Highly efficient and selective hydrogenation of unsaturated carbonyl compounds using Ni–Sn alloy catalysts†
Rodiansono
ab,
Syahrul
Khairi
a,
Takayoshi
Hara
a,
Nobuyuki
Ichikuni
a and
Shogo
Shimazu
*a
aGraduate School of Engineering, Chiba University, 1-33 Yayoi, Inage, Chiba 263-8522, Japan. E-mail: shimazu@faculty.chiba-u.jp; Fax: +81 43 290 3379; Tel: +81 43 290 3379
bDepartment of Chemistry, Lambung Mangkurat University, Jl. A. Yani Km 36 Banjarbaru, Indonesia 70714
First published on 13th June 2012
Abstract
Inexpensive Ni–Sn-based alloy catalysts, both bulk and supported, exhibited high selectivity in the hydrogenation of a wide range of unsaturated carbonyl compounds and produced unsaturated alcohols almost exclusively. For the bulk Ni–Sn alloy catalysts, a relatively high reaction temperature of 453 K was required to achieve an efficient hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O rather than C
O rather than C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. The catalyst that consisted of the Ni–Sn alloy dispersed on TiO2 allowed a remarkable reduction of the reaction temperature to 383 K. Both the Ni3Sn2 and Ni3Sn alloy phases were found to be responsible for the enhancement of the chemoselectivity. The Ni–Sn alloy catalysts were reusable without any significant loss of selectivity.
C. The catalyst that consisted of the Ni–Sn alloy dispersed on TiO2 allowed a remarkable reduction of the reaction temperature to 383 K. Both the Ni3Sn2 and Ni3Sn alloy phases were found to be responsible for the enhancement of the chemoselectivity. The Ni–Sn alloy catalysts were reusable without any significant loss of selectivity.
Introduction
The chemoselective hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in α,β-unsaturated ketones/aldehydes has been extensively studied because the unsaturated alcohols that the reaction forms are important in the production of a variety of fine chemicals.1 The group-9 and group-10 metals, such as Rh, Ir, Ni, Pd, and Pt, are well known to generally hydrogenate the C
O bond in α,β-unsaturated ketones/aldehydes has been extensively studied because the unsaturated alcohols that the reaction forms are important in the production of a variety of fine chemicals.1 The group-9 and group-10 metals, such as Rh, Ir, Ni, Pd, and Pt, are well known to generally hydrogenate the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond more easily than the C
C bond more easily than the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of α,β-unsaturated aldehydes.2 Despite extensive research,3 only Ir-, Os-, and Pt-based catalysts have thus far produced unsaturated alcohols.4 To improve the chemoselective hydrogenation of the C
O bond of α,β-unsaturated aldehydes.2 Despite extensive research,3 only Ir-, Os-, and Pt-based catalysts have thus far produced unsaturated alcohols.4 To improve the chemoselective hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, the modification of the previously mentioned metals is necessary, i.e., the addition of more electropositive metals5 or the use of oxide supports that strongly interact with the active metals.6 Although these modified catalyst systems have been effective, catalyst preparation critically depends on the precise control of the amounts of the second metal.7 Recently, the tin alloying of the Pt-group metals has been extensively studied and widely applied in various chemical transformations.8 Delbecq et al. have suggested that an increase in the charge density of Pt metals by the addition of hyperelectronic metals or by the formation of a metal alloy could enhance the affinity towards C
O group, the modification of the previously mentioned metals is necessary, i.e., the addition of more electropositive metals5 or the use of oxide supports that strongly interact with the active metals.6 Although these modified catalyst systems have been effective, catalyst preparation critically depends on the precise control of the amounts of the second metal.7 Recently, the tin alloying of the Pt-group metals has been extensively studied and widely applied in various chemical transformations.8 Delbecq et al. have suggested that an increase in the charge density of Pt metals by the addition of hyperelectronic metals or by the formation of a metal alloy could enhance the affinity towards C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O rather than towards the C
O rather than towards the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond to form unsaturated alcohols in the hydrogenation of α,β-unsaturated aldehydes.9 However, precious metals such as Pt were utilised in these catalyst systems. Therefore, alternative economical and eco-friendly heterogeneous catalysts that would ensure the preferred hydrogenation of the C
C bond to form unsaturated alcohols in the hydrogenation of α,β-unsaturated aldehydes.9 However, precious metals such as Pt were utilised in these catalyst systems. Therefore, alternative economical and eco-friendly heterogeneous catalysts that would ensure the preferred hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group over C
O group over C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C are highly desired.
C are highly desired.
Catalysts based on Ni, which is also a Pt-group metal, would be good candidates because of the similarity of their catalytic behaviour to that of Pt, and such catalysts have been widely used for numerous chemical reactions both in the laboratory and in industry.10 A few reports have shown that tin alloyed with Ni exhibited a unique catalytic performance for the hydrogenation of alkyne,11 the dehydrogenation of cyclohexane,12 reforming,13 oxidation,14 and carbonylation.15 Unlike tin alloyed with Pt, the utilisation of Ni–Sn alloy-based catalysts for the selective hydrogenation of unsaturated carbonyl compounds has been rarely investigated thus far.16
Recently, we reported the synthesis of Ni–Sn alloy catalysts supported on aluminium hydroxide (Ni–Sn/AlOH) by the hydrothermal treatment of a mixture of RANEY®nickel supported on aluminium hydroxide (R-Ni/AlOH) and SnCl2·2H2O solution. We subsequently applied the catalysts to the chemoselective hydrogenation of various unsaturated carbonyl compounds.17 We found that the chemoselectivity of Ni could be controlled precisely by changing the Ni/Sn ratio to form a Ni–Sn alloy that might play a key role in the enhancement of the chemoselectivity.
In the present report, we have extended our study to the preparation of Ni–Sn alloy catalysts supported on various inorganic compounds such as Al2O3, aluminium hydroxide (AlOH), active carbon (AC), SiO2, and TiO2. Both bulk and supported Ni–Sn alloy catalysts were prepared via the hydrothermal treatment of a solution that contained Ni and Sn species at 423 K for 24 h followed by H2 treatment at 573–873 K for 90 min. The effects of the Ni/Sn ratio and the supports on the activity and the selectivity in the hydrogenation of furfural (FFald) and various unsaturated carbonyl compounds were studied.
Results and discussion
Catalyst characterisations
Three classes of bulk Ni–Sn alloy catalysts (denoted Ni–Sn(x), x = Ni/Sn feeding ratio) were prepared via the hydrothermal treatment of solutions that contained Ni and Sn species with various Ni/Sn ratios. The physicochemical properties of the bulk and the supported Ni–Sn alloys are summarised in Table 1.| Entry | Catalysta | Compositionb/mol% | Major alloy phasec | H2d/μmol g−1 | S BET e/m2 g−1 | D f/nm |
|---|---|---|---|---|---|---|
| a The value in the parentheses is Ni/Sn ratio. b Determined by ICP-AES. c Based on the crystallographic databases,18 and mol% of alloy component was calculated by the Multi-Rietveld Analysis Program LH-Riet 7.00 method on the Rietica software.19 d H2 uptake at 273 K (noted after corrected for physical and chemical adsorption). e BET specific surface areas, determined by N2 physisorption at 77 K. f The average Ni–Sn crystallite sizes derived from the Scherrer's equation. g Ni3Sn(201). h Ni3Sn2(101). i Ni3Sn4(112). j Ni(111). | ||||||
| 1 | Ni–Sn(3.0) | Ni74.9Sn25.1 | Ni3Sn (66%) | 12.0 | 5 | 13g |
| 2 | Ni–Sn(1.5) | Ni59.9Sn40.1 | Ni3Sn2 (91%) | 8.6 | 12 | 17h |
| 3 | Ni–Sn(0.75) | Ni42.7Sn57.3 | Ni3Sn4 (87%) | 4.7 | 57 | 23i |
| 4 | Ni–Sn(1.5)/Al2O3 | Ni60.2Sn39.8 | Ni3Sn2 | 9.1 | 94 | 16h |
| 5 | Ni–Sn(1.5)/AlOH | Ni60.0Sn40.0 | Ni3Sn2 | 9.0 | 120 | 15h |
| 6 | Ni–Sn(1.5)/AC | Ni61.2Sn38.8 | Ni3Sn2 | 13.0 | 676 | 17h |
| 7 | Ni–Sn(1.5)/SiO2 | Ni58.7Sn41.3 | Ni3Sn2 | 13.5 | 234 | 10h |
| 8 | Ni–Sn(1.5)/TiO2 | Ni60.4Sn39.6 | Ni3Sn2 | 13.0 | 52 | 6h |
| 9 | R–Ni/AlOH | Ni47.6Sn52.4 | — | 104 | 151 | 11j |
Based on the ICP-AES analyses, the compositions of the bulk and supported Ni–Sn alloys were approximately equivalent to the feeding ratios of each precursor and were reflected in the composition of each Ni–Sn alloy phase (Table 1, entries 1–3). In the case of Ni–Sn(3.0), the major alloy phase was Ni3Sn (Fig. 1a), whereas Ni–Sn(1.5) and Ni–Sn(0.75) gave Ni3Sn2 and Ni3Sn4 as the major alloys formed, respectively (Fig. 1b and c).18 The simulated calculation using the multi-Rietveld analysis program LH-Riet in the Reitica software package19 for each of the XRD patterns of the synthesised bulk Ni–Sn alloy catalysts was performed to estimate the proportions of the different Ni–Sn alloy phases formed; the profiles are shown in Fig. S1–S3, ESI.†
 | ||
| Fig. 1 XRD patterns of the synthesised bulk Ni–Sn alloy catalysts after H2 treatment at 673 K with Ni/Sn ratios of (a) 3.0, (b) 1.5, and (c) 0.75.(★) Ni3Sn. (⊗) Ni3Sn2. (●) Ni3Sn4. | ||
The major alloy components were approximately 66% Ni3Sn for Ni–Sn(3.0), 91% Ni3Sn2 for Ni–Sn(1.5), and 87% Ni3Sn4 for Ni–Sn(0.75) after the treatment with H2 at 673 K. Komatsu et al. reported the formation of bulk Ni3Sn2 from the arc melting of Ni/Sn mixtures at 1433 K,11a whereas Masai et al. have reported the formation of a mixture of Ni3Sn, Ni3Sn2 and Ni3Sn4 alloys by H2 treatment at 773 K.12d In general, a Ni3Sn2 alloy is formed at temperatures greater than 1730 K because the melting points of Ni and Sn are 1730 K and 505 K, respectively.11a Ni3Sn, Ni3Sn2, and Ni3Sn4 alloy phases were successfully synthesised at relatively lower temperature using our simple method.
The H2 uptake, the BET surface area, and the average Ni–Sn crystallite sizes are also summarised in Table 1. With increasing Sn content (decreasing Ni/Sn ratio), the H2 uptake decreased, whereas the BET surface area and the average Ni–Sn crystallite sizes increased (Table 1, entries 1–3). The average Ni–Sn crystallite sizes of Ni3Sn, Ni3Sn2, and Ni3Sn4 were 13, 17, and 27 nm, respectively, which were comparable to the previous report.12b The H2 uptakes of bulk Ni3Sn, Ni3Sn2, and Ni3Sn4 were 12.0 μmol g−1, 8.6 μmol g−1, and 4.7 μmol g−1, respectively. Our results are consistent with the previous reports of Komatsu et al. that Ni3Sn exhibited a H2 uptake greater than that of Ni3Sn2 or Ni3Sn4.12c
Five types of supports (Al2O3, AlOH, AC, SiO2, and TiO2) were employed for the preparation of the supported Ni–Sn(1.5) alloy catalysts using a procedure similar to that used for the synthesis of the bulk phases. The physicochemical properties of the supported Ni–Sn(1.5) alloy catalysts are also summarised in Table 1 (entries 4–8), and the XRD patterns are shown in Fig. 2. The total loading amount of Ni–Sn was 2.3∼2.4 mmol g−1 (based on the ICP-AES results) for all of the supported Ni–Sn(1.5) samples (the composition (mol%) of Ni and Sn are listed in Table 1). The H2 uptake and the average Ni3Sn2 alloy crystallite sizes for Ni–Sn(1.5)/Al2O3 and Ni–Sn(1.5)/AlOH were almost equal to that of the bulk alloy (Table 1, entries 4–5), while the H2 uptakes for Ni–Sn(1.5)/AC, Ni–Sn(1.5)/SiO2, and Ni–Sn(1.5)/TiO2 were 13.0, 13.5, and 13.0 μmol g−1, respectively (entries 6–8). The XRD patterns also revealed that Ni3Sn2, a major alloy phase, was formed on the Al2O3, AlOH, and AC supports (Fig. 2a–c). In contrast, the XRD patterns Ni–Sn(1.5)/SiO2 and Ni–Sn(1.5)/TiO2 exhibited broadened peaks at 2θ = 30.8°, 42.5°, and 44.2°, which correspond to the Ni3Sn2(101), Ni3Sn2(102), and Ni3Sn2(110) diffraction peaks, respectively.18 These results suggest that the higher dispersions of the Ni–Sn alloy on the SiO2 and TiO2 were formed as roughly depicted in the average Ni3Sn2(101) crystallite sizes, which were 10 nm and 6 nm, respectively (Table 1, entries 7 and 8). In conclusion, the XRD analysis and H2 measurement results confirm that Ni–Sn alloy phases were also formed on the supports and that their characteristics were consistent with the results observed for the bulk material.
 | ||
| Fig. 2 XRD patterns of Ni–Sn(1.5) on various supports of (a)Al2O3, (b) AlOH, (c) AC, (d) SiO2, and (e) TiO2. | ||
Catalytic reactions
Results for the selective hydrogenation of FFald using bulk and supported Ni–Sn(1.5) alloy catalysts are summarised in Table 2, and the reaction pathways are shown in Scheme 1.| Entry | Catalyst | Conversion/% | Yielda/% | Selectivityb/% |
|---|---|---|---|---|
| Reaction conditions: FFald, 1.1 mmol; (FFald/Ni ratio = 15); iso-PrOH, 3 mL; H2, 3.0 MPa, 383 K, 75 min.a Yield of FFalc, determined by GC using an internal standard technique.b Selectivity to FFalc. The value in the parantheses is the selectivity to THFalc.c At 433 K.d At 403 K. | ||||
| 1 | Ni–Sn(3.0) | 72 | 70 | 97(3) |
| 2c | Ni–Sn(1.5) | 67 | 67 | 100(0) |
| 3c | Ni–Sn(0.75) | 16 | 12 | 75(25) |
| 4 | Ni–Sn(1.5)/Al2O3 | 85 | 84 | 99(1) |
| 5 | Ni–Sn(1.5)/AlOHd | 67 | 67 | 100(0) |
| 6 | Ni–Sn(1.5)/AC | 72 | 72 | 100(0) |
| 7 | Ni–Sn(1.5)/SiO2 | 32 | 62 | 99(1) |
| 8 | Ni–Sn(1.5)/TiO2 | >99 | >99 | 100(0) |
| 9d | R–Ni/AlOH | >99 | >99 | 0(100) |
| 10 | Sn/AlOH | 0 | 0 | 0 |
 | ||
| Scheme 1 Reaction pathways of FFald hydrogenation by Ni–Sn alloy catalysts. | ||
On the Ni–Sn(3.0) alloy catalyst, FFald conversion was 72% with a furfuryl alcohol (FFalc) yield of 70% (Table 2 entry 1), whereas, on the Ni–Sn(1.5) alloy, a 67% yield of FFalc was obtained without tetrahydrofurfuryl alcohol (THFalc) formation (entry 2). In contrast, the Ni–Sn(0.75) alloy gave only a 12% yield of FFalc (75% selectivity) (entry 3). These results suggest that the presence of Ni3Sn and Ni3Sn2 alloy catalysts played an important role in the selective hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O rather than C
O rather than C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups in the unsaturated carbonyl compounds.17
C groups in the unsaturated carbonyl compounds.17
Screening tests using supports other than the five types in Table 2 were also performed and resulted in insufficient results.20 For catalysts supported on AlOH, Al2O3, SiO2, and AC, relatively high FFald conversions and yields of FFalc were obtained (Table 2, entries 4–7). For the Ni–Sn(1.5)/Al2O3 catalyst, FFald conversion was 85% with a FFalc yield of 84% (Table 2, entry 4), whereas the Ni–Sn(1.5)/AlOH, Ni–Sn(1.5)/AC, and Ni–Sn(1.5)/SiO2 catalysts produced FFalc yields of 67%, 72%, and 62%, respectively (Table 2, entries 5–7). A remarkably high FFald conversion (>99%) and FFalc selectivity (100%) were obtained when Ni–Sn(1.5)/TiO2 was used under the same conditions (Table 2, entry 8). The high conversion of FFald and the high selectivity of FFalc over Ni–Sn(1.5)/TiO2 can be attributed to the relatively high dispersion of the Ni–Sn alloy on TiO2 giving rise to active sites with a significantly higher catalytic activity. Alternatively, the high conversion and selectivity may be a result of the strong interactions between the active metals and TiO2 generating significant interactions between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups and Ni–TiOx sites and leading to high selectivity to unsaturated alcohols.6a Kijenski et al. have reported that Pt catalysts supported on TiO2 gave higher selectivity to FFalc in the hydrogenation of FFald than did Pt supported on SiO2, ZrO2 or MgO.6f Recently, Corma et al. studied the chemoselectivity of Ni supported on TiO2 in the hydrogenation of substituted nitroaromatics.6f Moreover, the monometallic R-Ni/AlOH catalyst converted FFald to give >99% THFalc, which indicates that R-Ni/AlOH hydrogenated both C
O groups and Ni–TiOx sites and leading to high selectivity to unsaturated alcohols.6a Kijenski et al. have reported that Pt catalysts supported on TiO2 gave higher selectivity to FFalc in the hydrogenation of FFald than did Pt supported on SiO2, ZrO2 or MgO.6f Recently, Corma et al. studied the chemoselectivity of Ni supported on TiO2 in the hydrogenation of substituted nitroaromatics.6f Moreover, the monometallic R-Ni/AlOH catalyst converted FFald to give >99% THFalc, which indicates that R-Ni/AlOH hydrogenated both C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of FFald (entry 9), whereas Sn/AlOH was not active for the hydrogenation of FFald under the same conditions (entry 10). These results suggest that the addition of tin to form a Ni–Sn alloy retards the C
O of FFald (entry 9), whereas Sn/AlOH was not active for the hydrogenation of FFald under the same conditions (entry 10). These results suggest that the addition of tin to form a Ni–Sn alloy retards the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogenation activity of nickel. Swift et al. have reported that the formation of a Ni–Sn alloy by the addition of tin to a Ni/SiO2 catalyst remarkably changed the reactivity of Ni/SiO2 because of the change in the electron density of nickel metal.12f Delbecq et al. indicated that the C
C hydrogenation activity of nickel. Swift et al. have reported that the formation of a Ni–Sn alloy by the addition of tin to a Ni/SiO2 catalyst remarkably changed the reactivity of Ni/SiO2 because of the change in the electron density of nickel metal.12f Delbecq et al. indicated that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O hydrogenation selectivity in the hydrogenation of α,β-unsaturated aldehydes could be enhanced by the formation of a Pt–Sn alloy because of the higher affinity of the alloy towards C
O hydrogenation selectivity in the hydrogenation of α,β-unsaturated aldehydes could be enhanced by the formation of a Pt–Sn alloy because of the higher affinity of the alloy towards C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O rather than towards C
O rather than towards C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, as noted previously.9 Resasco et al. have reported that the selective hydrogenation of C
C bonds, as noted previously.9 Resasco et al. have reported that the selective hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O versus C
O versus C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in α,β-unsaturated aldehydes by a Pd–Cu alloy supported on silica was caused by the preferential η2-coordination of C
C in α,β-unsaturated aldehydes by a Pd–Cu alloy supported on silica was caused by the preferential η2-coordination of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to Pd.21
O to Pd.21
Effect of reaction temperature
The influence of reaction temperature on the yield of FFalc over bulk Ni–Sn(1.5), Ni–Sn(1.5)/AlOH, and Ni–Sn(1.5)/TiO2 alloy catalysts is shown in Fig. 3. Differences in the activity of each catalyst were clearly observed. On the bulk Ni–Sn(1.5) catalyst, the yield of FFalc gradually increased as temperature was increased, and complete conversion of FFald (∼99%) was achieved at 453 K. In the case of Ni–Sn(1.5)/AlOH, FFald was converted completely at 413 K. Surprisingly, Ni–Sn(1.5)/TiO2 demonstrated a remarkably high FFald conversion of >99% (>99% FFalc yield) at a significantly lower temperature of 383 K. Therefore, we conclude that the optimised reaction temperatures for FFald hydrogenation using bulk Ni–Sn(1.5), Ni–Sn(1.5)/AlOH, and Ni–Sn(1.5)/TiO2, were 453 K, 413 K, and 383 K, respectively. | ||
| Fig. 3 Effect of reaction temperature on the FFalc yield over the bulk and supported Ni–Sn(1.5) alloy catalysts. Reaction conditions: catalyst, 0.05 g; FFald, 1.1 mmol; iso-PrOH, 3 mL; H2, 3.0 MPa, 75 min. | ||
Effect of initial H2 pressure
The effect of the initial H2 pressure on the FFald conversion and product selectivity is shown in Fig. 4. FFald conversion and FFalc selectivity gradually increased as the initial H2 pressure increased, whereas the THFalc selectivity decreased smoothly to almost 0% between 2.5 and 3.0 MPa. | ||
| Fig. 4 Effect of initial H2 pressure on the conversion and selectivity in the hydrogenation of FFald over Ni–Sn(1.5)/TiO2 alloy catalyst. Reaction conditions: catalyst, 0.05 g; FFald, 1.1 mmol; iso-PrOH, 3 mL; 383 K, 75 min. | ||
Time profiles
The reaction profiles of FFald hydrogenation at 383 K on the bulk and supported Ni–Sn(1.5) alloy catalysts are shown in Fig. 5. When the bulk Ni–Sn(1.5) catalyst was used, FFalc was formed after a reaction time of 75 min and then gradually increased to achieve 45% FFalc yield after 180 min. The induction periods could be associated to the slow formation of oxidic tin (Snn+) from metallic tin (Sn0) as reported by Sordelli et al. (Rh–Sn)22 and Margitfalvi et al. (Pt–Sn).23 Since the crystallite size or dispersion of the Ni–Sn alloy could affect the length of induction period, Ni–Sn(1.5)/TiO2 showed a high activity at lower temperature (Fig. 3) without an induction period (Fig. 5). In the case of supported Ni–Sn(1.5)/AlOH catalyst, the induction period slightly diminished and 100% FFald conversion (>99% FFalc yield) was achieved after 120 min. Notably, the supported Ni–Sn(1.5)/TiO2 demonstrated a conversion of FFald 1.5 times greater than that of the Ni–Sn(1.5)/AlOH and approximately 50 times greater than that of the bulk catalyst. Furthermore, over the Ni–Sn(1.5)/TiO2 catalyst, the 100% selectivity of FFalc was retained when the reaction temperature was increased to 453 K (Fig. 3) or when the reaction time was extended to 180 min (Fig. 5).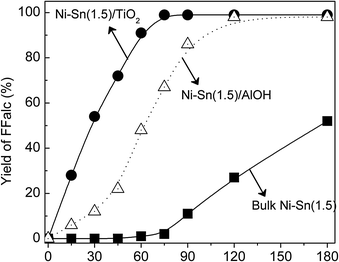 | ||
| Fig. 5 Time profile of the hydrogenation of FFald over the bulk and the supported Ni–Sn(1.5) alloy catalysts. Reaction conditions: catalyst, 0.05 g; FFald, 1.1 mmol; iso-PrOH, 3 mL; H2, 3.0 MPa, 383 K. | ||
Reusability test
A reusability test was performed on the Ni–Sn(1.5)/TiO2 catalyst, and the results are summarised in Table 3.| Run | 1 | 2 | 3 | 4 | 5 | 6a |
|---|---|---|---|---|---|---|
| Reaction conditions: FFald, 1.1 mmol; (FFald/Ni ratio = 15); iso-PrOH (3 mL); H2, 3.0 MPa, 383 K, 75 min.a The used catalyst was treated by H2 at 673 K for 1 h before reaction.b Selectivity to FFalc, determined by GC using an internal standard technique. | ||||||
| Conversion (%) | >99 | 62 | 51 | 46 | 43 | >99 |
| Selectivityb (%) | 100 | 96 | 97 | 99 | 99 | 99 |
The used Ni–Sn(1.5)/TiO2 catalyst was easily separated by either simple centrifugation or filtration after the reaction. The activity of the catalyst decreased while the high selectivity was maintained for at least five consecutive runs. The amount of Ni and Sn that leached into the reaction solution was 0.58% and 1.3% after four runs, respectively. Treatment of the used Ni–Sn(1.5)/TiO2 catalyst (after five runs) with H2 at 673 K for 1 h restored the catalyst's original activity and selectivity.24
Hydrogenation of various unsaturated carbonyl compounds
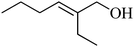
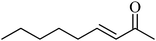
A comparison of the activity and selectivity of the bulk Ni–Sn(1.5) and supported Ni–Sn(1.5)/TiO2 alloy catalysts in the hydrogenation of various α,β-unsaturated aldehydes and ketones is summarised in Table 4.| Entry | Substrate | Product | Temp./K | Bulk Ni–Sn(1.5) | Ni–Sn(1.5)/TiO2 | ||
|---|---|---|---|---|---|---|---|
| Conv.a (%) | Select.a (%) | Conv.a (%) | Select. a(%) | ||||
| Reaction conditions: Substrate/Ni = 15; iso-PrOH, 3 mL; H2, 3MPa; time, 75 min.a Conversion and selectivity were determined by GC using an internal standard technique. | |||||||
| 1 |

|

|
383 | 29 | 90 | 98 | 94 |
| 2 |

|
|
403 | 32 | 73 | 93 | 96 |
| 3 |

|

|
403 | 34 | 89 | >99 | 90 |
| 4 |

|

|
403 | 28 | 80 | >99 | 91 |
| 5 |

|

|
403 | 45 | 74 | 70 | 88 |
| 6 |

|

|
383 | 13 | 100 | 41 | 100 |
| 7 |

|

|
403 | 78 | 97 | 65 | 100 |
| 8 |
|

|
383 | 52 | 89 | 88 | 96 |
| 9 |

|

|
383 | 78 | 89 | 91 | 73 |
| 10 |

|

|
383 | 49 | 100 | 89 | 100 |
The catalytic activity of supported Ni–Sn(1.5)/TiO2 in the hydrogenation of various α,β-unsaturated aliphatic aldehydes, including citronellal, 2-ethyl-2-hexenal, trans-2-octenenaldehyde, trans-2-hexenaldehyde, and crotonaldehyde, was approximately 1.5–3.5 times higher than that of the bulk Ni–Sn(1.5) (Table 4, entries 1–5). For example, the hydrogenation of citronellal over bulk Ni–Sn(1.5) gave 29% conversion (90% selectivity), whereas the same reaction over supported Ni–Sn(1.5)/TiO2 gave 98% conversion (94% selectivity) at 383 K (Table 4, entry 1). The hydrogenation of cinnamaldehyde over bulk Ni–Sn(1.5) and supported Ni–Sn(1.5)/TiO2 catalysts gave notable conversions of 13% and 41%, respectively, at 383 K with 100% selectivity to cinnamyl alcohol (entry 6). In addition, the hydrogenation of 3-cyclohexencarboxaldehyde over the bulk Ni–Sn(1.5) alloy gave 78% conversion (97% selectivity), and the same reaction over supported Ni–Sn(1.5)/TiO2 gave 65% conversion (100% selectivity) at 403 K (entry 7).
In the case of the hydrogenation of the α,β-unsaturated ketone 2-nonene-2-one over bulk Ni–Sn(1.5) and supported Ni–Sn(1.5)/TiO2 catalysts, 52% and 88% conversions were achieved, respectively, and the desired product of 2-nonene-2-ol was produced with selectivities of 89% and 96%, respectively (entry 8). In the hydrogenation of 2-cyclohexen-1-one, relatively high conversions of 78% (89% selectivity) and 91% (73% selectivity) were achieved for the bulk and supported catalysts, respectively. Notable selectivities towards cyclohexanone of 21% and 27% over the bulk and supported Ni–Sn(1.5)/TiO2 catalysts, respectively, were obtained at 383 K (entry 9). In addition, hydrogenation of the aromatic ketone acetophenone over both the bulk and supported Ni–Sn(1.5)/TiO2 alloy catalysts at 383 K gave a high selectivity to 1-phenylethanol (100%) at conversion rates of 78% and 89%, respectively (entry 10). Based on these results, Ni–Sn alloy catalysts can be concluded to be promising catalysts for the selective hydrogenation of a wide range of α,β-unsaturated aldehydes and ketones into the corresponding unsaturated alcohols.
Conclusions
Both bulk and supported Ni–Sn alloy catalysts were successfully synthesised using a simple method at a relatively low temperature. The dispersion of the Ni–Sn(1.5) alloy on TiO2 enhanced its activity 50-fold at a significantly lower temperature (383 K) compared to the activity of the bulk catalyst (453 K) in the selective hydrogenation of FFald. The Ni–Sn(1.5) alloy was also found to be reusable without any significant loss of selectivity. The activity of the used Ni–Sn alloy catalyst can be restored to the original performance after H2 treatment at 673 K. The hydrogenation of various unsaturated carbonyl compounds using both bulk and supported Ni–Sn alloy catalysts showed high selectivity towards the almost exclusive production of unsaturated alcohols.Experimental
General
Nickel(II) chloride hexahydrate (NiCl2·6H2O), tin(II) chloride dihydrate (SnCl2·2H2O), TiO2, active carbon (SBET = 815 m2 g−1), and aluminium hydroxide were purchased and used as received from WAKO Pure Chemical Industries, Ltd. unless otherwise stated. SiO2 (SBET = 200 m2 g−1) and Al2O3 (Al2O3, SBET = 100 m2 g−1) were purchased from Japan Aerosil Co.All organic chemical compounds were purified using standard procedures prior to use.
Catalyst preparation
A typical procedure for the synthesis of a bulk Ni–Sn (1.5 feeding ratio) alloy catalyst is described as follows: NiCl2·6H2O (7.2 mmol) was dissolved in deionised water (denoted as solution A), and SnCl2·2H2O (4.8 mmol) was dissolved in ethanol/2-methoxy ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (denoted as solution B) at room temperature. Solutions A and B were mixed at room temperature; the temperature was subsequently raised to 323 K and the mixture was stirred for 12 h. The pH of the mixture was adjusted to 12 through the dropwise addition of an aqueous solution of NaOH (3.1 M). The mixture was then placed into a sealed-Teflon autoclave for the hydrothermal reaction at 423 K for 24 h. The resulting black precipitate was filtered, washed with distilled water, and then dried under vacuum overnight. Prior to the catalytic reaction, the obtained black powder was treated under hydrogen at 673 K for 90 min.
1) (denoted as solution B) at room temperature. Solutions A and B were mixed at room temperature; the temperature was subsequently raised to 323 K and the mixture was stirred for 12 h. The pH of the mixture was adjusted to 12 through the dropwise addition of an aqueous solution of NaOH (3.1 M). The mixture was then placed into a sealed-Teflon autoclave for the hydrothermal reaction at 423 K for 24 h. The resulting black precipitate was filtered, washed with distilled water, and then dried under vacuum overnight. Prior to the catalytic reaction, the obtained black powder was treated under hydrogen at 673 K for 90 min.
Characterisation
Analytical GLC was performed on a Shimadzu GC-8A equipped with a flame ionisation detector and with Thermon 3000 and Silicone OV-101 packing. A Shimadzu 14A with a flame ionisation detector equipped with a RT-βDEXsa capillary column was used for product analyses of the hydrogenations of α,β-unsaturated aldehydes and ketones. GC-MS was performed on a Shimadzu GC-17B equipped with a thermal conductivity detector and with an RT-βDEXsm capillary column. 1H and 13C NMR spectra were obtained on a JNM-AL400 spectrometer at 400 MHz; samples for NMR were dissolved in chloroform-d1 with TMS as an internal standard. Products were confirmed by the comparison of their GC retention time, mass, 1H and 13C NMR spectra with those of authentic samples. XRD measurements were recorded on a Mac Science M18XHF instrument using monochromatic Cu Kα radiation (λ = 0.15418 nm). The XRD was operated at 40 kV and 200 mA with a step width of 0.02° and a scan speed of 4° min−1 (α1 = 0.154057 nm, α2 = 0.154433 nm). ICP measurements were performed on an SPS 1800H plasma spectrometer of Seiko Instruments Inc. (Ni: 221.7162 nm and Sn: 189.898 nm). The BET surface area (SBET) and pore volume (Vp) were measured using N2 physisorption at 77 K on a Belsorp Max (BEL Japan). The samples were degassed at 473 K for 2 h to remove physisorbed gases prior to the measurement. The amount of nitrogen adsorbed onto the samples was used to calculate the BET surface area via the BET equation. The pore volume was estimated to be the liquid volume of nitrogen at a relative pressure of approximately 0.995 according to the Barrett–Joyner–Halenda (BJH) approach based on desorption data.25 SEM images of the synthesised catalysts were taken on a JEOL JSM-610 SEM after the samples were coated using a JEOL JTC-1600 autofine coater.The H2 uptake was determined through irreversible H2 chemisorption. After the catalyst was heated at 393 K under vacuum for 30 min, it was treated at 673 K under H2 for 30 min. The catalysts were subsequently cooled to room temperature under vacuum for 30 min. The H2 measurement was conducted at 273 K, and H2 uptake was calculated according to the method described in the literature.26
Typical procedure for hydrogenation of unsaturated carbonyl compounds
Catalyst (0.05 g), FFald (1.1 mmol), and iso-PrOH (3 mL) as solvent were placed into a glass reaction tube, which fitted inside a stainless steel reactor. After H2 was introduced into the reactor with an initial H2 pressure of 3.0 MPa at room temperature, the temperature of the reactor was increased to 383 K. After 75 min, the conversion of FFald and the yield of FFalc were determined via GC analysis. The Ni–Sn(1.5)/TiO2 catalyst was easily separated using either simple centrifugation or filtration. The solvent was removed in vacuo, and the residue was purified via silica-gel column chromatography.Acknowledgements
Financial support from the Directorate General of Higher Education of the Republic of Indonesia through the DIKTI scholarship program to conduct a PhD course is kindly acknowledged.Notes and references
- J. Falbe, H. Bahrmann, W. Lipps and D. Meyer, in Ullmanns Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co, 2005, pp. 21, vol. 11 Search PubMed.
- (a) S. I. Fujita, Y. Sano, B. M. Bhanage and M. Arai, J. Catal., 2004, 225, 95–104 CrossRef CAS; (b) A. Ghosh and R. Kumar, Microporous Mesoporous Mater., 2005, 87, 33–44 CrossRef CAS.
- For review, see (a) P. Claus, Top. Catal., 1998, 5, 51–62 CrossRef CAS; (b) P. Gallezot and D. Richard, Catal. Rev. Sci. Eng., 1998, 40, 81–126 CrossRef CAS; (c) P. Mäki-Arvela, J. Hajek, T. Salmi and D. Yu Murzin, Appl. Catal., A, 2005, 292, 1–49 CrossRef.
- (a) C. Bianchini, E. Farnetti, M. Graziani, G. Nardin, A. Vacca and F. Zanobini, J. Am. Chem. Soc., 1990, 112, 9190–9197 CrossRef CAS; (b) J. Kaspar, M. Graziani, G. P. Escobar and A. Trovarelli, J. Mol. Catal., 1992, 72, 243–251 CrossRef CAS; (c) M. De bruyn, S. Coman, R. Bota, V. I. Parvulescu, D. E. De Vos and P. A. Jacobs, Angew. Chem., Int. Ed., 2003, 42, 5333–5336 CrossRef CAS; (d) D. V. Sokoskii, N. V. Anisimova, A. K. Zharmagambetova, S. G. Mukhamezhanova and L. N. Edygenova, React. Kinet. Catal. Lett., 1987, 33, 399–403 CrossRef.
- (a) W. M. H. Sachtler and R. A. Van Santen, Adv. Catal., 1977, 26, 69–119 CrossRef CAS; (b) R. Ferrando, J. Jellinek and R. L. Johnston, Chem. Rev., 2008, 108, 846–910 CrossRef.
- (a) A. Dandekar and M. A. Vannice, J. Catal., 1999, 183, 344–354 CrossRef CAS; (b) M. Consonni, D. Jokic, D. Yu Murzin and R. Touroude, J. Catal., 1999, 188, 165–175 CrossRef CAS; (c) R. Zanella, C. Louis, S. Giorgio and R. Touroude, J. Catal., 2004, 223, 328–339 CrossRef CAS; (d) P. Concepcion, A. Corma, J. S. Albero, V. Franco and J. Y. C. Ching, J. Am. Chem. Soc., 2004, 126, 5523–5532 CrossRef CAS; (e) J. Kijenski, P. Winiarek, T. Paryjczak, A. Lewicki and A. Mikolajska, Appl. Catal., A, 2002, 233, 171–182 CrossRef CAS; (f) A. Corma, P. Serna, P. Concepcion and J. J Calvino, J. Am. Chem. Soc., 2008, 130, 8748–8753 CrossRef CAS.
- (a) J. K. A. Clarke, Chem. Rev., 1975, 75, 291–305 CrossRef CAS; (b) P. Serp and P. Kalck, Chem. Rev., 2002, 102, 3085–3128 CrossRef CAS; (c) H. L. Jiang and Q. Xu, J. Mater. Chem., 2011, 21, 13705–13725 RSC.
- (a) J. Arana, P. Ramirez de la Piscina, L. Llorca, J. Sales and N. Homs, Chem. Mater., 1998, 10, 1333–1342 CrossRef CAS; (b) G. F. Santori, M. L. Casella and O. A. Ferretti, J. Mol. Catal. A: Chem., 2002, 186, 223–239 CrossRef CAS; (c) D. I. Jerdev, A. Olivas and B. E. Koel, J. Catal., 2002, 205, 278–288 CrossRef CAS; (d) L. Jiang, Z. Zhou, W. Li, W. Zhou, S. Song, H. Li, G. Sun and Q. Xin, Energy Fuels, 2004, 18, 866–871 CrossRef CAS; (e) T. Komatsu and H. Ikenaga, J. Catal., 2006, 241, 426–434 CrossRef CAS; (f) C. Dupont, Y. Jugnet, F. Delbecq and D. Loffreda, J. Catal., 2010, 273, 211–220 CrossRef CAS; (g) F. Vigne, J. Haubrich, D. Loffreda, P. Sautet and F. Delbecq, J. Catal., 2010, 275, 129–139 CrossRef CAS; (h) P. D. Zgolicz, V. I. Rodriguez, I. M. J. Vilella, S. R. de Miguel and O. A. Scelza, Appl. Catal., A, 2011, 392, 208–217 CrossRef CAS; (i) B. Merlo, V. Vetere, J. F. Ruggera and M. L. Casella, Catal. Commun., 2009, 10, 1665–1669 CrossRef; (j) A. B. Merlo, G. F. Santori, J. Sambeth, G. J. Siri, M. L. Cassela and O. A. Ferretti, Catal. Commun., 2006, 7, 204–208 CrossRef CAS.
- (a) F. Delbecq and P. Sautet, J. Catal., 1995, 152, 217–236 CrossRef CAS; (b) F. Delbecq and P. Sautet, J. Catal., 2003, 220, 115–126 CrossRef CAS.
- Heterogeneous Catalyst, in Modern Organonickel Chemsitry, ed. T. Osawa, Wiley-VCH Verlag GmbH & Co. KGaA, 2005, pp. 274 Search PubMed.
- (a) A. Onda, T. Komatsu and T. Yashima, Phys. Chem. Chem. Phys., 2000, 2, 2999–3005 RSC; (b) T. Komatsu and A. Onda, Catal. Surv. Asia, 2008, 12, 6–15 CrossRef CAS; (c) L. Deghedi, J. M. Basset, J. P. Candy, J. A. Dalmon, A. C. Dubreuil and L. Fischer, Chem. Eng. Trans., 2009, 17, 31–36 Search PubMed.
- (a) A. Onda, T. Komatsu and T. Yashima, Chem. Commun., 1998, 1507–1508 RSC; (b) A. Onda, T. Komatsu and T. Yashima, J. Catal., 2001, 201, 13–21 CrossRef CAS; (c) A. Onda, T. Komatsu and T. Yashima, J. Catal., 2003, 221, 378–385 CrossRef; (d) M. Masai, K. Mori, H. Muramoto, T. Fujiwara and S. Ohnaka, J. Catal., 1975, 38, 128–134 CrossRef CAS; (e) M. Masai, K. Mori, H. Muramoto, T. Fujiwara and S. Ohnaka, J. Catal., 1977, 50, 419–428 CrossRef CAS; (f) H. E. Swift and J. E. Bozik, J. Catal., 1968, 12, 5–14 CrossRef CAS.
- (a) J. W. Shabaker and J. A. Dumesic, Ind. Eng. Chem. Res., 2004, 43, 3105–3112 CrossRef CAS; (b) J. W. Shabaker, G. W. Huber and J. A. Dumesic, J. Catal., 2004, 222, 180–191 CrossRef CAS; (c) J. W. Shabaker, D. A. Simonetti, R. D. Cortright and J. A. Dumesic, J. Catal., 2005, 231, 67–76 CrossRef CAS; (d) F. Xie, X. Chu, H. Hu, M. Qiao, S. Yana, Y. Zhu, H. He, K. Fan, H. Li, B. Zong and X. Zhang, J. Catal., 2006, 241, 211–220 CrossRef CAS; (e) E. Nikolla, A. Holewinski, J. W. Schwank and S. Linic, J. Am. Chem. Soc., 2006, 128, 11354–11355 CrossRef CAS; (f) E. Nikolla, J. W Schwank and S. Linic, J. Catal., 2007, 250, 85–93 CrossRef CAS; (g) E. Nikolla, J. W Schwank and S. Linic, Catal. Today, 2008, 136, 243–248 CrossRef CAS; (h) E. Nikolla, J. W Schwank and S. Linic, J. Catal., 2009, 263, 220–227 CrossRef CAS.
- S. Pengpanich, V. Meeyoo, T. Rirksomboon and J. Schwank, Catal. Today, 2008, 136, 214–221 CrossRef CAS.
- T. C. Liu and S. J. Chiu, Ind. Eng. Chem. Res., 1994, 33, 488–492 CrossRef CAS.
- V. Hlukhyy, F. Raif, P. Clauss and T. F. Fässler, Chem.–Eur. J., 2008, 14, 3737–3744 CrossRef CAS.
- Rodiansono, T. Hara, N. Ichikuni and S. Shimazu, Chem. Lett Search PubMed , in press.
- Powder diffraction files, JCPDS-International center for diffraction data (ICDD), 1997.
- Rietica Web: http://www.rietica.org/links.htm/, Multi-Rietveld analysis program LH-Riet 7.200 on the Rietica software package, 2012.01.10.
- Supported Ni–Sn(1.5)/MCM-41 catalyst gave only 20%FFald conversion (Table S1, ESI† entry 1). Other supports such as ZrO2 and ZnO also gave relatively low FFald conversion (Table S1, ESI† entries 2 and 3), while Ni–Sn(1.5)/MgO did not give the hydrogenated products at the same conditions (Table S1, entry 4, ESI†).
- S. Sitthisa, T. Pham, T. Prasomsri, T. Sooknoi, R. G. Mallinson and D. E. Resasco, J. Catal., 2011, 280, 17–27 CrossRef CAS.
- L. Sordelli, R. Psaro, G. Vlaic, A. Cepparo, S. Recchia, A. Fusi and R. Zanoni, J. Catal., 1999, 182, 186–198 CrossRef CAS.
- J. L. Margitfalvi, A. Tompos, I. Kolosova and J. Valyon, J. Catal., 1998, 174, 246–249 CrossRef CAS.
- The XRD patterns of the recovered Ni–Sn(1.5)/TiO2 before and after H2 treatment at 673 K are shown in Fig. S4, ESI†.
- S. Lowell, J. E. Shields, M. A. Thomas and M. Thommes, Characterization of porous solids and powders: surface area, pore size and density, Kluwer Academic Publishers, Netherlands, 2004, ch. 8 Search PubMed.
- (a) C. H. Bartholomew, R. B. Pannel and J. L. Butler, J. Catal., 1980, 65, 335–347 CrossRef CAS; (b) C. H. Bartholomew and R. B. Pannel, J. Catal., 1980, 65, 390–401 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cy20216f |
| This journal is © The Royal Society of Chemistry 2012 |
