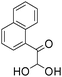
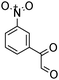
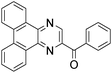
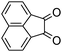
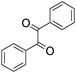
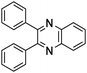
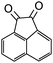
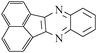
A rapid and eco-friendly synthesis of novel and known benzopyrazines using silica tungstic acid (STA) as a new and recyclable catalyst†
Saeed
Khodabakhshi
*a and
Bahador
Karami
b
aYoung Researchers Club, Gachsaran Branch, Islamic Azad University, Gachsaran, Iran. E-mail: saeidkhm@yahoo.com; Fax: +98 7423332003; Tel: +98 7423332033
bDepartment of Chemistry, Gachsaran Branch, Islamic Azad University, Gachsaran, Iran. E-mail: karamivk@yahoo.com; Fax: +98 7423332003; Tel: +98 7423332033
First published on 31st May 2012
Abstract
A novel silica tungstic acid (STA) as a green catalyst has been prepared and employed for the solvent-free synthesis of novel benzopyrazines. Catalyst loadings as low as 2 mol% could be used leading to high yields of pure products. The STA was characterized by X-ray fluorescence (XRF), X-ray diffraction (XRD), inductively coupled plasma (ICP-OES) and Fourier transform infrared spectroscopy (FT-IR).
Introduction
Loss of catalyst during organic reaction or a work-up process is a significant problem in many of the catalyzed organic transformations. Minimizing catalyst loss and avoidance of organic solvents require a fundamental understanding of green chemistry factors.1,2 These concepts provide direction for improvements in organic synthesis and addressing environmental and economic concerns.Organic synthesis based on green strategies has been widely investigated due to stringent environmental and economic regulations. In fact, methods involving conventional acids are inherently associated with problems such as high toxicity, corrosive and polluting reagents, non-reusability and waste of catalyst, and difficulty in product isolation. Therefore, the use of solid heterogeneous catalysts instead of these conventional acids is desirable to achieve effective catalyst handling, purification of products and to minimize waste production. The aim of this project is the introduction of a novel and safe solid acid and its catalytic application in solvent-free synthesis of novel benzopyrazines. The benzopyrazine scaffold is accessible via condensation reaction of 1,2-dicarbonyls with 1,2-aryldiamines. Some benzopyrazines are found in Nature and they are produced by bacteria such as Pseudomonas spp., Streptomyces spp., and Pantoea agglomerans. These natural products have been implicated in the virulence and competitive fitness of organisms producing them.3,4 Besides, some of them constitute the basis of many insecticides, anti-tumor agents, fungicides, herbicides, and receptor antagonists.5–9
Until now, several strategies have been developed for the synthesis of benzopyrazines including the use of different reagents such as silica bonded s-sulfonic acid (SBSSA),10 (NH4)6Mo7O24·4H2O,11 Fe/Al-MCM-41,12 CeCl3·7H2O,13 Zn[(L)proline],14 polyaniline-sulfate salt.15 However, many of these methods suffer from the absence of green chemistry, and have been associated with several shortcomings such as the use of toxic, expensive and unrecyclable catalysts, long reaction times, low product yields, difficult work-up procedures, and use of organic solvents. Recently, we prepared and used some green solid acids such as silica supported zirconium oxychloride (ZrOCl2·8H2O/SiO2),16 molybdate sulfuric acid (MSA)17 and tungstate sulfuric acid (TSA)18 in organic transformations. In this work, as can be seen in Scheme 1, from the reaction of silica gel and thionyl chloride, silica chloride (1) has been prepared.19 Accordingly, we found that anhydrous sodium tungstate can react with silica chloride (SiO2–Cl 1) to give silica tungstic acid (STA 2).
 | ||
| Scheme 1 Preparation of silica tungstic acid (2). | ||
Experimental
The silica chloride (1) was synthesized according to the published procedure in our laboratory.19 The chemicals were purchased from Merck and Aldrich chemical companies. The reactions were monitored by TLC (silica-gel 60 F254, hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc). Fourier transform infrared (FT-IR) spectroscopy spectra were recorded on a Shimadzu-470 spectrometer, using KBr pellets and the melting points were determined on a KRUSS model instrument. 1H NMR spectra were recorded on a Bruker Avance II 400 NMR spectrometer at 400 MHz, in which CDCl3 was used as solvent and TMS as the internal standard. The X-ray diffraction (XRD) pattern was obtained using a Philips X Pert Pro X diffractometer operated with a Ni-filtered Cu Kα radiation source. X-ray fluorescence (XRF) spectroscopy spectra were recorded using an X-Ray Fluorescence Analyzer, Bruker, S4 Pioneer, Germany. The inductively coupled plasma (ICP-OES) spectra were recorded using a PerkinElmer Optima 7300 DV series.
EtOAc). Fourier transform infrared (FT-IR) spectroscopy spectra were recorded on a Shimadzu-470 spectrometer, using KBr pellets and the melting points were determined on a KRUSS model instrument. 1H NMR spectra were recorded on a Bruker Avance II 400 NMR spectrometer at 400 MHz, in which CDCl3 was used as solvent and TMS as the internal standard. The X-ray diffraction (XRD) pattern was obtained using a Philips X Pert Pro X diffractometer operated with a Ni-filtered Cu Kα radiation source. X-ray fluorescence (XRF) spectroscopy spectra were recorded using an X-Ray Fluorescence Analyzer, Bruker, S4 Pioneer, Germany. The inductively coupled plasma (ICP-OES) spectra were recorded using a PerkinElmer Optima 7300 DV series.
Preparation of silica chloride 1
To an oven-dried (125 °C, vacuum) sample of silica-gel 60 (10 g) in a round bottomed flask (250 mL), equipped with a condenser and a drying tube, was added thionyl chloride (40 mL) and the mixture in the presence of CaCl2 as a drying agent was refluxed for 48 h. The resulting white-grayish powder was filtered and stored in a tightly capped bottle.19Preparation of silica tungstic acid 2
To a mixture of silica chloride (6.00 g) and sodium tungstic (7.03 g) was added n-hexane (10 mL). The reaction mixture was stirred under refluxing conditions for 4 h. After completion of the reaction, the reaction mixture was filtered and washed with distilled water, and dried and then stirred in the presence of 0.1 N HCl (40 mL) for an hour. Finally, the mixture was filtered, washed with distilled water, and dried to afford STA 2.Preparation of benzopyrazine 5
1 mmol of 1,2-dicarbonyl, 0.02 mmol STA and 1 mmol phenylenediamine were mixed and added to a mortar. The mixture was thoroughly ground with a pestle at room temperature. The progress of the reaction was monitored by TLC (AcOEt/hexane, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). After completion of the reaction, the mixture was dissolved in hot ethanol and the catalyst was filtered. The pure product was crystallized from ethanol. The catalyst was washed with diethyl ether, dried at 70 °C for 45 min, and reused in another reaction.
7). After completion of the reaction, the mixture was dissolved in hot ethanol and the catalyst was filtered. The pure product was crystallized from ethanol. The catalyst was washed with diethyl ether, dried at 70 °C for 45 min, and reused in another reaction.
Spectral data of novel compounds
5g: 1H NMR (400 MHz, CDCl3) δ (ppm): 9.42 (s, 1H), 9.13 (s, 1H), 8.58 (d, 1H, J = 7.6 Hz), 8.40 (d, 1H, J = 8 Hz), 8.21 (t, 2H, J = 8.4 Hz), 7.89–7.77 (m, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 149.11, 149.03, 142.51, 142.17, 142.12, 138.51, 133.08, 130.87, 130.50, 130.20, 129.79, 129.30, 124.68, 122.48.5a: 1H NMR (400 MHz, CDCl3) δ (ppm): 9.59 (s, 1H), 8.74 (s, 1H), 8.58 (s, 1H), 8.43 (dd, 1H, J = 8.6, 1.6 Hz), 8.32 (s, 2H), 8.09–8.05 (m, 2H), 7.95–7.93 (m, 3H), 7.68 (t, 1H, J = 7.6 Hz), 7.64–7.55 (m, 4H). 13C NMR (100 MHz, CDCl3) δ (ppm): 195.68, 153.21, 145.21, 144.58, 144.28, 140.63, 137.89, 137.18, 134.44, 133.56, 133.37, 132.95, 132.68, 132.39, 130.34, 130.18, 130.09, 129.25, 129.05, 128.57, 127.89, 127.70, 126.89, 124.41.
5f: 1H NMR (400 MHz, CDCl3) δ (ppm): 9.60 (s, 1H), 9.20 (s, 1H), 9.10 (s, 1H), 8.66–8.59 (m, 2H), 8.48 (d, 1H, J = 8 Hz), 8.38 (d, 1H, J = 8 Hz), 7.85 (t, 1H, J = 8.4 Hz). 13C NMR (100 MHz, CDCl3) δ (ppm): 151.68, 149.13, 148.07, 145.35, 144.67, 140.94, 137.33, 133.36, 131.44, 130.53, 125.76, 124.31, 122.63, 119.99.
5c: 1H NMR (400 MHz, CDCl3) δ (ppm): 9.42 (s, 1H), 8.52 (s, 1H), 8.292 (s, 2H), 7.93 (d, 2H, J = 7.2 Hz), 7.83 (t, 2H, J = 7.6 Hz), 7.67 (d, 1H, J = 7.6 Hz), 7.58–7.51 (m, 3H), 7.14 (dd, 1H, J = 8, 2 Hz), 3.98 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 195.67, 160.44, 153.13, 144.51, 144.13, 140.72, 137.94, 137.65, 137.15, 132.90, 132.33, 130.32, 130.27, 130.17, 130.11, 128.56, 120.13, 116.88, 112.86, 55.54.
5e: 1H NMR (400 MHz, CDCl3) δ (ppm): 9.51 (s, 1H), 9.18 (s, 1H), 8.64 (d, 1H, J = 7.6 Hz), 8.55 (s, 1H), 8.43 (dd, 1H, J = 8, 1.2 Hz), 8.34 (s, 2H), 7.94 (d, 2H, J = 7.2 Hz), 7.83 (t, 1H, J = 8 Hz), 7.69 (t, 1H, 7.2 Hz), 7.58 (t, 2H, 7.6 Hz). 13C NMR (100 MHz, CDCl3) δ (ppm): 195.47, 150.61, 149.09, 143.88, 143.57, 141.22, 138.79, 137.98, 136.93, 133.27, 133.08, 133.24, 130.79, 130.37, 130.24, 130.19, 128.63, 125.21, 122.68.
Results and discussion
The STA (2) was characterized by X-ray fluorescence (XRF), X-ray diffraction (XRD), inductively coupled plasma (ICP-OES) and Fourier transform infrared spectroscopy (FT-IR). The XRF data of STA (2) showed the composition of the catalyst as 81.25 (%w/w) SiO2 and 1.66 (%w/w) WO4 (Table 1, entry 2). The percentage of tungsten (W) in catalyst was also determined by ICP-OES at 8000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K which showed 1.78 (%w/w) tungsten.
000 K which showed 1.78 (%w/w) tungsten.
Also, Fig. 1 shows the XRD pattern of silica tungstic acid (2) which exhibits the presence of the tungstic acid crystalline phase supported on amorphous silica as a broad peak.
 | ||
| Fig. 1 XRD pattern of STA (2). | ||
The FT-IR spectra of anhydrous sodium tungstate, silica chloride, and silica tungstic acid (2) are shown in Fig. 2. The spectrum shows the XRD pattern for the silica tungstic acid (2) which exhibits the presence of the tungstic acid crystalline phase supported on amorphous silica as a broad peak around 22° (2θ) (θ is the Bragg's angle). The three peaks in the 23–25° region of the XRD spectrum could be attributed to the presence and linking of WO3 to the silica gel.20
 | ||
| Fig. 2 Comparison of FT-IR spectra of SiO2, STA (2), and Na2WO4. | ||
The absorptions at 3459, 1636, 1096, 970, 1620, and 799 cm–1 in the catalyst spectrum reveal both bonds in SiO2–Cl and WO4 groups. We evaluated the amounts of tungstic acid supported on SiO2 through two methods including (a) neutralization reaction (titration with 0.1 normal sodium hydroxide) and (b) weight difference between primary solid acid without chloride and new silica tungstic acid (2). After these experiments, we found that 1 gram of catalyst includes 0.05 grams of –OWO3H. Regarding the molecular weight of WO4H (249 g), therefore, 1 gram of catalyst is equal to 0.2 mmoles.
In regard to the importance of novel catalyst applications, after characterization of the catalyst, we investigated the use of STA (2) in synthesis of some benzopyrazines. We found that STA 2 can act as a highly efficient heterogeneous catalyst for the condensation of o-phenylenediamines 3 and 1,2-dicarbonyls 4 under solvent-free conditions to afford benzopyrazine derivatives 5 in excellent yields (Scheme 2).
 | ||
| Scheme 2 Synthesis of novel benzopyrazines using STA (2). | ||
A solvent-free or solid state reaction obviously reduces pollution, and helps to decrease cost due to the simplification of the experimental procedure, work-up technique and saving labour. Interest in the control of chemical processes based on green chemistry has increased remarkably during the past three decades as a response to public concern about the use of hazardous chemical materials. Generally, in this protocol, the STA 2 shows valuable features including: (i) the reaction can be carried out under solvent free conditions, (ii) high thermal stability, and (iii) a small amount of catalyst was used, and therefore this catalyst has made this reaction economic and eco-friendly. In order to optimize the reaction conditions, the synthesis of 5h was chosen as a model. After examination of various amounts of STA in the synthesis of 5h, it was found that this condensation reaction can be efficiently carried out by adding 2 mol% of catalyst at room temperature under solvent-free conditions in a short time span of 5 min. The use of excess amounts of the catalyst did not have a marked influence on the product yield or reaction rate. The probable reason for this is the coordination of excess catalyst to the nitrogen of amine groups. After optimizing the reaction conditions, in order to prove the versatility of this method, different 1,2-dicarbonyls were treated with o-phenylenediamines under solvent-free conditions. The results are summarized in Table 2.
The design and synthesis of recoverable catalysts is a highly challenging interdisciplinary field combining chemistry, materials science, and engineering from economic and environmental points of view. It should be noted that the main disadvantage of many of the reported methods for the synthesis of benzopyrazines is that the catalysts are destroyed in the work-up procedure and cannot be recovered or reused. In this process, the recycled catalyst was used for five cycles during which a little appreciable loss was observed in the catalytic activities (Fig. 3).
 | ||
| Fig. 3 Recyclability of STA (2) in the synthesis of 5h under solvent-free conditions. | ||
Although various kinds of reagents have been developed for the catalysis of condensation of 1,2-aryldiamines with 1,2-dicarbonyls, many of them must be used with organic solvents or require high temperatures. Furthermore, many of these processes require stoichiometric or excess amounts of unrecoverable catalysts or reagents which are not eco-friendly. In order to show the efficiency of the proposed method, in Table 3 some of our results are compared with some of those reported in the literature. It is evident from the results that the use of STA leads to an improved protocol in terms of compatibility with environment, cheapness, reaction time, and yield when compared with the other catalysts.
| Entry | Catalyst (amount) | Conditions | Time (min)/yield (%) |
|---|---|---|---|
| 1 | CeCl3·7H2O (10 mol%) | Glycerine, r.t. | 240/9513 |
| 2 | Zn–proline (10 mol%) | HOAc, r.t. | 10/9514 |
| 3 | Polyaniline-sulfate salt (5 wt%) | 1,2-dichloroethane, r.t. | 20/9515 |
| 4 | Ammonium chloride (50 mol%) | MeOH, r.t. | 7/10021 |
| 5 | Fe/Al-MCM-41 (0.1 g) | CH3CN/reflux | 10/9612 |
| 6 | (NH4)6Mo7O24·4H2O | EtOH/H2O, r.t. | 15/9511 |
| 7 | SBSSA (3.4 mol%) | EtOH/H2O, r.t. | 5/9610 |
| 8 | STA (2 mol%) | Solvent-free, r.t. | 10/95 [this work] |
It should be mentioned that our efforts for the synthesis of benzopyrazines by using aliphatic 1,2-dicarbonyls through this method were unsuccessful. The problem with aliphatic 1,2-dicarbonyls is likely that they can undergo enolization.
Conclusions
In conclusion, we have prepared a new and efficient heterogeneous catalyst and introduced its application for the eco-friendly synthesis of benzopyrazines. The significant aspects of our methodology for the benzopyrazines synthesis are efficiency, generality, high yield, short reaction time, avoidance of organic solvents, recyclability of catalyst, ease of product isolation, and ultimately conformity with the green chemistry protocols.Acknowledgements
The authors are very grateful to Mr Abbas Biglari (Institute for Advanced Studies in Basic Sciences, Zanjan, Iran) for his helpful cooperation, and financial support of this work from the Young Researchers Club, Islamic Azad University of Gachsaran, Iran, is acknowledged.Notes and references
- M. B. Gawande and S. Branco, Green Chem., 2011, 13, 3355–3359 RSC.
- C. J. B. Alejo, C. Fasciani, M. Grenier, J. C. Netto-Ferreira and J. C. Scaiano, Catal. Sci. Technol., 2011, 1, 1506–1511 CAS.
- J. M. Turner and A. J. Messenger, Adv. Microb. Physiol., 1986, 27, 211–275 CrossRef CAS.
- M. McDonald and D. V. Mavrodi, J. Am. Chem. Soc., 2001, 38, 9459–9460 CrossRef.
- H. Budzikiewicz, FEMS Microbiol. Lett., 1993, 104, 209–228 CrossRef CAS.
- N. Sato, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. F. V. Scriven, Pergamon, Oxford, 1996, vol. 6, p. 233 Search PubMed.
- A. Gazit, H. App, G. McMahon, J. Chen, A. Levitzki and F. D. Bohmer, J. Med. Chem., 1996, 39, 2170–2177 CrossRef CAS.
- G. Sakata, K. Makino and Y. Kurasawa, Heterocycles, 1998, 27, 2481–2515 Search PubMed.
- L. E. Seitz, W. J. Suling and R. C. J. Reynolds, J. Med. Chem., 2002, 45, 5604–5606 CrossRef CAS.
- K. Niknam, D. Saberi and M. Mohagheghnejad, Molecules, 2009, 14, 1915–1926 CrossRef CAS.
- A. Hasaninejad, A. Zare, M. R. Mohammadizadeh and Z. Karami, J. Iran. Chem. Soc., 2009, 6, 153–158 CrossRef CAS.
- M. M. Heravi, M. Hosseini, H. A. Oskooie and B. Baghernejad, J. Korean Chem. Soc., 2011, 55, 235–239 CrossRef CAS.
- A. V. Narsaiah and J. K. Kumar, Synth. Commun., 2012, 42, 883–892 CrossRef CAS.
- M. M. Heravi, M. H. Tehrani, K. Bakhtiari and H. A. Oskooie, Catal. Commun., 2007, 8, 1341–1344 CrossRef CAS.
- C. Srinivas, C. Naga, S. S. P. Kumar, V. J. Rao and S. Palaniappan, J. Mol. Catal. A: Chem., 2007, 265, 227–230 CrossRef CAS.
- B. Karami and M. Kiani, Catal. Commun., 2011, 14, 62–67 CrossRef CAS.
- (a) B. Karami, S. Khodabakhshi and M. Nikrooz, Polycyclic Aromat. Compd., 2011, 31, 97–109 CrossRef CAS; (b) B. Karami, Z. Haghighijou, M. Farahi and S. Khodabakhshi, Phosphorus, Sulfur Silicon Relat. Elem., 2012, 187, 754–761 CrossRef CAS.
- (a) B. Karami, S. Khodabakhshi, N. Safikhani and A. Arami, Bull. Korean Chem. Soc., 2012, 33, 123–127 CrossRef CAS; (b) B. Karami, S. Nikoseresht and S. Khodabakhshi, Chin. J. Catal., 2012, 33, 298–301 CrossRef CAS.
- A. Cornelis and P. Laszlo, Synthesis, 1985, 909–918 CrossRef CAS.
- C. Santato, M. Odziemkowski, M. Ulmann and J. Augustynski, J. Am. Chem. Soc., 2001, 123, 10639–10649 CrossRef CAS.
- H. R. Darabi, F. Tahoori, K. Aghapoor, F. Taala and F. J. Mohsenzadeh, J. Braz. Chem. Soc., 2008, 19, 1646–1652 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra of novel compounds. See DOI: 10.1039/c2cy20227a |
| This journal is © The Royal Society of Chemistry 2012 |