Enhanced photocatalytic degradation of methylene blue by ZnO–reduced graphene oxide–carbon nanotube composites synthesized via microwave-assisted reaction
Tian
Lv
,
Likun
Pan
*,
Xinjuan
Liu
and
Zhuo
Sun
Engineering Research Center for Nanophotonics and Advanced Instrument, Ministry of Education, Department of Physics, East China Normal University, Shanghai, China. E-mail: lkpan@phy.ecnu.edu.cn; Fax: +86 21 62234321; Tel: +86 21 62234132
First published on 1st June 2012
Abstract
ZnO–reduced graphene oxide (RGO)–carbon nanotube (CNT) composites were successfully synthesized via microwave-assisted reduction of a graphite oxide dispersion in zinc nitrate solution with a CNT suspension. Their photocatalytic performance in the degradation of methylene blue was investigated and the results show that the CNTs play an important role in the enhancement of the photocatalytic performance and the ZnO–RGO–CNT composite with 3.9 wt% CNTs achieves a maximum degradation efficiency of 96% under UV light irradiation for 260 min as compared with ZnO–RGO (88%) due to the increased light absorption and the reduced charge recombination with the introduction of CNTs.
1. Introduction
Photocatalysis has been widely applied as a technique for the destruction of organic pollutants due to its effectiveness, easy operation and ideally producing nontoxic end products.1–5 ZnO, a semiconductor with a direct bandgap of 3.37 eV, has been extensively investigated as the most active photocatalyst.6 However, a major limitation to achieving high photocatalytic efficiency is the quick recombination of photogenerated charge carriers. Recombination has faster kinetics than surface redox reactions and greatly reduces the quantum efficiency of photocatalysis. Therefore, currently a particularly attractive option is to design and develop composite materials based on ZnO to solve this problem.7–9Carbon based materials, such as carbon nanofibers10,11 and carbon nanotubes (CNTs),12–14 have been reported as hybrid components to be incorporated into ZnO due to their low cost, superior chemical stability, and good conductivity. Yan et al. synthesized composites of ZnO and multi-walled CNTs via electrostatic interaction and an in situ hydrothermal method and investigated the degradation of rhodamine B under UV irradiation.11 Jiang and Gao deposited ZnO nanoparticles on CNTs through noncovalent modification of CNTs with sodium dodecyl sulfate for the photocatalytic degradation of methylene blue (MB) and a degradation efficiency of ∼85% was achieved.12 Zhu et al. synthesized ZnO–CNT composites via a sol process for the photocatalytic degradation of methylene orange (MO) and a degradation efficiency of ∼98% was achieved.14 Byrappa et al. fabricated ZnO–CNT composites under mild hydrothermal conditions with an autogenous pressure and found that the composite was very effective in the degradation of indigo carmine dye.15 These carbon materials act as an excellent electron-acceptor/transport material in the process of photocatalysis to effectively facilitate the migration of photo-induced electrons and to hinder the charge recombination in electron-transfer processes, which enhances the photocatalytic performance of ZnO.
Recently, graphene, a rising star in the carbon family, has attracted a great deal of attention due to its excellent electronic properties, superior chemical stability and high specific surface area,16–19 which would enable it to be another excellent electron-transport material in the process of photocatalysis. Xu et al. synthesized ZnO–graphene composite by reducing graphite oxide (GO) coated on the surface of ZnO nanoparticles using hydrazine and found that the composite showed an improved photocatalytic efficiency (∼90%) in the degradation of MB as compared with pure ZnO.20 Yang et al. synthesized functionalized graphene sheet–ZnO composites via a thermal treatment method and investigated their photocatalytic performance in the degradation of rhodamine 6G under UV light irradiation.21 Li and Cao reported the incorporation of graphene into ZnO to form a ZnO–graphene composite by chemically reducing the mixture of GO dispersion and Zn(AcO)2 in aqueous solution using NaBH4 and studied the photocatalytic degradation of rhodamine B under UV and visible light irradiation using the composite.22 Kavitha et al. fabricated ZnO–graphene hybrids via a single source precursor (zinc benzoate dihydrazinate complex) on graphene at 200 °C, which demonstrated enhanced photocatalytic activity towards MB degradation (∼70%).23 In our previous work, ZnO–RGO composites were synthesized by reducing a GO dispersion with zinc nitrate using a microwave synthesis system and the composites achieved a maximum efficiency of 88% in the degradation of MB under UV light irradiation.24 Despite the progress to date, there is still more room to enhance the degradation performance of these composites for practical applications. It should be noticed that by now, most of the graphene materials used for photocatalysis are obtained by the chemical oxidation and reduction of graphite. However, in this method, incomplete chemical reduction is often observed,25–29 which causes poor electrical conductivity of graphene. Attempts to combine highly conductive CNTs and chemically reduced graphene have been carried out to solve this problem30–35 because CNTs can serve as an electrical conducting network, bridge the defects for electron transfer and increase the basal spacing between graphene sheets.36,37 Enhanced photocatalytic performance of ZnO is expected if such a graphene–CNT composite is combined with a ZnO photocatalyst.
In this work, the one-step synthesis of a ZnO–RGO–CNT composite was carried out through microwave-assisted reduction of a GO dispersion in zinc nitrate solution with a CNT suspension using a microwave system. Microwave irradiation can heat the reactant to a high temperature in a short time by transferring energy selectively to microwave absorbing polar solvents. Thus it can facilitate mass production in a short time with little energy cost38–41 and form an intimate contact between ZnO, RGO and CNTs.42,43 The as-synthesized ZnO–RGO–CNT composites exhibit enhanced photocatalytic performance in the degradation of MB under UV light irradiation as compared with pure ZnO, ZnO–CNT and ZnO–RGO.
2. Experimental details
Multi-walled CNTs were purchased from Nanotech Port Co., Ltd. (Shenzhen, China) with detailed specifications as follows: length (5–15 μm), diameter (40–60 nm) and purity (≥98%). Commercial graphite powder was used as the starting reagent for the synthesis of GO via a modified Hummers method, which has been described in our previous works.44–50 1.8 mg ml−1 GO suspension and a certain amount of CNTs were added into 20 ml 0.3 M Zn(NO3)2 solution and then the solution was sonicated for 30 min to produce a uniform dispersion. A dilute NaOH solution was dropped in the solution to form a brownish-black suspension with a pH value of 10–11. The solution was then put into an automated focused microwave system (Explorer-48, CEM Co.) and treated for 30 min at 100 °C. It was obviously found that the color of the suspension had changed into grayish-black, indicating the chemical reduction of the GO sheets.51 The as-synthesized ZnO–RGO–CNT samples were isolated by filtration, washed several times with distilled water, and finally dried in a vacuum oven at 60 °C for 24 h. The ZnO–RGO–CNT samples with 0, 2.4, 3.9, 6.1 and 7.6 wt% CNTs are named as ZGC-0, ZGC-1, ZGC-2, ZGC-3 and ZGC-4 and in all these samples, the weight ratio of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RGO was controlled to be 89.9
RGO was controlled to be 89.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 because such optimized components show the highest activity in the degradation of MB according to our previous report.24 Pure ZnO and ZnO–CNT (named as ZC) were also synthesized by direct microwave assisted reaction for comparison and the weight ratio of ZnO
1 because such optimized components show the highest activity in the degradation of MB according to our previous report.24 Pure ZnO and ZnO–CNT (named as ZC) were also synthesized by direct microwave assisted reaction for comparison and the weight ratio of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CNTs in ZC was controlled to be 89.9
CNTs in ZC was controlled to be 89.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
The surface morphology, structure and composition of the samples were characterized by field-emission scanning electron microscopy (FESEM, Hitachi S-4800), high-resolution transmission electron microscopy (HRTEM, JEOL-2010) and X-ray diffraction spectroscopy (XRD, Holland Panalytical PRO PW3040/60) with Cu-Kα radiation (V = 30 kV, I = 25 mA), respectively. The UV-vis absorption spectra were recorded using a Hitachi U-3900 UV-vis spectrophotometer.
The photocatalytic performance of the as-prepared samples was evaluated by photocatalytic degradation of MB under UV light irradiation. The samples (1.5 g l−1) were dispersed in 100 ml MB aqueous solution (5 mg l−1). The mixed suspensions were magnetically stirred for 0.5 h in the dark to reach an adsorption–desorption equilibrium. Under ambient conditions and stirring, the mixed suspensions were exposed to UV irradiation produced by a 500 W high pressure Hg lamp with the main wave crest at 365 nm. At certain time intervals, 2 ml of the mixed suspensions were extracted and centrifuged to remove the photocatalyst. The degradation process was monitored by measuring the absorption of MB in the filtrate at 663 nm using a UV-vis absorption spectrometer.
3. Results and discussion
The FESEM images of RGO, ZGC-0, ZGC-1, ZGC-2, ZGC-3 and ZGC-4 are shown in Fig. 1(a)–(f). The RGO nanosheets are curled and corrugated and ZnO displays a hexagonal nanorod structure. It is clearly observed that ZnO nanorods were supported on the surface of RGO sheets with wrinkles and folds. The long CNT wires weave between RGO sheets and ZnO nanorods31 and their outer diameters are about 40–60 nm. | ||
| Fig. 1 FESEM images of (a) RGO, (b) ZGC-0, (c) ZGC-1, (d) ZGC-2, (e) ZGC-3 and (f) ZGC-4. | ||
The low-magnification and high-magnification HRTEM images of ZGC-2 are shown in Fig. 2(a) and (b). It can be observed that RGO sheets retain the two-dimensional sheet structure with wrinkles and ZnO nanorods are attached to RGO sheets with CNTs wound between them. The lattice fringe spacing of the nanocrystal is indexed as the (100) plane of ZnO (JCPDS 79-0206) in Fig. 2(b).19
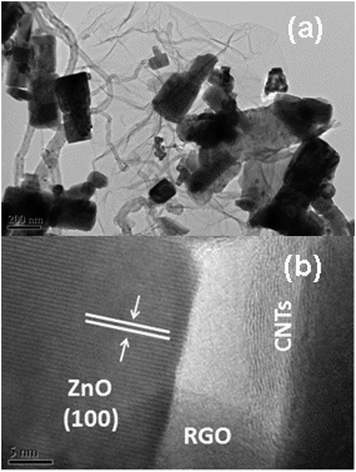 | ||
| Fig. 2 (a) Low-magnification and (b) high-magnification HRTEM images of ZGC-2. | ||
The XRD patterns of RGO, ZnO, ZGC-0, ZGC-1, ZGC-2, ZGC-3 and ZGC-4 are shown in Fig. 3. RGO nanosheets exhibit a (002) diffraction peak at 26° and a (100) peak at 44.5°.52 The XRD analysis further shows that the main diffraction peaks of ZnO–RGO–CNT composites are similar to those of pure ZnO and correspond to the hexagonal phase of ZnO (JCPDS 36-1451). No typical diffraction peaks of RGO nanosheets are observed due to the low amount of RGO in the composite.53 From the comparison between the XRD patterns of ZGC-2 and ZGC-0, as shown in the inset of Fig. 3, a weak (002) diffraction peak at 26.2° should be contributed from the CNTs.54
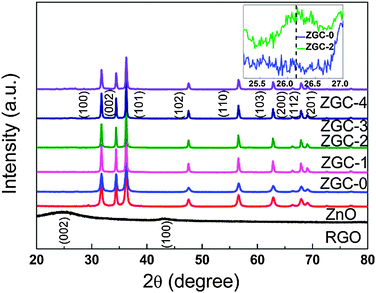 | ||
| Fig. 3 XRD patterns of RGO, ZnO, ZGC-0, ZGC-1, ZGC-2, ZGC-3 and ZGC-4. Inset is the magnified patterns of ZGC-0 and ZGC-2. | ||
The UV-vis absorption spectra of RGO, ZnO, ZGC-0, ZGC-1, ZGC-2, ZGC-3, and ZGC-4 are shown in Fig. 4. The absorption peak of RGO at 265 nm is generally regarded as the excitation of the π-plasmon of the graphitic structure.19 The absorption intensity is enhanced and the peak at 365 nm exhibits a small red-shift when CNT are introduced into the composite, which should be due to the chemical interaction between the CNTs and the semiconductor photocatalyst. This phenomenon is similar to the result in the case of TiO2–CNT composite materials.55,56
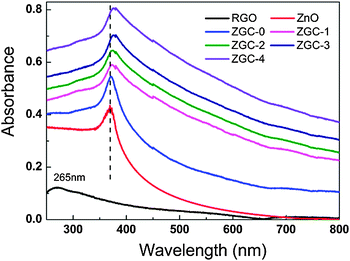 | ||
| Fig. 4 UV-vis absorption spectra of RGO, ZnO, ZGC-0, ZGC-1, ZGC-2, ZGC-3 and ZGC-4. | ||
The photocatalytic degradation of MB under UV irradiation was used to evaluate the photocatalytic performance of ZnO, ZC, ZGC-0, ZGC-1, ZGC-2, ZGC-3 and ZGC-4, as shown in Fig. 5. It is observed that the concentration of MB is hardly reduced under UV light irradiation in the absence of the photocatalyst. The photocatalytic degradation efficiencies are calculated to be 88% and 81.6% for ZGC-0 (ZnO–RGO) and ZC (ZnO–CNT), respectively. When CNTs are introduced into ZnO–RGO, the degradation efficiency is increased to 91.5% for ZGC-1 and reaches the maximum value of 96% for ZGC-2. It is known that during photocatalysis, the light absorption and the charge transportation and separation are crucial factors.11 The enhancement of the photocatalytic performance should be mainly ascribed to the increase of the light absorption in the presence of CNTs in the ZnO–RGO and the stepwise structure of the energy levels constructed in the ZnO–RGO–CNT composite,36 as shown in Fig. 6. The conduction band of ZnO is −4.05 eV (vs. vacuum) and valence band −7.25 eV (vs. vacuum).57 The work functions of RGO and CNTs are −4.42 eV and −4.8 eV.58,59 On the basis of the relevant band positions of ZnO, RGO and CNTs, photo-induced electrons easily transfer from the ZnO conduction band to CNTs via RGO, which could efficiently separate the photo-induced electrons and hinder the charge recombination in electron-transfer processes, thus enhancing the photocatalytic performance. Another possible contribution to the enhanced photocatalytic performance is the synergistic effect of RGO and CNTs. The incorporation of CNTs into RGO forms a conductive network structure to bridge the gaps between RGO nanosheets. Such a conjugated network significantly increases the electrical conductivity and provides fast electronic conducting channels for photocatalysis.34,35 When the CNT content is further increased above its optimum value, the photocatalytic performance deteriorates. This is due to the following reasons: (i) a larger amount of CNTs may filter light and decreases the number of charge carriers in the composite to be photogenerated.60 (ii) the excessive CNTs can act as a kind of recombination center instead of providing an electron pathway.13
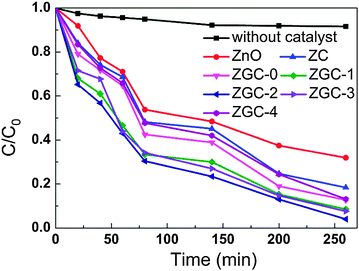 | ||
| Fig. 5 Photocatalytic degradation of MB by ZnO, ZC, ZGC-0, ZGC-1, ZGC-2, ZGC-3 and ZGC-4 under UV light irradiation. | ||
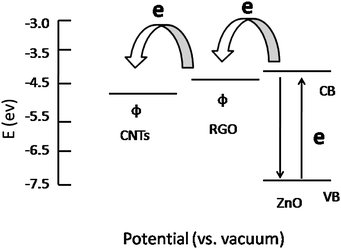 | ||
| Fig. 6 Schematic diagram of the energy levels of ZnO, RGO and CNTs. CB, VB and Φ are the conduction band, valence band, and work function. | ||
The pseudo-first-order kinetic equation was also used to evaluate the photocatalytic activity by fitting the experimental data for ZnO, ZC, ZGC-0, ZGC-1, ZGC-2, ZGC-3 and ZGC-4. The values of rate constants (Kapp) can be obtained directly from the fitted straight-line plots of ln(Ct/C0) versus reaction time t and follow the order: ZGC-2 (0.011 min−1) > ZGC-3 (0.01033 min−1) > ZGC-1 (0.00874 min−1) > ZGC-0 (0.00778 min−1) > ZGC-4 (0.00736 min−1) > ZC (0.00644 min−1) > ZnO (0.00442 min−1), where C0 and Ct are the initial concentration and the concentration of MB at reaction time t (mg l−1), respectively.24 ZGC-2 exhibits the best photocatalytic activity with a rate constant much higher than those of ZnO, ZnO–CNT and ZnO–RGO under UV light irradiation.
4. Conclusions
ZnO–RGO–CNT composites were successfully synthesized via microwave-assisted reduction of a GO dispersion in zinc nitrate solution with a CNT suspension. The results of photocatalytic experiments indicate that the incorporation of CNTs can enhance the photocatalytic performance and the ZnO–RGO–CNT composite with 3.9 wt% CNTs achieves a maximum degradation efficiency of 96%. The increased light absorption and the reduced charge recombination with the introduction of CNTs are responsible for the enhanced photocatalytic activity of the ZnO–RGO composite.Acknowledgements
This work was supported by a Special Project for Nanotechnology of Shanghai (No. 1052nm02700).Notes and references
- K. Inumaru, M. Yasui, T. Kasahara, K. Yamaguchi, A. Yasuda and S. Yamanaka, J. Mater. Chem., 2011, 21, 12117 RSC.
- H. Q. Li, J. Qu, Q. Z. Cui, H. Xu, H. M. Luo, M. F. Chi, R. A. Meisner, W. Wang and S. Dai, J. Mater. Chem., 2011, 21, 9487 RSC.
- S. Y. Yang, P. N. Zhu, A. S. Nair and S. Ramakrishna, J. Mater. Chem., 2011, 21, 6541 RSC.
- Y. W. L. Lim, Y. X. Tang, Y. H. Cheng and Z. Chen, Nanoscale, 2010, 2, 2751 RSC.
- Y. Liao, W. Que, Z. Tang, W. Wang and W. Zhao, J. Alloys Compd., 2011, 509, 1054 CrossRef CAS.
- O. Akhavan, M. Mehrabian, K. Mirabbaszadeh and R. Azimirad, J. Phys. D: Appl. Phys., 2009, 42, 225305 CrossRef.
- D. L. Jian, P. H. Gao, W. J. Cai, B. S. Allimi, S. P. Alpay, Y. Ding, Z. L. Wang and C. Brooks, J. Mater. Chem., 2009, 19, 970 RSC.
- Y. H. Sun, Q. Zhao, J. Y. Gao, Y. Ye, W. Wang, R. Zhu, J. Xu, L. Chen, J. Yang, L. Dai, Z. M. Liao and D. P. Yu, Nanoscale, 2011, 3, 4418 RSC.
- N. M. Mahmoodi, M. Arami and J. Zhang, J. Alloys Compd., 2011, 509, 4754 CrossRef CAS.
- J. Mu, C. Shao, Z. Guo, Z. Zhang, M. Zhang, P. Zhang, B. Chen and Y. Liu, ACS Appl. Mater. Interfaces, 2011, 3, 590 CAS.
- Y. Yan, T. Chang, P. C. Wei, S. Z. Kang and J. Mu, J. Dispersion Sci. Technol., 2009, 30, 198 CrossRef CAS.
- L. Jiang and L. Gao, Mater. Chem. Phys., 2005, 91, 313 CrossRef CAS.
- X. J. Wang, S. W. Yao and X. B. Li, Chin. J. Chem., 2009, 27, 1317 CrossRef CAS.
- L. P. Zhu, G. H. Liao, W. Y. Huang, L. L. Ma, Y. Yang, Y. Yu and S. Y. Fu, Mater. Sci. Eng., B, 2009, 163, 194 CrossRef CAS.
- K. Byrappa, A. S. Dayananda, C. P. Sajan, B. Basavalingu, M. B. Shayan, K. Soga and M. Yoshimura, J. Mater. Sci., 2008, 43, 2348 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498 CrossRef CAS.
- O. Akhavan, Carbon, 2011, 49, 11 CrossRef CAS.
- J. L. Wu, X. P. Shen, L. J. Jiang, K. Wang and K. M. Chen, Appl. Surf. Sci., 2010, 256, 2826 CrossRef CAS.
- T. G. Xu, L. W. Zhang, H. Y. Cheng and Y. F. Zhu, Appl. Catal., B, 2011, 101, 382 CrossRef CAS.
- Y. Yang, L. L. Ren, C. Zhang, S. Huang and T. X. Liu, ACS Appl. Mater. Interfaces, 2011, 3, 2779 CAS.
- B. J. Li and H. Q. Cao, J. Mater. Chem., 2011, 21, 3346 RSC.
- T. Kavitha, A. I. Gopalan, K. P. Lee and S. Y Park, Carbon, 2012, 50, 2994 CrossRef CAS.
- T. Lv, L. K. Pan, X. J. Liu, T. Lu, G. Zhu and Z. Sun, J. Alloys Compd., 2011, 509, 10086 CrossRef CAS.
- G. Zhu, L. K. Pan, H. C. Sun, X. J. Liu, T. Lv, T. Lu, J. Yang and Z. Sun, ChemPhysChem, 2012, 13, 769 CrossRef CAS.
- W. Yang, E. Widenkvist, U. Jansson and H. Grennberg, New J. Chem., 2011, 35, 780 RSC.
- D. R. Dreyer, S. Park, C. W. Bielawsk and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228 RSC.
- W. Cai, R. D. Piner, F. J. Stadermann, S. Park, M. A. Shaibat, Y. Ishii, D. Yang, A. Velamakanni, S. J. An, M. Stoller, J. An, D. Chen and R. S. Ruoff, Science, 2008, 321, 1815 CrossRef CAS.
- T. K. Hong, D. W. Lee, H. J. Choi, H. S. Shin and B. S. Kim, ACS Nano, 2010, 4, 3861 CrossRef CAS.
- V. C. Tung, L. M. Chen, M. J. Allen, J. K. Wassei, K. Nelson, R. B. Kaner and Y. Yang, Nano Lett., 2009, 9, 1949 CrossRef CAS.
- C. Li, Z. Li, H. Zhu, K. Wang, J. Wei, X. Li, P. Sun, H. Zhang and D. Wu, J. Phys. Chem. C, 2010, 114, 14008 CAS.
- H. Y. Jeong, D. S. Lee, H. K. Choi, D. H. Lee, J. E. Kim, J. Y. Lee, W. J. Lee, S. O. Kim and S. Y. Choi, Appl. Phys. Lett., 2010, 96, 213105 CrossRef.
- D. Cai, M. Song and C. Xu, Adv. Mater., 2008, 20, 1706 CrossRef CAS.
- T. Lu, L. K. Pan, H. B. Li, C. Y. Nie, M. F. Zhu and Z. Sun, J. Electroanal. Chem., 2011, 661, 270 CrossRef CAS.
- G. Zhu, L. K. Pan, T. Lu, T. Xu and Z. Sun, J. Mater. Chem., 2011, 21, 14869 RSC.
- Q. Su, Y. Liang, X. Feng and K. Mullen, Chem. Commun., 2010, 46, 8279 RSC.
- L. L. Zhang, Z. G. Xiong and X. S. Zhao, ACS Nano, 2010, 4, 11 CrossRef.
- H. Wu, P. Cao, W. Li, N. Ni, L. Zhu and X. Zhang, J. Alloys Compd., 2011, 509, 1261 CrossRef CAS.
- R. Dwivedi, A. Maurya, A. Verma, R. Prasad and K. S. Bartwal, J. Alloys Compd., 2011, 509, 6848 CrossRef CAS.
- G. V. Bazuev, A. P. Tyutyunnik, I. F. Berger, I. V. Nikolaenko and B. G. Golovkin, J. Alloys Compd., 2011, 509, 6158 CrossRef CAS.
- G. Zhu, L. K. Pan, T. Xu and Z. Sun, ACS Appl. Mater. Interfaces, 2011, 3, 1472 CAS.
- G. Zhu, L. K. Pan, T. Xu, Q. F. Zhao, B. Lu and Z. Sun, Nanoscale, 2011, 3, 2188 RSC.
- T. Lu, L. K. Pan, H. B. Li, G. Zhu, T. Lv, X. J. Liu, Z. Sun, T. Chen and D. H. C. Chua, J. Alloys Compd., 2011, 509, 5488 CrossRef CAS.
- H. B. Li, T. Lu, L. K. Pan, Y. P. Zhang and Z. Sun, J. Mater. Chem., 2009, 19, 6773 RSC.
- T. Lu, Y. P. Zhang, H. B. Li, L. K. Pan, Y. L. Li and Z. Sun, Electrochim. Acta, 2010, 55, 4170 CrossRef CAS.
- H. B. Li, L. K. Pan, T. Lu, Y. K. Zhan, C. Y. Nie and Z. Sun, J. Electroanal. Chem., 2011, 653, 40 CrossRef CAS.
- G. Zhu, T. Xu, T. Lv, L. K. Pan, Q. F. Zhao and Z. Sun, J. Electroanal. Chem., 2011, 650, 248 CrossRef CAS.
- X. J. Liu, L. K. Pan, T. Lv, G. Zhu, Z. Sun and C. Q. Sun, Chem. Commun., 2011, 47, 11984 RSC.
- X. J. Liu, L. K. Pan, T. Lv, G. Zhu, T. Lu, Z. Sun and C. Q. Sun, RSC Adv., 2011, 1, 1245 RSC.
- X. J. Liu, L. K. Pan, T. Lv, T. Lu, G. Zhu, Z. Sun and C. Q. Sun, Catal. Sci. Technol., 2011, 1, 1189 CAS.
- H. A. Becerril, J. Mao, Z. Liu, R. M. Stoltenberg, Z. Bao and Y. Chen, ACS Nano, 2008, 2, 463 CrossRef CAS.
- L. Tang, Y. Wang, Y. Li, H. Feng, J. Lu and J. Li, Adv. Funct. Mater., 2009, 19, 2782 CrossRef CAS.
- Y. L. Chen, Z. A. Hu, Y. Q. Chang, H. W. Wang, Z. Y. Zhang, Y. Y. Yang and H. Y. Wu, J. Phys. Chem. C, 2011, 115, 2563 CAS.
- J. Yan, L. F. Yi and W. R. Zhong, J. Serb. Chem. Soc., 2005, 70, 277 CrossRef CAS.
- Y. Guo, H. S. Wang, C. L. He, L. J. Qiu and X. B. Cao, Langmuir, 2009, 25, 4678 CrossRef CAS.
- P. N. Zhu, A. Sreekumaran, S. Y. Yang and R. Seeram, Mater. Res. Bull., 2011, 46, 588 CrossRef CAS.
- B. J. Li and H. Q. Cao, J. Mater. Chem., 2011, 21, 3346 RSC.
- R. Czerw, B. Foley, D. Tekleab, A. Rubio, P. M. Ajayan and D. L. Carroll, Phys. Rev. B: Condens. Matter, 2002, 66, 033408 CrossRef.
- Y. Yao, G. Li, S. Ciston, R. M. Lueptow and K. A. Gray, Environ. Sci. Technol., 2008, 42, 4952 CrossRef CAS.
- Y. J. Xu, Y. B. Zhuang and X. Z. Fu, J. Phys. Chem. C, 2010, 114, 2669 CAS.
| This journal is © The Royal Society of Chemistry 2012 |
