprene was also conducted using the C4/MMAO system (Table 5), and it was observed that the activities of the co-polymerization slowly decreased on increasing the concentration of isoprene, which was in contrast with previous reports in the literature.62 The molecular weights of ethylene-isoprene co-polymers decreased on increasing the monomer concentration, which was consistent with the above co-polymerizations with 1-hexene or 1-octene.
Table 5 Ethylene/Isoprene co-polymerization by the C4/MMAO systema
| Run |
Concentration |
Polym/g |
Actb |
T
m
c/°C |
M
w
d
,
e
|
M
w/Mne |
|
Conditions: 1 μmol complex C4, toluene (total volume 100 mL), Al/Ti = 5000, 30 min, 60 °C, 10 atm.
105 g mol−1 (Ti) h−1.
Determined by DSC.
104 g mol−1.
Determined by GPC. n.d.: polymer partly unsolvable.
|
| 1 |
0.4 M |
0.697 |
13.9 |
134.2 |
63.2 |
2.24 |
| 2 |
0.7 M |
0.509 |
10.2 |
133.9 |
33.6 |
2.84 |
| 3 |
1 M |
0.394 |
7.88 |
133.0 |
n.d. |
n.d. |
The 13C NMR spectra (Fig. 8) of the co-polymer (Run 2, Table 5) indicated a random co-polymer containing mainly PE units, and cis-1,4-IP-alt-E sequences with small amounts of trans-1,4-IP-alt-E (cis![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans = 75.8%
trans = 75.8%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.2%), without 2-IP-alt-E or 3,4-IP-alt-E units.
25.2%), without 2-IP-alt-E or 3,4-IP-alt-E units.
The monomer compositions were illustrated by comparing the integrals of the 1H NMR spectra (Fig. 9) by using the following formula (eqn (1)) according to the literature.63
| | |
Mol IP% = {(IH1 + 0.5IH2)/(IH1 + 0.5IH2 + 0.25(IH5 − IH2))} × 100
| (1) |
in which
IH1 is the integration of the resonance at 5.13 ppm (one
vinyl proton of the 1,4-isoprene unit),
IH2 is the integration of the resonance at 4.72 ppm (two
vinyl protons of the 3,4-isoprene unit) in the
1H NMR spectrum, and
IH5 is the integration of the broad signals at 1.3 ppm as the total
methylene protons in both 3,4-isoprene units and
ethylene units in the
1H NMR spectrum. The
1H NMR spectrum indicated a 1.75 mol%
isoprene incorporation in the resultant
polymer (Run 2,
Table 5).
Conclusion
A series of half-titanocene dichloride 2-(o-benzhydrylaryl- imino)quinolin-8-olates was synthesized and fully characterized. All half-titanocene complexes showed good activities for ethylene polymerization using MMAO as co-catalyst. The bulky ortho-substituents of the arylimino group prevented access of ethylene to the active titanium centre, thereby resulting in the lower observed activity. Longer chain alkyl-substituents (R and R3) enhanced the solubility of the complexes and resulted in better observed activity. The positive effect of the co-monomer was observed in the co-polymerization, however, the catalytic activities decreased with higher co-monomer concentrations. Comparison to the related half-titanocene54,55 and CGC pre-catalysts,8,9 the current system showed a better thermo-stability for ethylene polymerization, and also good activity toward ethylene/isoprene copolymerization.
Experimental section
General considerations
All manipulations of air and/or moisture-sensitive compounds were performed under nitrogen atmosphere in a glove-box or using standard Schlenk techniques. Methylaluminoxane (MAO, 1.46 M in toluene) was purchased from Albemarle and other reagents were purchased from Aldrich or Acros Chemicals. Modified methylaluminoxane (MMAO, 1.93 M in heptane) was purchased from Akzo Nobel Corp. Potassium hydride (KH), purchased from Beijing Regent Chemicals, was washed with hexane before use to remove contained mineral oil. Toluene, n-hexane, n-heptane and isoprene were refluxed over sodium and benzophenone, distilled, and then stored over activated molecular sieves (4 Å) for 24 h under nitrogen atmosphere. Dichloromethane (CH2Cl2), 1-hexene, and 1-octene were refluxed over calcium hydride, distilled, and then stored over activated molecular sieves (4 Å) for 24 h in a glove-box prior to use. CDCl3 was dried over activated 4 Å molecular sieves. IR spectra were recorded on a Perkin Elmer FT-IR 2000 spectrometer using a KBr disc in the range of 4000–400 cm−1. Elemental analysis was performed on a Flash EA 1112 microanalyzer. 1H NMR and 13C NMR spectra were recorded on a Bruker DMX 400 MHz instrument at ambient temperature using TMS as an internal standard. Assignments are based on COSY, HSQC and HMBC experiments. DSC trace and melting points of polyethylene were obtained from the second scanning run on a Perkin-Elmer DSC-7 at a heating rate of 10 °C min−1. 13C NMR spectra of the polymers were recorded on a Bruker DMX-300 MHz instrument at 112 °C in deuterated 1,2-dichlorobenzene with TMS as an internal standard. The molecular weight and molecular weight distribution of the polymers were determined by gel permeation chromatography (GPC) using a Waters Alliance GPC 2000 instrument equipped with a refractive index (RI) detector and a set of u-Styragel HT columns of 106, 105, 104, and 103 pore size in series. The measurement was performed at 150 °C with 1,2,4-trichlorobenzene as the eluent at a flow rate of 0.95 mL min−1. Narrow-molecular-weight PS samples were used as standards for calibration.
Synthesis of 2-((2-benzhydryl-4,6-dimethylphenylimino)methyl)quinolin-8-ol (L1)
A solution of 2-benzhydryl-4,6-dimethylbenzenamine (0.689 g, 2.4 mmol) and 8-hydroxyquinoline-2-carbaldehyde (0.346 g, 2 mmol) in ethanol was refluxed for 12 h, then the solvent was removed in vacuo. The product, 2-((2-benzhydryl-4,6-dimethylphenylimino)methyl)quinolin-8-ol (L1), was purified by column chromatography (silica gel, petroleum ether/ethyl acetate = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the second part to elute was collected and concentrated to give a yellow green solid in 69.1% yield (0.63 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.29–8.21 (m, 2H), 8.02 (s, 1H), 7.84 (s, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.37 (d, J = 8.1 Hz, 1H), 7.23–7.05 (m, 7H), 7.04 (d, J = 6.8 Hz, 4H), 6.98 (s, 1H), 6.63 (s, 1H), 5.57 (s, 1H), 2.25 (s, 3H), 2.14 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 164.3, 152.6, 152.1, 147.8, 143.8, 137.7, 136.7, 134.1, 133.4, 129.9, 129.8, 129.7, 129.2, 129.1, 128.6, 128.3, 128.2, 126.2, 126.0, 118.8, 117.9, 110.5, 52.3, 21.2, 18.4. IR (KBr, cm−1): 3410 (m), 2916 (w), 1974 (w), 1636 (m), 1561 (m), 1503 (m), 1469 (s), 1363 (s), 1329 (m), 1299 (m), 1237 (s), 1202 (m), 1135 (m), 1103 (m), 1036 (m), 846 (s), 793 (m), 745 (s), 697 (s). Mp: 88–89 °C. Anal. Calcd. For C31H26N2O: C, 84.13; H, 5.92; N, 6.33. Found: C, 84.11; H, 5.98; N, 6.29.
1), the second part to elute was collected and concentrated to give a yellow green solid in 69.1% yield (0.63 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.29–8.21 (m, 2H), 8.02 (s, 1H), 7.84 (s, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.37 (d, J = 8.1 Hz, 1H), 7.23–7.05 (m, 7H), 7.04 (d, J = 6.8 Hz, 4H), 6.98 (s, 1H), 6.63 (s, 1H), 5.57 (s, 1H), 2.25 (s, 3H), 2.14 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 164.3, 152.6, 152.1, 147.8, 143.8, 137.7, 136.7, 134.1, 133.4, 129.9, 129.8, 129.7, 129.2, 129.1, 128.6, 128.3, 128.2, 126.2, 126.0, 118.8, 117.9, 110.5, 52.3, 21.2, 18.4. IR (KBr, cm−1): 3410 (m), 2916 (w), 1974 (w), 1636 (m), 1561 (m), 1503 (m), 1469 (s), 1363 (s), 1329 (m), 1299 (m), 1237 (s), 1202 (m), 1135 (m), 1103 (m), 1036 (m), 846 (s), 793 (m), 745 (s), 697 (s). Mp: 88–89 °C. Anal. Calcd. For C31H26N2O: C, 84.13; H, 5.92; N, 6.33. Found: C, 84.11; H, 5.98; N, 6.29.
2-(1-(2,6-dibenzhydryl-4-methylphenylimino)methyl)quinolin-8-ol (L2)
Using the above procedure, but 2,6-dibenzhydryl-4-methyl-benzenamine was used instead of 2-benzhydryl-4,6-dimethyl-benzenamine, the 2-(1-(2,6-dibenzhydryl-4-methylphenylimino) methyl)quinolin-8-ol (L2) was obtained as a yellow green solid in 72.4% yield (0.88 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.16 (d, J = 8.1 Hz, 1H), 8.08 (d, J = 8.3 Hz, 1H), 7.85 (s, 1H), 7.48 (t, J = 7.6 Hz, 1H), 7.33 (d, J = 7.9 Hz, 1H), 7.25–7.11 (m, 14H), 7.02 (d, J = 6.0 Hz, 8H), 6.68 (s, 2H), 5.45 (s, 2H), 2.17 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 165.5, 152.7, 151.7, 147.9, 143.8, 142.9, 136.5, 133.2, 132.9, 129.8, 129.7, 129.4, 129.2, 129.1, 128.6, 128.4, 126.7, 126.3, 118.8, 117.9, 110.4, 52.5, 21.5. IR (K Br, cm−1): 3411 (m), 3021 (w), 2166 (w), 2029 (w), 1636 (m), 1598 (m), 1565 (m), 1508 (m), 1493 (s), 1471 (m), 1444 (m), 1327 (m), 1239 (s), 1202 (m), 1078 (m), 1032 (m), 915 (m), 839 (s), 757 (m), 739 (s), 697 (s). Mp: 99–101 °C. Anal. Calcd. For C43H34N2O: C, 86.84; H, 5.76; N, 4.71. Found: C, 86.81; H, 5.78; N, 4.68.
2-(1-(2,6-dibenzhydryl-4-isopropylphenylimino)methyl)quinolin-8-ol (L3)
Similarly, the 2-(1-(2,6-dibenzhydryl-4-isopropylphenylimino)methyl)quinolin-8-ol (L3) was obtained as a yellow green solid in 81.8% yield (1.04 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.16 (d, J = 8.6 Hz, 1H), 8.07 (d, J = 8.6 Hz, 1H), 7.85 (s, 1H), 7.48 (t, J = 7.9 Hz, 1H), 7.34 (d, J = 8.2 Hz, 1H), 7.24–7.12 (m, 14H), 7.00 (d, J = 7.1 Hz, 8H), 6.72 (s, 2H), 5.45 (s, 2H), 2.74–2.67 (m, 1H), 1.05 (d, J = 6.9 Hz, 6H). 13C NMR (CDCl3, 100 MHz, ppm): δ 165.1, 152.7, 151.7, 148.1, 143.9, 137.6, 136.5, 133.1, 129.8, 129.2, 128.3, 126.4, 126.3, 118.9, 117.8, 110.4, 52.4, 33.7, 24.1. IR (KBr, cm−1): 3382 (w), 3025 (w), 2959 (w), 1632 (m), 1600 (m), 1569 (m), 1508 (m), 1493 (s), 1445 (s), 1371 (m), 1327 (m), 1287 (m), 1235 (s), 1199 (m), 1077 (m), 1030 (m), 841 (m), 738 (s), 696 (s). Mp: 101–102 °C. Anal. Calcd. For C45H38N2O: C, 86.78; H, 6.15; N, 4.50. Found: C, 86.74; H, 6.11; N, 4.53.
2-(1-(2-benzhydryl-4,6-dimethylphenylimino)ethyl)quinolin-8-ol (L4)
The mixture of 0.374 g (2.0 mmol) 1-(8-hydroxyquinolin-2-yl)ethanone, (0.689 g, 2.4 mmol) 2-benzhydryl-4,6-dimethyl-benzenamine and a catalytic amount of p-toluenesulfonic acid (0.10 g) in 20 m L toluene was refluxed for 24 h. After toluene evaporation, 2-(1-(2-benzhydryl-4,6-dimethylphenylimino)ethyl)quinolin-8-ol (L4) was purified on column chromatography (silica gel, petroleum ether/ethyl acetate = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a yellow green solid in 46.1% (0.42 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.48 (d, J = 8.6 Hz, 1H), 8.23 (d, J = 8.6 Hz, 1H), 8.07 (s, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.28–7.19 (m, 2H), 7.12–7.10 (m, 6H), 6.99–6.96 (m, 4H), 6.65 (s, 1H), 5.46 (s, 1H), 2.26 (s, 3H), 1.97 (s, 3H), 1.58 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 168.3, 154.0, 152.5, 145.9, 143.8, 142.7, 137.0, 136.4, 133.3, 132.4, 130.0, 129.7, 129.5, 129.1, 128.8, 128.4, 128.2, 127.9, 126.3, 126.2, 125.0, 119.6, 118.0, 110.3, 52.5, 21.2, 17.9, 16.3. IR (KBr, cm−1): 3406 (m), 2916 (w), 2165 (w), 1976 (w), 1637 (m), 1567 (m), 1505 (m), 1465 (s), 1365 (s), 1327 (m), 1298 (m), 1238 (s), 1201 (m), 1138 (m), 1100 (m), 1030 (m), 843 (s), 790 (m), 747 (s), 699 (s). Mp: 92–93 °C. Anal. Calcd. For C32H28N2O: C, 84.18; H, 6.18; N, 6.14. Found: C, 84.12; H, 6.15; N, 6.19.
1) and obtained as a yellow green solid in 46.1% (0.42 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.48 (d, J = 8.6 Hz, 1H), 8.23 (d, J = 8.6 Hz, 1H), 8.07 (s, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.28–7.19 (m, 2H), 7.12–7.10 (m, 6H), 6.99–6.96 (m, 4H), 6.65 (s, 1H), 5.46 (s, 1H), 2.26 (s, 3H), 1.97 (s, 3H), 1.58 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 168.3, 154.0, 152.5, 145.9, 143.8, 142.7, 137.0, 136.4, 133.3, 132.4, 130.0, 129.7, 129.5, 129.1, 128.8, 128.4, 128.2, 127.9, 126.3, 126.2, 125.0, 119.6, 118.0, 110.3, 52.5, 21.2, 17.9, 16.3. IR (KBr, cm−1): 3406 (m), 2916 (w), 2165 (w), 1976 (w), 1637 (m), 1567 (m), 1505 (m), 1465 (s), 1365 (s), 1327 (m), 1298 (m), 1238 (s), 1201 (m), 1138 (m), 1100 (m), 1030 (m), 843 (s), 790 (m), 747 (s), 699 (s). Mp: 92–93 °C. Anal. Calcd. For C32H28N2O: C, 84.18; H, 6.18; N, 6.14. Found: C, 84.12; H, 6.15; N, 6.19.
2-(1-(2,6-dibenzhydryl-4-methylphenylimino)ethyl)quinolin-8-ol (L5)
Using the above procedure, but 2,6-dibenzhydryl-4-methyl-benzenamine was used instead of 2-benzhydryl-4,6-dimethyl-benzenamine, the 2-(1-(2,6-dibenzhydryl-4-methylphenylimino)ethyl)quinolin-8-ol (L5) was obtained as a yellow green solid in 47.7% yield (0.58 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.23–8.16 (m, 2H), 8.00 (s, 1H), 7.50 (t, J = 7.9 Hz, 1H), 7.38 (d, J = 8.1 Hz, 1H), 7.29–7.15 (m, 13H), 7.03 (d, J = 6.8 Hz, 4H), 7.00 (d, J = 6.8 Hz, 4H), 6.70 (s, 2H), 5.28 (s, 2H), 2.19 (s, 3H), 1.05 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 169.6, 153.9, 152.6, 146.1, 143.9, 142.9, 142.6, 137.0, 136.3, 132.2, 132.0, 130.0, 129.7, 129.6, 129.0, 128.8, 128.6, 128.5, 128.2, 126.7, 126.4, 126.2, 119.7, 117.9, 110.2, 52.5, 21.5, 16.8. IR (KBr, cm−1): 3405 (w), 3025 (w), 2167 (w), 1638 (m), 1599 (m), 1494 (s), 1445 (s), 1371 (w), 1334 (m), 1242 (s), 1077 (s), 1030 (m), 848 (m), 753 (s), 697 (s). Mp: 98–99 °C. Anal. Calcd. For C44H36N2O: C, 84.18; H, 6.18; N, 6.14. Found: C, 84.12; H, 6.15; N, 6.19.
2-(1-(2,6-dibenzhydryl-4-isopropylphenylimino)ethyl)quinolin-8-ol (L6)
Using the above procedure, but 2,6-dibenzhydryl-4-isopropyl-benzenamine was used instead of 2,6-dibenzhydryl-4-methylbenzenamine, the 2-(1-(2,6-dibenzhydryl-4-isopropyl-phenylimino)ethyl)quinolin-8-ol (L6) was obtained as a yellow green solid in 29.1% yield (0.37 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.23–8.16 (m, 2H), 8.01 (s, 1H), 7.50 (t, J = 7.9 Hz, 1H), 7.38 (d, J = 8.1 Hz, 1H), 7.25–7.15 (m, 13H), 7.03 (d, J = 6.8 Hz, 4H), 6.99 (d, J = 6.8 Hz, 4H), 6.75 (s, 2H), 5.28 (s, 2H), 2.76–2.69 (m, 1H), 1.09 (s, 3H), 1.06 (d, J = 3.28 HZ, 6H). 13C NMR (CDCl3, 100 MHz, ppm): δ 169.5, 153.9, 152.6, 146.3, 143.9, 143.0, 142.7, 137.0, 136.3, 131.9, 130.0, 129.6, 129.0, 128.8, 128.4, 128.1, 126.3, 126.2, 126.1, 119.8, 117.9, 110.2, 52.5, 33.6, 24.2, 16.8. IR (KBr, cm−1): 3407 (w), 2959 (w), 1632 (m), 1605 (m), 1564 (m), 1508 (m), 1493 (s), 1445 (s), 1371 (m), 1327 (m), 1287 (m), 1235 (s), 1199 (m), 1077 (m), 1030 (m), 841 (m), 738 (s), 696 (s). Mp: 104–105 °C. Anal. Calcd. For C46H40N2O: C, 86.76; H, 6.33; N, 4.40. Found: C, 86.74; H, 6.35; N, 6.37.
Synthesis of nickel complexes (η5-cyclopentadienyl)titanium dichloride 2-((2-benzhydryl-4,6-dimethylphenylimino)methyl)quinolin-8-olate (C1)
To a 30 mL toluene solution of 0.442 g (1.00 mmol) 2-((2-benzhydryl-4,6-dimethylphenylimino)methyl)quinolin-8-ol, 0.040 g (1.00 mmol) KH was added at −78 °C. The mixture was stirred for additional 4 h, and then 0.219 g (1.00 mmol) CpTiCl3 was added at −78 °C. The resultant mixture was slowly warmed up to room temperature and stirred for additional 6 h. After the solvent evaporation, the residue was extracted with CH2Cl2 (3 × 20 mL), and the filtrate was combined and concentrated to about 20 mL. The concentrated solution was covered with n-heptane and kept for three days to obtain brown crystals of (η5-cyclopentadienyl)titanium dichloride 2-((2-benzhydryl-4,6-dimethylphenylimino)methyl)quinolin-8-olate (C1) in 58.1% yield (0.37 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.32 (d, J = 8.4 Hz, 1H), 7.77 (t, J = 8.0 Hz, 1H), 7.44 (d, J = 8.1 Hz, 1H), 7.35 (t, J = 7.4 Hz, 1H), 7.28–7.22 (m, 5H), 7.17–7.11 (m, 4H), 7.08 (s, 2H), 6.99–6.93 (m, 1H), 6.96 (s, Cp, 5H), 6.78 (s, 1H), 6.56 (s, 1H), 2.39 (s, 3H), 2.31 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 168.6, 167.4, 149.2, 144.6, 143.2, 141.0, 139.2, 137.5, 136.7, 135.9, 133.3, 130.9, 130.7, 130.6, 130.0, 129.2, 128.9, 128.6, 128.4, 126.6, 126.5, 125.4, 125.1, 122.3, 121.5, 116.7, 111.8, 49.9, 21.3, 20.5. Anal. Calcd. For C36H30N2Cl2OTi: C, 69.14; H, 4.83; N, 4.48. Found: C, 69.11; H, 4.87; N, 4.47.
(η5-Cyclopentadienyl)titanium dichloride 2-(1-(2,6-dibenz-hydryl-4-methylphenylimino)methyl)quinolin-8-olate (C2)
Using the same procedure for C1, C2 was prepared by using L2 instead of L1 as brown crystals in 0.49 g (62.1%). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.27 (d, J = 8.1 Hz, 1H), 8.02 (d, J = 8.3 Hz, 1H), 7.48 (t, J = 7.6 Hz, 1H), 7.33 (d, J = 7.9 Hz, 1H), 7.25–7.11 (m, 14H), 7.02 (d, J = 6.0 Hz, 8H), 6.97 (s, Cp, 5H), 6.68 (s, 2H), 5.49 (s, 2H), 2.11 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 168.5, 165.7, 150.7, 147.4, 143.2, 142.5, 136.9, 133.1, 132.4, 129.8, 129.4, 129.8, 129.1, 128.9, 128.1, 128.2, 126.3, 125.9, 118.1, 117.9, 110.4, 53.5, 21.1. Anal. Calcd. For C48H38N2Cl2OTi: C, 74.14; H, 4.93; N, 3.60. Found: C, 74.16; H, 4.95; N, 3.65.
(η5-Cyclopentadienyl)titanium dichloride 2-(1-(2,6-dibenz-hydryl-4-isopropylphenylimino)methyl)quinolin-8-olate (C3)
Using the same procedure for C1, C3 was prepared by using L3 instead of L1 as brown crystals in 56.3% yield (0.46 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.41 (d, J = 8.3 Hz, 1H), 7.89 (t, J = 7.9 Hz, 1H), 7.66 (d, J = 8.3 Hz, 1H), 7.28–7.22 (m, 11H), 7.22–7.16 (m, 4H), 7.12–7.08 (t, J = 7.9 Hz, 4H), 7.01 (s, Cp, 5H), 6.81–6.76 (m, 4H), 6.04 (s, 1H), 2.76–2.71 (m, 1H), 1.07 (d, J = 6.8 Hz, 6H). 13C NMR (CDCl3, 100 MHz, ppm): δ 170.7, 162.8, 148.6, 146.7, 144.9, 144.0, 143.1, 140.8, 137.5, 130.7, 130.2, 129.8, 129.2, 128.5, 128.3, 127.6, 126.7, 126.5, 112.0, 50.5, 33.7, 23.9. Anal. Calcd. For C50H42N2Cl2OTi: C, 74.54; H, 5.25; N, 3.48. Found: C, 74.57; H, 5.21; N, 3.46.
(η5-Cyclopentadienyl)titanium dichloride 2-((2-benzhydryl-4,6-dimethylphenylimino)ethyl)quinolin-8-olate (C4)
Using the same procedure for C1, C4 was prepared by using L4 instead of L1 as brown crystals in 80.1% yield (0.51 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.45 (d, J = 8.6 Hz, 1H), 7.79 (t, J = 8.0 Hz, 1H), 7.49 (d, J = 8.5 Hz, 1H), 7.39–7.33 (m, 3H), 7.25–7.21 (m, 2H), 7.18–7.11 (m, 6H), 7.03 (d, J = 7.4 Hz, 2H), 6.96 (s, 1H), 6.81 (s, Cp, 5H), 6.66 (s, 1H), 2.32 (s, 3H), 2.28 (s, 3H), 1.12 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 171.3, 153.1, 152.8, 145.2, 143.6, 142.4, 137.5, 136.9, 133.1, 132.8, 130.4, 129.5, 129.3, 129.1, 128.8, 128.7, 128.2, 127.4, 126.6, 126.1, 125.4, 119.8, 118.1, 110.6, 54.1, 21.9, 17.2, 16.1. Anal. Calcd. For C37H32N2Cl2OTi: C, 69.50; H, 5.04; N, 4.38. Found: C, 69.54; H, 5.07; N, 4.34.
(η5-Cyclopentadienyl)titanium dichloride 2-(1-(2,6-dibenz-hydryl-4-methylphenylimino)ethyl)quinolin-8-olate (C5)
Using the same procedure for C1, C5 was prepared by using L5 instead of L1 as brown crystals in 41.8% yield (0.33 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.27–8.11 (m, 2H), 7.53 (t, J = 7.9 Hz, 1H), 7.32 (d, J = 8.1 Hz, 1H), 7.29–7.15 (m, 13H), 7.02 (d, J = 6.8 Hz, 4H), 7.00 (d, J = 6.8 Hz, 4H), 6.74 (s, Cp, 5H), 6.71 (s, 2H), 5.23 (s, 2H), 2.17 (s, 3H), 1.04 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 170.6, 154.3, 152.1, 145.7, 144.1, 142.3, 142.9, 138.1, 135.3, 132.8, 132.1, 130.3, 129.4, 129.6, 129.1, 128.8, 128.6, 128.5, 128.1, 126.9, 126.2, 125.9, 119.9, 118.1, 110.4, 53.1, 21.8, 16.3. Anal. Calcd. For C49H40N2Cl2OTi: C, 74.34; H, 5.09; N, 3.54. Found: C, 74.36; H, 5.05; N, 3.51.
(η5-Cyclopentadienyl)titanium dichloride 2-(1-(2,6-dibenz-hydryl-4-isopropylphenylimino)ethyl)quinolin-8-olate (C6)
Using the same procedure for C1, C6 was prepared by using L6 instead of L1 as brown crystals in 55.1% yield (0.45 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.33 (d, J = 11.3 Hz, 1H), 7.76 (t, J = 11.3 Hz, 1H), 7.44 (d, J = 10.9 Hz, 1H), 7.38–7.29 (m, 6H), 7.21–7.14 (m, 6H), 7.10–7.08 (m, 7H), 6.99–6.94 (m, 3H), 6.91 (s, 5H, Cp), 6.84 (s, 2H), 6.68 (s, 1H), 5.85 (s, 1H), 2.84–2.66 (m, 1H), 1.25 (s, 3H), 1.19 (d, J = 9.16 Hz, 3H), 1.05 (d, J = 9.08 Hz, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 171.5, 153.3, 152.6, 146.3, 143.9, 143.1, 142.7, 137.0, 136.3, 131.7, 130.2, 129.4, 129.6, 128.4, 128.2, 128.1, 126.7, 126.2, 126.1, 119.4, 118.0, 110.4, 52.1, 33.1, 24.8, 16.1. Anal. Calcd. For C51H44N2Cl2OTi: C, 74.73; H, 5.41; N, 3.42. Found: C, 74.77; H, 5.46; N, 3.45.
X-ray crystallographic study
Crystals of C2 and C4 suitable for single-crystal X-ray analysis were obtained by laying n-heptane on the dichloromethane solutions. Single-crystal X-ray diffraction for C2 and C4 was performed on a Rigaku RAXIS Rapid IP diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) at 173(2) K. Cell parameters were obtained by global refinement of the positions of all collected reflections. Intensities were corrected for Lorentz and polarization effects and empirical absorption. The structures were solved by direct methods and refined by full-matrix least-squares on F2. All non-hydrogen atoms were refined anisotropically. Structure solution and refinement were performed by using the SHELXL-97 package.64 Crystal data collection and refinement details are given in Table 6.
| |
C2
|
C4
|
| Empirical formula |
C48H38Cl2N2OTi |
C37H32Cl2N2OTi |
| Formula weight |
777.60 |
639.45 |
| Crystal color |
Red |
Red |
|
T/K |
173(2) |
173(2) |
| Wavelength (Å) |
0.71073 |
0.71073 |
| Crystal system |
Orthorhombic |
Monoclinic |
| Space group |
Pbca
|
C2/c |
|
a/Å |
17.529(4) |
53.675(11) |
|
b/Å |
18.752(4) |
10.021(2) |
|
c/Å |
25.656(5) |
24.886(5) |
|
α/° |
90 |
90 |
|
β/° |
90 |
100.29(3) |
|
γ/° |
90 |
90 |
| Volume/Å3 |
8433(3) |
13170(5) |
|
Z
|
8 |
16 |
| Dcalc/Mg m−3 |
1.225 |
1.290 |
|
μ/mm−1 |
0.366 |
0.454 |
|
F(000) |
3232 |
5312 |
| Crystal size/mm |
0.35 × 0.20 × 0.05 |
0.32 × 0.20 × 0.18 |
|
θ range/° |
1.59–25.34 |
0.77−25.00 |
| Limiting indices |
−18 ≤ h ≤ 20, |
−63 ≤ h ≤ 63, |
| −22 ≤ k ≤ 12, |
−11 ≤ k ≤ 11, |
| −30 ≤ l ≤ 20 |
−29 ≤ l ≤ 19 |
| No. of rflns collected |
24720 |
25332 |
| No. of unique rflns |
7661 |
11535 |
|
R
int
|
0.0828 |
0.0639 |
| Completeness to θ/% |
99.3 (θ = 25.34) |
99.5(θ = 25.00) |
| Goodness-of-fit on F2 |
1.153 |
1.069 |
| Final R indices [I > 2σ(I)] |
R1 = 0.1065, |
R1 = 0.0932, |
| wR2 = 0.2577 |
wR2 = 0.2446 |
|
R indices (all data) |
R1 = 0.1428, |
R1 = 0.1193, |
| wR2 = 0.2817 |
wR2 = 0.2632 |
Procedures for ethylene homo- and co-polymerization
A stainless steel autoclave (0.5 L) equipped with a mechanical stirrer and a temperature controller was heated in vacuum at 80 °C and recharged with ethylene three times, then cooled to room temperature under ethylene atmosphere. A toluene solution of the titanium pre-catalyst (with co-monomer) was transferred into the reactor. After the desired reaction temperature was reached and the required amount of co-catalyst (with 100 mL volume in total) was added, the autoclave was immediately pressurized to 10 atm. The ethylene pressure was kept constant during the reaction through the feeding of ethylene. After the required time, the feeding of ethylene was stopped, and the autoclave was placed in a water-ice bath for one hour. The resultant mixture was poured into acidic ethanol containing 10% hydrochloric acid, and the solid polymer was collected and washed with water and ethanol several times and dried under vacuum to constant weight.
Acknowledgements
This work is supported by NSFC No. 20904059 as well as the MOST 863 program No. 2009AA034601. The EPSRC are thanked for the awarded of a travel grant (to CR).
References
- W. P. Long and D. S. Breslow, J. Am. Chem. Soc., 1960, 82, 1953 CrossRef CAS.
- H. Sinn and W. Kaminsky, Adv. Organomet. Chem., 1980, 18, 99 CrossRef CAS.
- H. Sinn, W. Kaminsky and H. J. Vollmer, Angew. Chem., Int. Ed. Engl., 1980, 19, 390 CrossRef.
- H. H. Brintzinger, D. Fischer, R. Mülhaupt, B. Rieger and R. M. Waymouth, Angew. Chem., Int. Ed. Engl., 1995, 34, 1143 CrossRef CAS.
- W. Kaminsky, Macromol. Chem. Phys., 1996, 197, 3907 CrossRef CAS.
- W. Kaminsky and M. Arndt, Adv. Polym. Sci., 1997, 127, 143 CrossRef CAS.
- J. Suhm, J. Heinemann, C. Wörner, P. Müller, F. Stricker, J. Kressler, J. Okuda and R. Mülhaupt, Macromol. Symp., 1998, 129, 1 CrossRef CAS.
- P. J. Shapiro, E. Bunel, W. P. Schaefer and J. E. Bercaw, Organometallics, 1990, 9, 867 CrossRef CAS.
- P. J. Shapiro, W. D. Cotter, W. P. Schaefer, J. A. Labinger and J. E. Bercaw, J. Am. Chem. Soc., 1994, 116, 4623 CrossRef CAS.
- A. L. McKnight and R. M. Waymouth, Chem. Rev., 1998, 98, 2587 CrossRef CAS.
- H. Braunschweig and F. M. Breitling, Coord. Chem. Rev., 2006, 250, 2691 CrossRef CAS.
- J. C. Stevens, F. J. Timmers, D. R. Wilson, G. F. Schmidt, P. N. Nickias, R. K. Rosen, G. W. McKnight and S. Lai, (Dow). Eur Pat Appl., 1991, 0416815A2 Search PubMed.
-
J. A. M. Canich, (Exxon). U S Patent 5026798, 1991 Search PubMed.
- G. J. P. Britovsek, V. C. Gibson and D. F. Wass, Angew. Chem., Int. Ed., 1999, 38, 428 CrossRef CAS.
- V. C. Gibson and S. K. Spitzmesser, Chem. Rev., 2003, 103, 283 CrossRef CAS.
- M. J. Ferreaira and A. M. Martins, Coord. Chem. Rev., 2006, 250, 118 CrossRef.
- K. Nomura, J. Liu, S. Padmanabhan and B. Kitiyanan, J. Mol. Catal. A: Chem., 2007, 267, 1 CrossRef CAS.
- W. Wang, M. Fujiki and K. Nomura, J. Am. Chem. Soc., 2005, 127, 4582 CrossRef CAS.
- K. Nomura, J. Liu, M. Fujiki and A. Takemoto, J. Am. Chem. Soc., 2007, 129, 14170 CrossRef CAS.
- S. Zhang, W. E. Piers, X. Gao and M. Parvez, J. Am. Chem. Soc., 2000, 122, 5499 CrossRef CAS.
- J. Richter, F. T. Edelmann, M. Noltemeyer, H.-G. Schmidt, M. Schmulinson and M. S. Eisen, J. Mol. Catal. A: Chem., 1998, 130, 149 CrossRef CAS.
- R. Vollmerhaus, P. Shao, N. J. Taylor and S. Collins, Organometallics, 1999, 18, 2731 CrossRef CAS.
- L. R. Sita and R. Babcock, Organometallics, 1998, 17, 5228 CrossRef CAS.
- K. C. Jayaratne, R. J. Keaton, D. A. Henningsen and L. R. Sita, J. Am. Chem. Soc., 2000, 122, 10490 CrossRef CAS.
- R. K. J. Bott, D. L. Hughes, M. Schomann, M. Bochmann and S. J. Lancaster, J. Organomet. Chem., 2003, 665, 135 CrossRef CAS.
- J. Huang, B. Lian, Y. Qian, W. Zhou, W. Chen and G. Zheng, Macromolecules, 2002, 35, 4871 CrossRef CAS.
- S. Doherty, R. J. Errington, A. P. Jarvis, S. Collins, W. Clegg and M. R. J. Elsegood, Organometallics, 1998, 17, 3408 CrossRef CAS.
- T. Yasumoto, T. Yamagata and K. Mashima, Organometallics, 2005, 24, 3375 CrossRef CAS.
- T. Yasumoto, T. Yamagata and K. Mashima, Chem. Lett., 2007, 36, 1030 CrossRef CAS.
- J. Kim, J. W. Hwang, Y. Kim, M. H. Lee, Y. Han and Y. Do, J. Organomet. Chem., 2001, 620, 1 CrossRef CAS.
- D. Jones, K. Cavell and W. Keim, J. Mol. Catal. A: Chem., 1999, 138, 37 CrossRef CAS.
- S. Matsui, Y. Tohi, M. Mitani, J. Saito, H. Makio, H. Tanaka, M. Nitabaru, T. Nakano and T. Fujita, Chem. Lett., 1999, 1065 CrossRef CAS.
- H. Makio, N. Kashiwa and T. Fujita, Adv. Synth. Catal., 2002, 344, 477 CrossRef CAS.
- T. Matsugi and T. Fujita, Chem. Soc. Rev., 2008, 37, 1264 RSC.
- H. Makio, H. Terao, A. Iwashita and T. Fujita, Chem. Rev., 2011, 111, 2363 CrossRef CAS.
- J. Tian and G. W. Coates, Angew. Chem., Int. Ed., 2000, 39, 3626 CrossRef CAS.
- J. Tian, P. D. Hustad and G. W. Coates, J. Am. Chem. Soc., 2001, 123, 5134 CrossRef CAS.
- J. B. Edson, Z. G. Wang, E. J. Kramer and G. W. Coates, J. Am. Chem. Soc., 2008, 130, 4968 CrossRef CAS.
- Y. Yoshida, S. Matsui, Y. Takagi, M. Mitani, M. Nitabaru, T. Nakano, H. Tanaka and T. Fujita, Chem. Lett., 2000, 1270 CrossRef CAS.
- Y. Yoshida, J. Mohri, S. Ishii, M. Mitani, J. Saito, S. Matsui, H. Makio, T. Nakano, H. Tanaka, M. Onda, Y. Yamamoto, A. Mizuno and T. Fujita, J. Am. Chem. Soc., 2004, 126, 12023 CrossRef CAS.
- Y. Yoshida, S. Matsui and T. Fujita, J. Organomet. Chem., 2005, 690, 4382 CrossRef CAS.
- D. M. Dawson, D. A. Walker, M. T. Pett and M. Bochmann, J. Chem. Soc., Dalton Trans., 2000, 459 RSC.
- K. Itagaki, M. Fujiki and K. Nomura, Macromolecules, 2007, 40, 6489 CrossRef CAS.
- H. Zhang, D. J. Byun and K. Nomura, Dalton Trans., 2007, 1802 RSC.
- H. Zhang, S. Katao, K. Nomura and J. Huang, Organometallics, 2007, 26, 5967 CrossRef CAS.
- K. Nomura, Dalton Trans., 2009, 8811 RSC.
- S. Liu, W.-H. Sun, Y. Zeng, D. Wang, W. Zhang and Y. Li, Organometallics, 2010, 29, 2459 CrossRef CAS.
- W.-H. Sun, S. Liu, W. Zhang, Y. Zeng, D. Wang and T. Liang, Organometallics, 2010, 29, 732 CrossRef CAS.
- S. Liu, J. Yi, W. Zuo, K. Wang, D. Wang and W.-H. Sun, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3154 CrossRef CAS.
- W. Zuo, M. Zhang and W.-H. Sun, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 357 CrossRef CAS.
- W. Zuo, S. Zhang, S. Liu, X. Liu and W.-H. Sun, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3396 CrossRef CAS.
- W.-H. Sun, M. Shen, W. Zhang, W. Huang, S. Liu and C. Redshaw, Dalton Trans., 2011, 40, 2645 RSC.
- W. Huang, W. Zhang, S. Liu, T. Liang and W.-H. Sun, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1887 CrossRef CAS.
- W. Huang, W.-H. Sun and C. Redshaw, Dalton Trans., 2011, 40, 6802 RSC.
- W. Huang, B. Li, Y. Wang, W. Zhang, L. Wang, Y.-S. Li, W.-H. Sun and C. Redshaw, Catal. Sci. Technol., 2011, 1, 1208 CAS.
- P. Hu, F. Wang and G.-X. Jin, Organometallics, 2011, 30, 1008 CrossRef CAS.
- E. Y. X. Chen and T. J. Marks, Chem. Rev., 2000, 100, 1391 CrossRef CAS.
- S. Koltzenburg, J. Mol. Catal. A: Chem., 1997, 116, 355 CrossRef CAS.
- J. Tian and G. W. Coates, Angew. Chem., Int. Ed., 2000, 39, 3626 CrossRef CAS.
- G. B. Galland, P. Quijada, R. S. Mauler and S. C. de Menezes, Macromol. Rapid Commun., 1996, 17, 607 CrossRef CAS.
- M. R. Seger and G. E. Maciel, Anal. Chem., 2004, 76, 5734 CrossRef CAS.
- C. Capacchione, D. Saviello, A. Avagliano and A. Proto, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 4200 CrossRef CAS.
- X. Li, M. Nishiura, L. Hu, K. Mori and Z. Hou, J. Am. Chem. Soc., 2009, 131, 13870 CrossRef CAS.
-
G. M. Sheldrick, SHELXTL-97, Program for the Refinement of Crystal Structures, University of Göttingen, Germany, 1997 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N functionality in complexes C1–C3 when compared to those observed for the parent ligands L1–L3 (164–165 ppm), whilst there was a 1–3 ppm downfield shift for CCH3
N functionality in complexes C1–C3 when compared to those observed for the parent ligands L1–L3 (164–165 ppm), whilst there was a 1–3 ppm downfield shift for CCH3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the complexes C4–C6cf. to the ligands L4–L6 (168–169 ppm). Elemental analytical data were consistent with complexes of molecular formula CpTiLCl2. Representative molecular structures were confirmed for complexes C2 and C4via single crystal X-ray diffraction.
N in the complexes C4–C6cf. to the ligands L4–L6 (168–169 ppm). Elemental analytical data were consistent with complexes of molecular formula CpTiLCl2. Representative molecular structures were confirmed for complexes C2 and C4via single crystal X-ray diffraction.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at a temperature of 60 °C over 30 min at 10 atm, the activity was observed to be 1.9 × 105 g mol−1 (Ti) h−1 in the presence of MAO, and 6.1 × 105 g mol−1 (Ti) h−1 when using MMAO. Given these results, further investigations were conducted using MMAO as co-catalyst.
1 at a temperature of 60 °C over 30 min at 10 atm, the activity was observed to be 1.9 × 105 g mol−1 (Ti) h−1 in the presence of MAO, and 6.1 × 105 g mol−1 (Ti) h−1 when using MMAO. Given these results, further investigations were conducted using MMAO as co-catalyst.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, were conducted at 60 °C over 30 min (Runs 2–7, Table 2), which indicated that the highest activity (9.4 × 105 g mol−1 (Ti) h−1) was achieved at an Al/Ti ratio of 5000
1, were conducted at 60 °C over 30 min (Runs 2–7, Table 2), which indicated that the highest activity (9.4 × 105 g mol−1 (Ti) h−1) was achieved at an Al/Ti ratio of 5000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. GPC measurements showed that the obtained polyethylenes had lower molecular weights on increasing the molar ratio of Al/Ti (Fig. 3), consistent with the explanation that a higher Al/Ti molar ratio enhances chain transfer to aluminium, i e. termination.57,58
1. GPC measurements showed that the obtained polyethylenes had lower molecular weights on increasing the molar ratio of Al/Ti (Fig. 3), consistent with the explanation that a higher Al/Ti molar ratio enhances chain transfer to aluminium, i e. termination.57,58


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 60 °C under 10 atm over 30 min, all the pre-catalysts C1–C6 were screened for ethylene polymerization (Table 3). The bulkier the ligand used, the lower the activity observed, thus C1 (R1 = Me, R2 = Ph2CH) > C2 (R1 = R2 = Ph2CH), C4 (R1 = Me, R2 = Ph2CH) > C5 (R1 = R2 = Ph2CH), consistent with bulkier ortho-substituents at the arylimino group preventing access of ethylene to the active centre. For the same ortho-substituent at the arylimino group, pre-catalysts possessing a para-isopropyl substituent at the arylimino showed higher activity than did analogues possessing a para-methyl substituent at the arylimino group, i e.C3 (R3 = i-Pr, R1 = R2 = Ph2CH) > C2 (R3 = Me, R1 = R2 = Ph2CH) and C6 (R3 = i-Pr, R1 = R2 = Ph2CH) > C5 (R3 = Me, R1 = R2 = Ph2CH), indicating the better activity was caused by the better soluble para-isopropyl group. In addition, better catalytic activities were observed for the ketimine systems versus the aldimines, i e.C4 (R = Me) > C1 (R = H), C5 (R = Me) > C2 (R = H), C6 (R = Me) > C3 (R = H). These were consistent with our previous observations for half-titanocene dichloride 2-aryliminoquinolin-8-olates, where higher activities were achieved by 2-(1-(arylimino)propyl)quinolin-8-olates54 over 2-(1-(arylimino)ethyl)quinolin-8-olates.55 Besides the better solubility of the complexes bearing ligands with longer alkyl groups, the longer chain alkyl substituents can also be considered better electron-donors capable of stabilizing electron-deficient titanium complexes.59,60
1 at 60 °C under 10 atm over 30 min, all the pre-catalysts C1–C6 were screened for ethylene polymerization (Table 3). The bulkier the ligand used, the lower the activity observed, thus C1 (R1 = Me, R2 = Ph2CH) > C2 (R1 = R2 = Ph2CH), C4 (R1 = Me, R2 = Ph2CH) > C5 (R1 = R2 = Ph2CH), consistent with bulkier ortho-substituents at the arylimino group preventing access of ethylene to the active centre. For the same ortho-substituent at the arylimino group, pre-catalysts possessing a para-isopropyl substituent at the arylimino showed higher activity than did analogues possessing a para-methyl substituent at the arylimino group, i e.C3 (R3 = i-Pr, R1 = R2 = Ph2CH) > C2 (R3 = Me, R1 = R2 = Ph2CH) and C6 (R3 = i-Pr, R1 = R2 = Ph2CH) > C5 (R3 = Me, R1 = R2 = Ph2CH), indicating the better activity was caused by the better soluble para-isopropyl group. In addition, better catalytic activities were observed for the ketimine systems versus the aldimines, i e.C4 (R = Me) > C1 (R = H), C5 (R = Me) > C2 (R = H), C6 (R = Me) > C3 (R = H). These were consistent with our previous observations for half-titanocene dichloride 2-aryliminoquinolin-8-olates, where higher activities were achieved by 2-(1-(arylimino)propyl)quinolin-8-olates54 over 2-(1-(arylimino)ethyl)quinolin-8-olates.55 Besides the better solubility of the complexes bearing ligands with longer alkyl groups, the longer chain alkyl substituents can also be considered better electron-donors capable of stabilizing electron-deficient titanium complexes.59,60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at 60 °C over 30 min under 10 atm of ethylene, whilst at the same time changing the concentration of 1-hexene (Table 4). A positive effect of the co-monomer was observed, leading to better activities for the co-polymerization than for ethylene homo-polymerization. However, the catalytic activities decreased with increasing co-monomer concentration (runs 1–3, Table 4), consistent with the observations for half-titanocene pre-catalysts featuring amidate48 or imino-indolate ligands.51 GPC analysis showed that the molecular weights of the obtained polymers decreased with increasing concentration of 1-hexene, suggesting possibly that chain termination occurred during the insertion of co-monomer. According to DSC analysis, the melting points of the obtained polymers were lower, indicating more branching on increasing the concentration of 1-hexene. Similar catalytic tendencies were also observed for the ethylene/1-octene co-polymerization (Table 4), though the activities for 1-octene were slightly lower than those observed for 1-hexene.
1 at 60 °C over 30 min under 10 atm of ethylene, whilst at the same time changing the concentration of 1-hexene (Table 4). A positive effect of the co-monomer was observed, leading to better activities for the co-polymerization than for ethylene homo-polymerization. However, the catalytic activities decreased with increasing co-monomer concentration (runs 1–3, Table 4), consistent with the observations for half-titanocene pre-catalysts featuring amidate48 or imino-indolate ligands.51 GPC analysis showed that the molecular weights of the obtained polymers decreased with increasing concentration of 1-hexene, suggesting possibly that chain termination occurred during the insertion of co-monomer. According to DSC analysis, the melting points of the obtained polymers were lower, indicating more branching on increasing the concentration of 1-hexene. Similar catalytic tendencies were also observed for the ethylene/1-octene co-polymerization (Table 4), though the activities for 1-octene were slightly lower than those observed for 1-hexene.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) trans = 75.8%
trans = 75.8%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25.2%), without 2-IP-alt-E or 3,4-IP-alt-E units.
25.2%), without 2-IP-alt-E or 3,4-IP-alt-E units.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the second part to elute was collected and concentrated to give a yellow green solid in 69.1% yield (0.63 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.29–8.21 (m, 2H), 8.02 (s, 1H), 7.84 (s, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.37 (d, J = 8.1 Hz, 1H), 7.23–7.05 (m, 7H), 7.04 (d, J = 6.8 Hz, 4H), 6.98 (s, 1H), 6.63 (s, 1H), 5.57 (s, 1H), 2.25 (s, 3H), 2.14 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 164.3, 152.6, 152.1, 147.8, 143.8, 137.7, 136.7, 134.1, 133.4, 129.9, 129.8, 129.7, 129.2, 129.1, 128.6, 128.3, 128.2, 126.2, 126.0, 118.8, 117.9, 110.5, 52.3, 21.2, 18.4. IR (KBr, cm−1): 3410 (m), 2916 (w), 1974 (w), 1636 (m), 1561 (m), 1503 (m), 1469 (s), 1363 (s), 1329 (m), 1299 (m), 1237 (s), 1202 (m), 1135 (m), 1103 (m), 1036 (m), 846 (s), 793 (m), 745 (s), 697 (s). Mp: 88–89 °C. Anal. Calcd. For C31H26N2O: C, 84.13; H, 5.92; N, 6.33. Found: C, 84.11; H, 5.98; N, 6.29.
1), the second part to elute was collected and concentrated to give a yellow green solid in 69.1% yield (0.63 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.29–8.21 (m, 2H), 8.02 (s, 1H), 7.84 (s, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.37 (d, J = 8.1 Hz, 1H), 7.23–7.05 (m, 7H), 7.04 (d, J = 6.8 Hz, 4H), 6.98 (s, 1H), 6.63 (s, 1H), 5.57 (s, 1H), 2.25 (s, 3H), 2.14 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 164.3, 152.6, 152.1, 147.8, 143.8, 137.7, 136.7, 134.1, 133.4, 129.9, 129.8, 129.7, 129.2, 129.1, 128.6, 128.3, 128.2, 126.2, 126.0, 118.8, 117.9, 110.5, 52.3, 21.2, 18.4. IR (KBr, cm−1): 3410 (m), 2916 (w), 1974 (w), 1636 (m), 1561 (m), 1503 (m), 1469 (s), 1363 (s), 1329 (m), 1299 (m), 1237 (s), 1202 (m), 1135 (m), 1103 (m), 1036 (m), 846 (s), 793 (m), 745 (s), 697 (s). Mp: 88–89 °C. Anal. Calcd. For C31H26N2O: C, 84.13; H, 5.92; N, 6.33. Found: C, 84.11; H, 5.98; N, 6.29.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and obtained as a yellow green solid in 46.1% (0.42 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.48 (d, J = 8.6 Hz, 1H), 8.23 (d, J = 8.6 Hz, 1H), 8.07 (s, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.28–7.19 (m, 2H), 7.12–7.10 (m, 6H), 6.99–6.96 (m, 4H), 6.65 (s, 1H), 5.46 (s, 1H), 2.26 (s, 3H), 1.97 (s, 3H), 1.58 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 168.3, 154.0, 152.5, 145.9, 143.8, 142.7, 137.0, 136.4, 133.3, 132.4, 130.0, 129.7, 129.5, 129.1, 128.8, 128.4, 128.2, 127.9, 126.3, 126.2, 125.0, 119.6, 118.0, 110.3, 52.5, 21.2, 17.9, 16.3. IR (KBr, cm−1): 3406 (m), 2916 (w), 2165 (w), 1976 (w), 1637 (m), 1567 (m), 1505 (m), 1465 (s), 1365 (s), 1327 (m), 1298 (m), 1238 (s), 1201 (m), 1138 (m), 1100 (m), 1030 (m), 843 (s), 790 (m), 747 (s), 699 (s). Mp: 92–93 °C. Anal. Calcd. For C32H28N2O: C, 84.18; H, 6.18; N, 6.14. Found: C, 84.12; H, 6.15; N, 6.19.
1) and obtained as a yellow green solid in 46.1% (0.42 g). 1H NMR (CDCl3, 400 MHz, ppm): δ 8.48 (d, J = 8.6 Hz, 1H), 8.23 (d, J = 8.6 Hz, 1H), 8.07 (s, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.39 (d, J = 8.2 Hz, 1H), 7.28–7.19 (m, 2H), 7.12–7.10 (m, 6H), 6.99–6.96 (m, 4H), 6.65 (s, 1H), 5.46 (s, 1H), 2.26 (s, 3H), 1.97 (s, 3H), 1.58 (s, 3H). 13C NMR (CDCl3, 100 MHz, ppm): δ 168.3, 154.0, 152.5, 145.9, 143.8, 142.7, 137.0, 136.4, 133.3, 132.4, 130.0, 129.7, 129.5, 129.1, 128.8, 128.4, 128.2, 127.9, 126.3, 126.2, 125.0, 119.6, 118.0, 110.3, 52.5, 21.2, 17.9, 16.3. IR (KBr, cm−1): 3406 (m), 2916 (w), 2165 (w), 1976 (w), 1637 (m), 1567 (m), 1505 (m), 1465 (s), 1365 (s), 1327 (m), 1298 (m), 1238 (s), 1201 (m), 1138 (m), 1100 (m), 1030 (m), 843 (s), 790 (m), 747 (s), 699 (s). Mp: 92–93 °C. Anal. Calcd. For C32H28N2O: C, 84.18; H, 6.18; N, 6.14. Found: C, 84.12; H, 6.15; N, 6.19.
