Biopolymer–metal complex wool–Pd as a highly active catalyst for Suzuki reaction in water†
Heng-chang
Ma
*,
Wei
Cao
,
Zhi-kang
Bao
and
Zi-Qiang
Lei
*
Key Laboratory of Polymer Materials of Gansu Province, Key Laboratory of Eco-Enviroment-Related Polymer Materials Ministry of Education, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou 730070, People's Republic of China. E-mail: mahc@nwnu.edu.cn; leizq@ nwnu.edu.cn; Fax: (+86)-0931-7970-359
First published on 22nd May 2012
Abstract
A heterogeneous biopolymer complex wool–Pd catalyst has been applied in water-mediated coupling reactions of aryl iodides and bromides with arylboronic acid. The results showed that the reactions could be conducted in neat water under atmospheric conditions with water-insoluble or even solid aryl halides. More importantly, the catalyst system has the advantages of excellent yields, environmental friendliness, and catalyst recyclability.
Introduction
The Suzuki–Miyaura cross-coupling reaction is one of the preeminent methods for the carbon(sp2)–carbon(sp2) bond formation.1 In recent years, this transformation has been extensively used in numerous synthetic ventures,2 and also various modifications have been introduced.3Minimization of chemical waste from the reaction mixture, of which 80% is estimated to be solvents,4 is a constant challenge as environmental concerns are increasingly brought into focus. Therefore, the endeavour to replace volatile organic solvents in organometallic catalysis by alternative more practical and environmentally friendly solvents must be a priority.5 Although there are some examples reported concerning palladium-catalyzed cross-coupling reactions of aryl halogenates with aryl boronic acids using purely aqueous reaction conditions, most of these reactions were conducted in organic solvents.6 Several sulfonated phosphine derivatives and water-soluble ligands have been prepared and used in cross-coupling reactions conducted in water–organic biphasic solvent systems.7 These homogeneous catalyst systems suffer from some issues in terms of the separation and recycling of the catalysts. Additionally, they induce contamination of the ligand residue in the products. Therefore, the development of polymer-supported and insoluble transition metal catalysts has attracted a great deal of attention in organic chemistry.8 The functionalized Wang resin represents the most commonly used synthetic support, to which phosphines, amines, carbenes etc. were successfully immobilized, and some promising results were achieved.9 However, problems still existed. For instance, the requirements of several steps to synthesize supporter itself with appropriate bore diameter, cross-linking degrees, the lower loading of palladium and severe catalyst leaching, and the poor swell property prevents the reactions from taking place in the neat aqueous phase.
In previous research, some natural biopolymers, such as chitosan,10 cellulose,11 wool,12etc. have been used as efficient polymer supports in several important palladium-catalyzed transformations. Among them, we have focused on the natural animal fibers (wool) due to the facts that the animal fibers themselves could be used as a solid phase ligand with no need for further functionalization. Furthermore, the loaded palladium particles could be distributed evenly on the surface of the fibers because of the structurally ordered amino acid chains, so the formation and aggregation of Pd-black could be prevented, which was regarded as the most critical problem to the performance of palladium-catalyzed conversions. In our previous research,13 we reported an effective catalyst system composed of wool–palladium (wool–Pd) complex in aqueous media with very small amount of PEG-400 for the Heck reaction using NaAc as a base. The results showed that aryl bromides could carry out the coupling reaction with a variety of alkenes at 80 °C in aqueous media under atmospheric conditions. Encouraged by these results, we have recently applied the catalyst system to the Suzuki–Miyaura cross-coupling reaction.
Herein, we report an effective bio-polymer-supported palladium catalyst in neat water for the Suzuki reaction using K2CO3 as a base. The reaction can be conducted under atmospheric conditions without any specific protection, and no phase-transfer agents are necessary. Moreover, the catalyst is easy to separate from the reaction mixture by simple filtration. More importantly, the cheap catalyst was stable, showed negligible metal leaching, and retained good activity for at least three successive runs without any additional activation treatment.
Results and discussion
Synthesis and characterization of the catalyst
The synthesis of the catalyst can be found in our previous research.13Fig. 1 shows the powder XRD patterns of the three samples of supported Pd catalysts. The peaks at 2θ values of 40° and 42° in the spectrum of the wool–Pd(0) are related to the (111) and (200) reflection of crystalline Pd(0). This sample was obtained from the treatment of the newly prepared wool–Pd(II) with benzyl alcohol at 70 °C for 6 h. Of note is that in the fresh wool–supported PdCl2 catalyst, the weak reflections of the crystalline Pd(0) also had peaks at the corresponding 40° and 42°, which was possibly due to the existence of thiol (–SH) groups in the wool fibers. Obviously, after several recycling uses, the representative 2θ values of crystalline Pd(0) also could be observed. The average particle sizes of reused catalyst are almost 60 nm (see ESI).†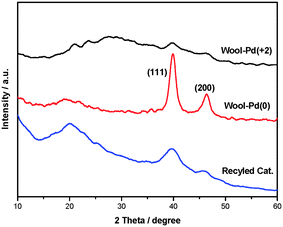 | ||
| Fig. 1 XRD patterns of the three samples of supported Pd catalysts. | ||
The binding energies of wool, PdCl2 and wool–Pd complex were obtained by XPS analysis (Table 1). The binding energy of the Pd3d 3/2 and Pd3d 5/2 in the wool–Pd complex increase 0.75 eV and 0.87 eV, respectively; the change of Pd3d binding energy means the decrease of its electron density. Little change of the binding energy of the Cl2p was observed, this means there are unreactive Cl though the chemical bond formed in this process. There are two kinds of nitrogen-containing group, –NH–CO– and –NH2 in wool, and their N1s binding energies are different. Such data in wool–Pd are also differed from those in wool. The difference of the N1s binding energies between –NH–CO– in wool and –NH–CO– in wool–Pd is 0.32 eV, and that between –NH2 in wool and –NH2 in wool–Pd is 0.55 eV. In the same way, there are three kinds of S-containing group, –SO3H, –SH and –S–S– in wool, and their S2p binding energies are different. The difference of S2p binding energy between –SO3H in wool and –SO3H in wool–Pd is only 0.31 eV, that between –SH in wool and –SH in wool–Pd is 0.8 eV and that between –S–S– in wool and –S–S– in wool–Pd is 0.7 eV. The difference of O1s binding energy between wool and wool–Pd also could not be detected. These results show that coordination or ionic bonds are formed by the connection of N atoms (in –NH2) and S atoms (in –SH and –S–S–) with Pd atoms in the wool–Pd complex. (Scheme 1)
| XPS Peaks | Binding energy (eV) | ΔEb (eV) | |||
|---|---|---|---|---|---|
| PdCl2 | Wool | Wool–Pd complex | |||
| Pd3d | Pd3d 3/2 | 342.80 | 343.55 | +0.75 | |
| Pd3d 5/2 | 337.43 | 338.30 | +0.87 | ||
| Cl2p | 198.55 | 198.80 | +0.25 | ||
| N1s | NHCO | 400.05 | 399.80 | −0.32 | |
| NH2 | 400.37 | 399.25 | −0.55 | ||
| S2p | SO3H | 168.18 | 167.87 | −0.31 | |
| S–S | 165.05 | 165.75 | +0.70 | ||
| SH | 163.80 | 163.00 | −0.80 | ||
| O1s | 531.93 | 532.12 | +0.19 | ||
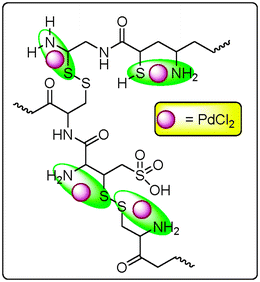 | ||
| Scheme 1 The possible structure of wool–Pd. | ||
Suzuki reaction in water
To explore the optimal reaction conditions, the coupling of bromobenzene (1a) with phenylboronic acid (1b) was determined as the model reaction (Table 2). The preliminary results indicated that temperature and base had a significant effect on the coupling reaction. The lower temperature disfavored the transformation, (Table 2, entries 1–2). Higher yields of cross-coupling product could be achieved at elevated temperature (75 °C), however, over-heated reaction mixture led to the self-coupling of phenylboronic acid to form biphenyl.14 (Table 2, entry 4). Under the optimized reaction temperature, several bases were examined, such as NaAc, NaOH, Na2CO3, K2CO3 and pyridine. Notably, the most commonly used base K2CO3 was effective in providing the corresponding cross-coupling product with better yield 99% (Table 2, entry 8). We also found that an appropriate amount of catalyst was necessary for the transformation, with 0.55% catalyst, 1c was obtained in 99% isolated yield. Minimization of catalyst amount to 0.45%, 1c was achieved with 85% yield and 188 TON value. Notably in the aqueous phase, the gradually formed 1c precipitated during stirring. After consumption of 1a, 1c was colleted by simple filtration, obviating the need for additional chromatograph separation steps. Finally, the general reaction condition is optimized as bromobenzene (0.2 mmol), phenylboronic acid (0.22 mmol), K2CO3 (0.3 mmol), 1 mg of wool–Pd complex catalyst (0.55 mol%), stirred in 5 mL water at 75 °C under atmosphereic conditions.| Entry | Base | T/°C | Cat. (mol%) | Yieldb (%) | TON (mol mol−1) |
|---|---|---|---|---|---|
| a Reaction conditions: Bromobenzene (0.2 mmol), phenylboronic acid (0.22 mmol), K2CO3 (0.3 mmol), water (5 mL), under atmospheric conditions, time (5 h). b Yield according to GC. | |||||
| 1 | NaAc | 35 | 0.55 | 8 | 15 |
| 2 | NaAc | 55 | 0.55 | 26 | 47 |
| 3 | NaAc | 75 | 0.55 | 53 | 96 |
| 4 | NaAc | 85 | 0.55 | 43 | 78 |
| 5 | Pyridine | 75 | 0.55 | 21 | 38 |
| 6 | NaOH | 75 | 0.55 | 78 | 141 |
| 7 | Na2CO3 | 75 | 0.55 | 81 | 147 |
| 8 | K2CO3 | 75 | 0.55 | 99 | 180 |
| 9 | K2CO3 | 75 | 0.45 | 85 | 188 |
| 10 | K2CO3 | 55 | 0.11 | 77 | 140 |
| 11 | K2CO3 | 25 | 0.11 | 18 | 33 |
To demonstrate the scope of the catalyst system, different aryl halides were cross-coupled with phenylboronic acids (Scheme 2). The results showed that the catalytic system was applicable to various aryl iodides and bromides and tolerant to a broad range of functional groups, including of H, NO2, NH2, OR, OH, COMe and CHO (Scheme 2, 1c–11c). Using 0.055 mol% catalyst, the coupling of electron-donating aryl iodides and phenylboronic acids provided the corresponding biaryl products in >91% yields. A temperature of 75 °C was essential for the successful coupling reaction of electron-withdrawing group (–NO2) substituted substrates; the excellent yield of 96% (4c) and 98% (8c) were obtained within 5 h. When different temperatures were screened, 2c and 4c could be produced at even lower temperatures of 55, 45 and 25 °C, however, longer reaction time was required. At 25 °C, 2c and 4c were achieved in 10% and 20% isolated yields respectively within 5 h. Of particular importance was that the coupling of substrates containing hydrophilic functional groups, which are insoluble in organic solvents and are present in many pharmaceutically interesting compounds, were particularly efficient partners, for instance, 2c, 5c, 6c and 9c resulted in 94, 92, 94 and 91% yields, respectively. Of note, the insoluble biaryl products precipitated almost quantitatively in the aqueous phase, obviating the need for additional separation and protection/deprotection steps. Aryl bromides with diverse electron-donating and electron-withdrawing substituents delivered the cross-coupled products in high yields using 0.55 mol% catalyst at 75 °C for longer reaction time. Relatively, electron deficient substrates performed better than those with electron-rich functional groups. Typically, 3c and 11c were isolated in 90% yields, 5c, 9c, 10c and 16c were given with 76, 79, 69 and 78% yields, respectively. Successful application to hindered substrate combinations, however, has been scarcely reported. To ascertain whether our palladium catalyst system could address this limitation, in a similar fashion, the hindered substrate of aryl bromides fragments were evaluated against different arylboronic acids. 2,4-Dimethoxy aryl bromide was successfully coupled to form an ortho-substituted biaryl in an excellent yield with only 0.55 mol% Pd (Scheme 2, 13c and 19c). Obviously, the electron-rich arylboronic acids performed well under the optimized reaction conditions, giving the corresponding products with excellent yields. For the more electron-deficient substrates, 4-Cl arylboronic acid predominantly coupled to CHO, OH, Me, OEt, NO2, COMe substituted aryl bromides with good to excellent yields. Due to the ubiquity of heterocycles in biologically active molecules we focused our attention on using heteroaryl halides as substrates. Typically, high catalyst loading is required to overcome catalyst deactivation through heteroatom coordination. With 1 mol% of wool–Pd, pyridine, thiofuran substrates all afforded their cross-coupled products in moderate yields (14c/65%, 15c/42%, 20c/72%, 21c/50%).
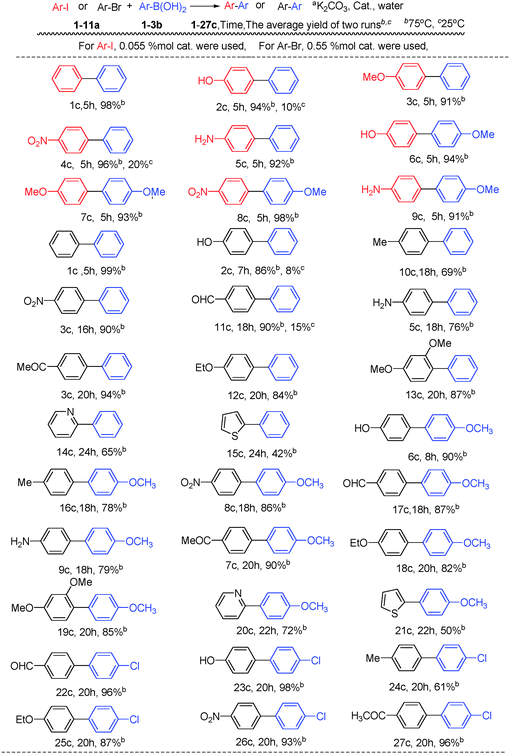 | ||
| Scheme 2 Scope of the Suzuki reactions with wool–Pd complex catalyst.a | ||
It has been known that hydrophobic interactions can have a significant influence on organic reactions.15 It was discovered in several organic reactions in water that such reactions often proceed with much higher rates than in organic solvents. The accelerating effect of water has been ascribed to a number of factors, including the hydrophobic effect as well as hydrogen bonding between water molecules and reactants. In our reaction protocols, the difference between the non-hydrophobic and hydrophobic conditions was remarkable (Table 3). The isolated yields of 11c were significantly lower for reaction in THF or THF–water than in the aqueous phase, despite the aqueous reactions being heterogeneous. We reasoned that in water hydrophobic surfaces associate strongly as a result of the tendency of water to exclude nonpolar species and thus minimize the Gibbs energy of solvation.15 In other words, reactions in water may be predisposed to favor transition states that optimize hydrophobic interactions. The great efficiency of the water-modulated reaction attributed to the hydrophobic effect because of the biologically lipophilic fibers-wool.
THF, DMSO and toluene are the most commonly used reaction media in Suzuki coupling reactions.16 However, in our disclosed catalyst system such non-protic solvents with better solubility, higher boiling point and stronger polarity disfavor the transformation (Fig. 2). A further solvent screening experiment revealed that except for water, protic solvents, such as MeOH and EtOH are very effective in promoting the C–C bond formation. The observations raise an interesting question. Could the cheap and environmentally friendly alcohols be used as solvent? It is well known that alcohols are used to act as receptors for halide anions, and in particular MeOH, EtOH and H2O are good hydrogen bond donors (HBD) as judged by their high ENT values.17 This belief was strengthened by a study of the coupling reaction in more than 10 solvents, which demonstrated that hydrogen bond donating, protic solvents indeed accelerate C–C bond formation. As shown in Fig. 2, the higher the ENT values, the faster this reaction becomes. Hydrogen bond donors always play an essential role in the arylation of aromatic halides, which probably promotes the dissociation of the intermediate I, resulting from the oxidative addition of halide with palladium species and thus formation of intermediate II or III. After liberation from I, X− serves as an electron-pair donor (EPD) and is stabilized by HBDs, such as H2O, MeOH and EtOH (Fig. 3).
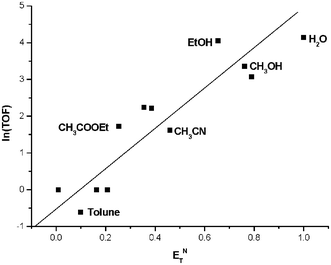 | ||
| Fig. 2 The effect of solvent on Suzuki reaction. | ||
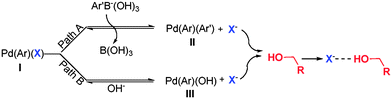 | ||
| Fig. 3 An equilibrium of Pd(Ar)(X) in Suzuki reaction. | ||
We also found that the decrease in the arylation rate with increasing bromide concentration, which suggested that the Br− generated from each arylation cycle must be effectively scavenged. The trapping of the bromide anions most likely arises from possible hydrogen bonding between ROH and Br−, rendering the equilibrium in favor of the Pd(II) cations (Fig. 3, II or III). Evidence in support of this equilibrium comes from reactions in the presence of added KBr. As shown in Fig. 4, the arylation of phenylboronic acid with 4-bromobenzaldehyde was notably slower when 1 equiv of Br− was introduced into the reaction system. With the increase of volume ratio of THF, the yields of 11c were reduced gradually. This is consistent with there being an equilibrium, which is shifted towards the left on addition of the halide ions (Fig. 3). Similar observations have been made in arylation reactions such as the Heck reaction. Thus, we reasoned that introduction of a potential hydrogen bond donor could enhance the reaction rates. Especially, with the property of hydrophobic effect and as also a hydrogen bond donor, water is screened as the most effective reaction media for the Suzuki coupling reaction.
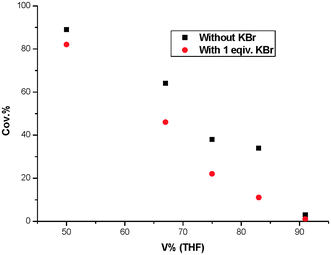 | ||
| Fig. 4 The effect of KBr on Suzuki reaction. | ||
Recycling of the catalyst
An important point concerning the use of heterogeneous catalysts is its lifetime, particularly for industrial and pharmaceutical applications of the Suzuki reaction. For the recycling study, the reaction was performed with 1a and 1b, maintaining the same reaction conditions as described above. The successive operations were carried out as follows: after the end of the reaction, the mixture was cooled down to room temperature and filtrated. Then the filtrated aqueous phase and the used wool–Pd were added to the next run. TLC monitored the reaction progress, and the conversion and product selectivity were determined using GC analysis. This indicated that the catalyst can be recycled more than 3 times, and the total TON value is 680 (Fig. 5).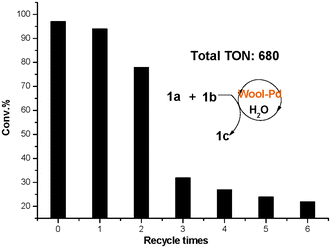 | ||
| Fig. 5 Recycling test of wool–Pd complex catalyst. | ||
Experimental section
Apparatus
All NMR spectra are recorded on MERCURY (400 MHz for 1H NMR, 100 MHz for 13C NMR) spectrometers; chemical shifts are expressed in ppm (δ units) relative to TMS signal as internal reference in CDCl3. Gas chromatography (GC) analysis was performed on a Shimadzu GC-2010 equipped with a 15 m × 0.53 mm × 1.5 μm RTX-1 capillary column and an oxyhydrogen flame detector. X-ray photoelectron spectroscopy analysis (XPS) measurements were recorded on a PHI5702 photoelectron spectrometer. Scanning electron microscopy (SEM) was performed with field-emission scanning electron microscopy (FESEM, JSM-6701F, Japan) at an accelerating voltage of 5.0 kV. X-ray diffraction (XRD) of samples was performed on a diffractometer (D/Max-2400, Rigaku) advance instrument using Cu-Kα radiation (k = 1.5418 Å) at 40 kV, 100 mA.General experimental procedure for the Suzuki reaction
In air, aryl halide (0.2 mmol), arylboronic acid (0.22 mmol), K2CO3 (0.3 mmol), 5 mL of distilled water, and 2 mg of wool–Pd complex catalyst (Pd 0.0022 mmol) were combined in a 10 mL round-bottomed flask. The reaction mixture was magnetically stirred and the temperature was maintained at 75 °C with an oil bath. Reaction progress was monitored by TLC. After reaction was completed, the reaction mixture was cooled to room temperature and filtrated. The solid was washed with water (3 × 5 mL), then with EtAc (3 × 5 mL), and the organic phase was combined. The catalyst was separated by filtration, washed with water, and dried in vacuum. The combined organic layer was dried with anhydrous MgSO4, and the solvent was removed under reduced pressure to give the product.Conclusion
In summary, we have developed an efficient catalyst system for Suzuki–Miyaura cross-coupling reactions. Our catalyst system had the following advantages: (1) using a natural biopolymer as a solid ligand and catalyst support, and the catalyst was synthesized via a facile method; (2) the reaction conditions were mild and neat water was used as the solvent; (3) the catalyst was reusable and easy to separate; (4) the product could be obtained by simple filtration. Further efforts to study the detailed mechanism and extend the application of the system to other coupling transformations are under way in our laboratory.Notes and references
- A. Suzuki, Pure Appl. Chem., 1985, 57, 1749 CrossRef CAS
; A. Suzuki, Pure Appl. Chem., 1991, 63, 419 CrossRef
; A. Suzuki, Pure Appl. Chem., 1994, 66, 213 CrossRef
; N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457 CrossRef
; S. P. Stanforth, Tetrahedron, 1998, 54, 263 CrossRef
; N. Miyaura, Top. Curr. Chem., 2002, 219, 11 CrossRef
; S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633 CrossRef
; M. Sasaki and H. Fuwa, Synlett, 2004, 1851 CrossRef
; F. Bellina, A. Carpita and R. Rossi, Synthesis, 2004, 2419 Search PubMed
; A. Suzuki, Chem. Commun., 2005, 4759 RSC
.
- P. Lloyd-Williams and E. Giralt, Chem. Soc. Rev., 2001, 30, 145 RSC
; K. C. Nicolau, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442 CrossRef CAS
; O. Baudoin, Eur. J. Org. Chem., 2005, 4223 CrossRef
.
- T. I. Wallow and B. M. Novak, J. Org. Chem., 1994, 59, 5034 CrossRef CAS
; S. Darses, J.-P. Genêt, J. L. Brayer and J.-P. Demoute, Tetrahedron Lett., 1997, 38, 4393 CrossRef
; M. A. Carroll and A. B. Holmes, Chem. Commun., 1998, 1395 RSC
; S. Darses, G. Michaud and J. P. Genêt, Eur. J. Org. Chem., 1999, 1875 CrossRef
; Y. Li and M. A. El-Sayed, J. Phys. Chem. B, 2001, 105, 8938 CrossRef
; I. F. J. Vankelecom, Chem. Rev., 2002, 102, 3779 CrossRef
; U. E. W. Lange, W. M. Braje, W. Amberg and G. Kettschau, Bioorg. Med. Chem. Lett., 2003, 13, 1721 CrossRef
; N. N. Romanova, A. G. Gravis and N. V. Zyk, Russ. Chem. Rev., 2005, 74, 969 CrossRef
; G. Cravotto, M. Beggiato, A. Penoni, G. Palmisano, S. Tollari, J. M. Lévêque and W. Bonrath, Tetrahedron Lett., 2005, 46, 2267 CrossRef
; J. H. Ryu, C. J. Jang, Y. S. Yoo, S. G. Lim and M. Lee, J. Org. Chem., 2005, 70, 8956 CrossRef
; L. J. Geng, J. T. Li, S. X. Chin and Wang, J. Org. Chem., 2005, 25, 608 Search PubMed
; D. E. Bergbreiter and S. D. Sung, Adv. Synth. Catal., 2006, 348, 1352 CrossRef
; B. M. L. Dioos, I. F. J. Vankelecom and P. A. Jacobs, Adv. Synth. Catal., 2006, 348, 1413–1446 CrossRef
; F. X. Felpin, T. Ayad and S. Mitra, Eur. J. Org. Chem., 2006, 2679 CrossRef
; V. Calò, A. Nacci and A. Monopoli, Eur. J. Org. Chem., 2006, 3791 CrossRef
; D. Korolev and N. A. Bumagin, Tetrahedron Lett., 2006, 47, 4225 CrossRef
; Y. M. Yamada, Y. Maeda and Y. Uozumi, Org. Lett., 2006, 8, 4259 CrossRef
.
- K. H. Shaughnessy and R. B. DeVasher, Curr. Org. Chem., 2005, 9, 585 CrossRef CAS
.
- R. A. Sheldon, Green Chem., 2005, 7, 267 RSC
; C. K. Z. Andrade and L. M. Alves, Curr. Org. Chem., 2005, 9, 195 CrossRef CAS
; F. Alonso, et al. , Tetrahedron, 2008, 64, 3047 CrossRef
.
- C. J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS
; C. J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68 RSC
.
- A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176 CrossRef CAS
; S. D. Walker, T. E. Barder, J. R. Martinelli and S. L. Buchwald, Angew. Chem., Int. Ed., 2004, 43, 1871 CrossRef
; S. Harkal, F. Rataboul, A. Zapf, C. Fuhrmann, T. Riermeier, A. Monsees and M. Beller, Adv. Synth. Catal., 2004, 346, 1742 CrossRef
; J. Dupont, C. S. Consorti and J. Spencer, Chem. Rev., 2005, 105, 2527 CrossRef
; U. Christmann and R. Vilar, Angew. Chem., Int. Ed., 2005, 44, 366 CrossRef
; K. W. Anderson and S. L. Buchwald, Angew. Chem., Int. Ed., 2005, 44, 6173 CrossRef
; N. Kudo, M. Perseghini and G. C. Fu, Angew. Chem., Int. Ed., 2006, 45, 1282 CrossRef
; R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461 CrossRef
; B. H. Lipshutz and S. Ghorai, Aldrichimica Acta, 2008, 41, 59 Search PubMed
; F. Alonso, I. P. Beletskaya and M. Yus, Tetrahedron, 2008, 64, 3047 CrossRef
.
- G. Marck, A. Villiger and R. Buchecker, Tetrahedron Lett., 1994, 35, 3277 CrossRef CAS
; K. Mori, K. Yamaguchi, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2002, 124, 11572 CrossRef
; F. X. Felpin, T. Ayad and S. Mitra, Eur. J. Org. Chem., 2006, 2679 CrossRef
; A. Corma and H. García, Adv. Synth. Catal., 2006, 348, 1391 CrossRef
; D. E. Bergbreiter and S. D. Sung, Adv. Synth. Catal., 2006, 348, 1352 CrossRef
; B. M. L. Dioos, I. F. J. Vankelecom and P. A. Jacobs, Adv. Synth. Catal., 2006, 348, 1413 CrossRef
; B. Helms and J. M. J. Fréchet, Adv. Synth. Catal., 2006, 348, 1125 CrossRef
.
- U. E. W. Lange, W. M. Braje, W. Amberg and G. Kettschau, Bioorg. Med. Chem. Lett., 2003, 13, 1721 CrossRef CAS
.
- M. Y. Yin, G. L. Yuan, Y. Q. Wu, M. Y. Huang and Y. Y. Jiang, J. Mol. Catal. A: Chem., 1999, 147, 93 CrossRef CAS
; D. Q. Zhou, M. He, Y. H. Zhang, M. Y. Huang and Y. Y. Jiang, Polym. Adv. Technol., 2003, 14, 287 CrossRef
.
- Y. Xu, L. Zhang and Y. Cui, J. Appl. Polym. Sci., 2008, 110, 2996 CrossRef CAS
.
- L. Xue, B. Jia, L. Tang, X. F. Ji, M. Y. Huangand and Y. Y. Jiang, Polym. Adv. Technol., 2004, 15, 346 CrossRef CAS
; B. Jia, X. Yang, M. Y. Huang and Y. Y. Jiang, React. Funct. Polym., 2003, 57, 163 CrossRef
; S. Wang, Z. Zhang, C. Chi, G. Wu, J. Ren, Z. Wang, M. Huang and Y. Jiang, React. Funct. Polym., 2008, 68, 424 CrossRef
.
- S. Wu, H. C. Ma, X. J. Jia, Y. M. Zhong and Z. Q. Lei, Tetrahedron, 2011, 67, 250 CrossRef CAS
.
- H. G. Kuvila and K. V. Nahabedian, J. Am. Chem. Soc., 1961, 83, 2159 CrossRef
; H. G. Kuvila, J. F. Reuwer and J. A. Mangravite, J. Am. Chem. Soc., 1964, 86, 2666 CrossRef
.
- S. Soomro, C. Rohlich and K. Kohlerb, Adv. Synth. Catal., 2011, 353, 767 CrossRef CAS
; J. W. Ruan and J. L. Xiao, Acc. Chem. Res., 2011, 44, 614 CrossRef
.
- T. E. Barder, S. D. Walker, J. R. Martinelli and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4685 CrossRef CAS
; H. Doucet, Eur. J. Org. Chem., 2008, 2013 CrossRef
; T. Kinzel, Y. Zhang and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 14073 CrossRef
.
- C. Reichardt, Chem. Rev., 1994, 94, 2319 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cy20126g |
| This journal is © The Royal Society of Chemistry 2012 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: