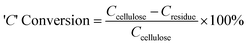Conversion of cellulose to polyols over promoted nickel catalysts†
Abhijit
Shrotri‡
a,
Akshat
Tanksale‡
*b,
Jorge Norberto
Beltramini
a,
Hanmant
Gurav
c and
Satyanarayana V.
Chilukuri
c
aARC Centre of Excellence for Functional Nanomaterials, University of Queensland, Brisbane, QLD 4072, Australia. E-mail: a.tanksale@uq.edu.au; Fax: +61 7 3346 3973; Tel: +61 7 3346 3807
bDepartment of Chemical Engineering, Monash University, Clayton, VIC 3800, Australia. E-mail: akshat.tanksale@monash.edu; Fax: +61 3 9905 5686; Tel: +61 3 9902 4388
cNational Chemical Laboratory, Dr Homi Bhabha Road, Pune 411008, India. E-mail: sv.chilukuri@ncl.res.in; Fax: +91 20 2590 2633; Tel: +91 20 2590 2019
First published on 14th May 2012
Abstract
Sorbitol is one of the key platform chemicals that can be applied to several industrial applications, including bio-fuels and hydrogen production. Presently there is no commercial heterogeneous catalytic process to produce sorbitol from cellulose due to the low yield and high cost of noble metals required for the conversion. In this paper we describe an aqueous phase hydrolysis–hydrogenation process to convert cellulose to sorbitol using a cheap Ni based catalyst. Monometallic Ni catalysts showed little activity for the reaction, but with the addition of a small amount of Pt to the Ni catalyst (Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt = 22
Pt = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 atom ratio), the activity was greatly enhanced. Results showed that the bimetallic Ni–Pt catalysts supported on mesoporous alumina gave a hexitol (sorbitol + mannitol) yield of 32.4% compared to only 5% with a Ni catalyst. Moreover, Ni–Pt supported on a mesoporous beta zeolite support provided even higher yield of 36.6%. These results were obtained after only 6 hours of run at 200 °C and 50 bar H2 pressure (at room temperature). The presence of a small amount of Pt promotes the protonation of water and hydrogen molecules, which spill over to Ni sites creating in situ acid sites to catalyse hydrolysis of cellulose.
1 atom ratio), the activity was greatly enhanced. Results showed that the bimetallic Ni–Pt catalysts supported on mesoporous alumina gave a hexitol (sorbitol + mannitol) yield of 32.4% compared to only 5% with a Ni catalyst. Moreover, Ni–Pt supported on a mesoporous beta zeolite support provided even higher yield of 36.6%. These results were obtained after only 6 hours of run at 200 °C and 50 bar H2 pressure (at room temperature). The presence of a small amount of Pt promotes the protonation of water and hydrogen molecules, which spill over to Ni sites creating in situ acid sites to catalyse hydrolysis of cellulose.
1. Introduction
Carbohydrates are one of the most abundant and economical bio-materials available in Nature, yet their non-food utilization is confined to textile, paper and coating industries. As carbohydrates are nontoxic, and biodegradable compounds, they can provide important environmental benefits to synthesise new platform chemicals.1 Carbohydrates form a large family of compounds with considerable structural variety that combines a number of carbon atom backbones with a high density of functional groups. Polysaccharides form the bulk of the renewable lignocellulosic biomass. However, the key platform chemicals1 are usually made of low molecular weight compounds (≤C6), so they are more readily obtained from low molecular weight carbohydrates such as C5 or C6 sugars. The challenge therefore is to utilise the polysaccharides like cellulose, starch, inulin and xylan that are inexpensive and available in large quantities but difficult to process as a feedstock for selective conversion to desired products. In this paper we describe a method for converting cellulose into key platform chemicals – sorbitol, mannitol and glycerol, which can be further converted to make value added chemicals, liquid fuels or hydrogen (Fig. 1). Sorbitol is an important platform chemical1 because it can be converted to several value added chemicals like iso-sorbide2,3 or renewable fuels like hydrogen4 and can be used unmodified as a sweetener in consumer products like toothpaste. | ||
| Fig. 1 Conversion of cellulose to key platform chemical sorbitol, which can be converted to hydrogen and value added chemicals. | ||
Traditionally, cellulose has been converted to glucose by dilute or concentrated acid hydrolysis.5–7 However, this process suffers from acid corrosion and glucose degradation at high acid concentration. Recently, several researchers have focused on using heterogeneous catalysts for hydrolysis of cellulose, following initial reports by Dhepe et al.8,9 whereby cellulose can be directly converted to sorbitol over a supported metal catalyst, in the presence of a hydrogen rich gas. This process has several advantages over the conversion of cellulose to glucose. In the presence of acids and under hydrothermal conditions, glucose is not stable and leads to the formation of various by-products, mainly due to the dehydration reaction. By using a hydrogen rich environment, glucose is rapidly converted to sorbitol, which is relatively stable under these conditions. Various types of supported metal catalysts have been tested for this reaction. Initially zeolite supports were considered for the reaction,8 but they are not very active due to the large number of pores in the microporous range. Most of the mesoporous catalysts require acid functionalization with sulphonic groups such as sulphonated silicas9 and carbon.10 However, under hot compressed water conditions the functionalised mesoporous silicas are not stable.11,12 Fukuoka and Dhepe reported that Pt and Ru supported on γ-alumina and SiO2–Al2O3 were among the best supports for high sorbitol yield.8 Ru supported on nitric acid functionalised carbon nanotubes has also been reported with some success.13 However, there is a need for a low cost catalyst, which can effectively replace or reduce the precious metal catalysts. In this regard we tested Ni-based catalysts, which are widely used in existing industries due to their low cost and availability. Ni is known to be a good hydrogenation catalyst, therefore, it is expected that Ni may perform well as a catalyst for glucose hydrogenation to sorbitol, as glucose is an intermediate product in this reaction. However, since the rate determining step is cellulose hydrolysis which forms glucose,14 an acid site is required to catalyse the hydrolysis reaction. Molecular hydrogen is known to dissociatively adsorb on transition metals and produce hydrogen atoms. These atoms spill over on supports and migrate away from the metal site where the atom donates its electron to a Lewis acid site to form a proton.15,16 This proton acts as an active site for many acid catalysed reactions.17 Dhepe and Fukuoka14 recently demonstrated that supported precious metals such as Pt and Ru can protonate hydrogen and water molecules during the reaction to produce in situ acid sites, which may catalyse the hydrolysis reaction. Therefore, in this research we tested the effect of Pt promotion on Ni catalysts supported on alumina nano-fibre, mesoporous alumina and mesoporous beta zeolites with different ratios of Si/Al. These supports were chosen because of their different level of acidity. We prepared monometallic catalysts of Pt and Ni and co-impregnated Ni–Pt catalysts on these supports. In an earlier study we reported the promoting effect of Pt and Pd on Ni catalysts supported on alumina nano-fibre for the aqueous phase reforming of sorbitol to produce hydrogen.4,18 It showed that when Ni and Pt are co-impregnated on an alumina support, the catalytic activity is enhanced significantly. This is due to the increased reducibility of Ni, reduction in the heat of CO-chemisorption, and promotion of the water gas shift reaction.4 In this study we report the synergistic effect of Ni–Pt bimetallic catalysts on hydrolysis–hydrogenation of cellulose to produce sorbitol.
2. Experimental
2.1 Catalyst preparation and testing
Alumina nano-fibre (Alnf) was prepared using the method described in our previous report.4 Mesoporous alumina (Al2O3) was prepared by sol–gel synthesis. 4 g of Laponite clay, which was used as a pillaring agent, was suspended in 200 mL of water and stirred until a clear solution was achieved. 20 g of polyethylene oxide surfactant Tergitol 15-S-9 was added to the solution and stirred for 2 hours. To this solution 20 mL of Locron-L solution was added drop-wise with continuous stirring. The resulting solution was transferred to an autoclave to age for 2 days at 100 °C. The resulting white precipitate was centrifuged and washed with deionised water six times. The resulting white cake was calcined at 500 °C for 2 hours with a heating rate of 2 °C min−1. A beta zeolite (Beta_N, where N = Si/Al) with N = 38 was acquired from M/s Zeolyst International, PA, USA. To increase the Si/Al ratio dealumination was carried out by steam treating the as received beta zeolite followed by removal of dislodged aluminium. Three types of Beta_N supports with different Si/Al ratios – 38, 75 and 150 – were used in this research. Ni, Pt and Pd metals were impregnated using appropriate quantities of their chemical precursors, Ni(NiO3)2·6H2O (Univar), 8 wt% H2PtCl6 in water (Sigma) and 10 wt% Pd(NO3)2 in 10 wt% HNO3 (Sigma), respectively. The precursors were added to the supports and heated to 50 °C in the presence of a small amount of water and stirred constantly for 5–6 hours. The resultant solution was dried overnight in a 90 °C oven and calcined at 500 °C for 5 h. The catalysts were then reduced in a fixed bed reactor at 400 °C for 5 h prior to the catalytic run.Catalysts were tested for cellulose conversion in a Parr high pressure batch reactor at 50 bar H2 pressure (at room temperature) and 200 °C for 6 hours. 20 g L−1 of Sigmacell Cellulose 20 μm (Sigma) was prepared in 300 mL of deionised water and 1.5 g of catalyst was added to the reactor before pressurising and heating. After the reaction was complete, the resulting solution was filtered using a 0.45 μm filter to recover the catalyst mixed with unconverted cellulose. The solution was filtered again with a 0.22 μm filter for HPLC analysis.
2.2 Post run characterisation
HPLC analysis was carried out in a Shimadzu Prominence HPLC system using a Rezex RCM Monosaccharide column (7.8 × 300 mm) with 8% Ca cross linking with an oven temperature set at 65 °C and a water (mobile phase) flow rate of 0.6 mL min−1. The products from the reaction were detected using a Shimadzu low temperature ELSDII detector at 40 °C and 370 kPa N2 pressure. The sorbitol and mannitol yield was calculated by dividing the measured concentration of sorbitol or mannitol (in mg L−1) by 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1, which was the initial cellulose concentration.
000 mg L−1, which was the initial cellulose concentration.
Carbon conversion was calculated using two methods – Total Organic Carbon (TOC) analysis in the aqueous phase and elemental carbon analysis in the solid residue. TOC was measured using a Shimadzu TOC-VCHS analyser. The total carbon content was analysed by injecting 50 μL of solution and oxidising in the presence of a catalyst at 680 °C to produce CO2, which was detected on a non-dispersive infrared (NDIR) detector. Total inorganic carbon was analysed by reacting 50 μL of solution with HCl, which is volatilised by sparging, and the emitted CO2 detected on an NDIR detector. TOC is calculated by subtracting total inorganic carbon from the total carbon content. TOC conversion was calculated using the following formula
Solid carbon content in the catalyst residue was measured using a Thermo Electron Corporation FlashEA® 1112 series CHNS-O analyser. 2–3 mg of sample was placed in a tin container, which was combusted in a furnace at 900 °C. The product gases were separated in a chromatographic column and analysed on a Thermal Conductivity Detector. The ‘C’ conversion, based on solid elemental analysis, was calculated using the following formula
2.3 Fresh catalyst characterisation
The supports were characterised with N2 physisorption using a Quantachrome Quadrasorb SI instrument at liquid nitrogen temperature, for pore size distribution, pore volume and surface area. Powder X-Ray Diffraction (XRD) of the calcined catalysts was carried out in a Rigaku MiniFlex instrument to analyse the support and metal particle size and phases. These XRD results were also used as a reference for the post run XRD of the used catalysts, which contained unconverted cellulose particles. The metal content of fresh and used catalysts was analysed by digesting 40 mg of catalyst in 5 mL of nitric acid and 4 mL of hydrofluoric acid using a CEM MSP 1000 microwave digester. Samples were diluted to 50 mL. Digested solid samples were analysed using a Varian Vista Pro ICPOES instrument.Ammonia (NH3) Temperature Programmed Desorption (TPD) measurements were carried out using Micromeritics Autochem 2920. About 100 mg of catalyst was activated in a helium flow for 2 hours at 550 °C. The sample was then cooled to 100 °C for the NH3 sorption. Mixed gas containing 10% NH3 in helium (20 mL min−1) was passed through the sample for 0.5 hour, followed by passing pure helium for 0.5 hour to remove physisorbed NH3. The TPD experiments were carried out in the temperature range of 100 °C to 600 °C at a heating rate of 10 °C min−1. The resultant peaks were integrated in the instrument software and the amount of NH3 desorbed was calculated against a calibration curve, which is made by passing pulses of known NH3 standard.
Carbon monoxide (CO) TPD was carried out to determine the dispersion of metals on a bimetallic beta zeolite catalyst. In a typical method about 200 mg of catalyst was loaded on the reaction cell and was reduced at 400 °C for 4 hours using a hydrogen and nitrogen gas mixture (50 mL min−1). The catalyst was purged with helium at 400 °C to remove adsorbed H2. The sample was cooled to room temperature under He flow. A 10% CO–He mixture was passed for 15 min to complete adsorption of CO on metal sites. Residual CO was purged with helium until CO base line reading was constant. Temperature programmed desorption was carried out in the temperature range of 30 °C to 500 °C at a heating rate of 10 °C min−1 under He flow of 40 mL min−1. The desorbed gas was detected using a thermal conductivity detector. Metal dispersion was calculated assuming that surface concentration of metals was equal to bulk concentration.19
3. Results and discussion
3.1 Catalyst properties
The catalyst properties shown in Table 1 include support acidity, BET surface area, BJH pore volume and catalyst metal loadings. The BET surface area does not change much with the changing Si/Al ratio in the beta zeolites. The N2 adsorption isotherm of beta zeolites (Fig. 2) shows that the catalyst is microporous and there is increased adsorption in the mesoporous range after the dealumination process. Appearance of large pores in Beta_75 and Beta_150 suggested significant changes in structure of catalysts after dealumination. High resolution TEM images of beta zeolites (see Fig S1, ESI†) show an increase in the pore width after the dealumination treatment. TEM images also inferred that the large pores seen in N2 adsorption analysis were interparticle channels formed due to breaking and loose agglomeration of particles during the dealumination process. Results in Table 1 show that reducing the alumina concentration has a reducing effect on the acidity of the beta zeolite supports. The highest total acidity (weak + strong acidity) was 0.794 mmol g−1 for Beta_38 (Si/Al = 38), which reduces to less than 1/3rd to 0.226 mmol g−1 for Beta_150 (Si/Al = 150). The metal concentration as calculated from the ICPOES analysis is approximately the same for all the catalysts. The Pt loading was kept at 3% w/w approximately and in the bi-metallic Ni–Pt catalyst Ni and Pt loadings were equal to 7% w/w and 1% w/w, respectively.| Support | Metal loading (% w/w) | Support | ||||||
|---|---|---|---|---|---|---|---|---|
| Mono-metallic (Pt) | Bi-metallic Co-impregnated (Ni–Pt) | Al in sample, mmol g−1 | BJH pore volume, cm3 g−1 | BET surface area, m2 g−1 | Acidity by NH3 TPD, mmol g−1 | |||
| Ni | Pt | Weak | Strong | |||||
| Alnf | 2.91 | 6.50 | 0.96 | — | 1.19 | 303 | 0.106 | 0.091 |
| Al2O3 | 3.08 | 7.32 | 0.98 | — | 0.93 | 531 | 0.103 | 0.090 |
| Beta_38 | 2.72 | 7.67 | 0.90 | 0.839 | 0.71 | 524 | 0.502 | 0.292 |
| Beta_75 | 3.44 | 7.31 | 0.86 | 0.434 | 0.63 | 538 | 0.256 | 0.215 |
| Beta_150 | 3.16 | 8.22 | 1.17 | 0.219 | 0.64 | 535 | 0.142 | 0.084 |
 | ||
| Fig. 2 N2 physisorption isotherm of beta zeolite supports with various Si/Al ratios; the inset shows pore size distribution of these supports – circles: Si/Al = 38, squares: Si/Al = 75 and triangles: Si/Al = 150. | ||
XRD diffraction patterns of all the catalysts supported on the beta zeolite supports with different Si/Al ratios are shown in Fig. 3. The large peaks at 2θ values of 8.8° and 26.25° match the beta zeolite peaks according to JCPDS cards and also those reported in the literature.20,21 The peaks at 2θ values of 46.6°, 54.5° and 80.3° correspond to PtO2, while the peaks at 43.2°, 50.8° and 74.3° correspond to NiO. PtO2 peaks are not visible for the bi-metallic catalysts due to the relatively low concentration of loading. These catalysts were not reduced before the XRD run; therefore the active metal species are present in their oxide form.
 | ||
| Fig. 3 XRD patterns of catalysts supported on beta zeolites with Si/Al ratios of 38 (Beta_38), 75 (Beta_75) and 150 (Beta_150). | ||
CO TPD results of bimetallic catalysts on beta zeolite supports show the CO desorption peak centered at about 150 °C (Fig. 4). These peaks represent the active metal sites with low heat of adsorption.4 Ni–Pt/Beta_38 showed least activity for CO adsorption in this region. This may be due to formation of large metal particles on the outer surface of the catalyst as the micropores in beta zeolites are too small for particle formation inside via the impregnation method. Dealumination treatments of the Beta_38 support increased the pore width and also created interparticle channels which assisted in formation of smaller particles, increasing the metal surface area. The metal dispersion of the catalysts was in the following increasing order – Ni–Pt/Beta_38 (3.90%) < Ni–Pt/Beta_150 (5.00%) < Ni–Pt/Beta_75 (5.75%).
 | ||
| Fig. 4 Rate of CO desorption for bimetallic catalysts on beta zeolite supports. Ni–Pt/Beta_38 (○), Ni–Pt/Beta_75 (□), Ni–Pt/Beta_150 (△). | ||
3.2 Cellulose conversion over alumina supported catalysts
The yields of sorbitol and mannitol as determined by HPLC analysis; and cellulose “C” conversion as determined by carbon elemental analysis from the solid residue are shown in Fig. 5. The Ni monometallic catalysts did not show good activity for the conversion of cellulose, which was less than 24% for both the Ni catalysts. The Pt supported catalysts showed higher conversion of cellulose with 36.4% and 40.7% for Alnf and mesoporous Al2O3 supported catalysts, respectively. At the same time, the yields of sorbitol and mannitol also increased substantially to a combined yield of 26.8% and 32.1% for Alnf and Al2O3 supported catalysts, respectively. If the selectivity of C6 polyols (hexitols) is defined asthen we find that the selectivity for the two Pt supported catalysts is 74% and 79% respectively. The conversion, yield and selectivity values for the Al2O3 supported catalyst are slightly higher than those for the Alnf supported catalyst. This may be due to the higher surface area of the support, which may provide higher Pt particle dispersion. Bi-metallic Ni–Pt catalysts provided the highest conversion of 44.0% and 48.7% for the Alnf and Al2O3 supported catalysts, respectively. However, the selectivity is lower compared to that of Pt catalysts of 65.8% and 66.5%, respectively. Despite lower selectivity, the overall yield is slightly higher due to the higher conversion. XRD analysis was done on the used catalysts (Fig. 6) which contained unconverted cellulose to confirm the cellulose conversion results (also see Fig. S2, ESI†). The bottom curve shows the XRD pattern of the feed cellulose powder, which shows a microcrystalline structure with three prominent peaks at 2θ = 18.2°, 26.1° and 40.3°. The corresponding peak intensities reduce in the order of Ni/Alnf > Pt/Alnf and Ni–Pt/Alnf for the used catalysts. For the Ni–Pt/Alnf catalyst the cellulose peaks are not visible at this scale. This indicates that the amount of microcrystalline cellulose is very small compared to the other two catalysts, which supports the ‘C’ conversion results.
 | ||
| Fig. 5 Sorbitol and mannitol yield and cellulose ‘C’ conversion (circles) from the aqueous phase hydrolysis–hydrogenation of cellulose at 200 °C and 50 bar H2 pressure (at room temperature) for 6 hours, using supported alumina catalysts. | ||
 | ||
| Fig. 6 XRD pattern of 20 μm cellulose powder and the used Ni, Pt and Ni–Pt catalysts supported on alumina nano-fibre (Alnf). After the reaction is complete the used catalysts were filtered with a 0.45 μm filter and then washed with deionised water to remove soluble products. | ||
Hexitol yield obtained with the bimetallic Ni–Pt catalysts is significantly higher than that with the Ni catalysts. Therefore it shows that a small amount of Pt promotes the Ni catalyst significantly to improve its catalytic activity. This is due to the synergistic effects between Pt and Ni particles when they are in close vicinity.4,18,22
In previous studies it was shown that the bi-metallic catalysts showed very different properties than the corresponding monometallic catalyst, which changes the binding energies of the adsorbed species.22–24 Supported Pt particles are known to assist in the protonation of water and molecular H2 by the hydrogen spillover effect, which provides in situ acidic sites for hydrolysis of cellulose.14
Our results suggest that the Ni monometallic catalyst is unable to generate this acidity and therefore the cellulose conversion is low. In Ni–Pt bimetallic catalysts Pt atoms promote protonation of water and H2 to initiate hydrolysis. In the H2 rich atmosphere Pt sites dissociate molecular H2 into atomic hydrogen (H+), which spills over to the Ni sites to create in situ acidity for hydrolysis reaction. The role of Pt promotion studied earlier showed that bi-metallic Ni–Pt catalysts have lower reduction temperature than Ni catalysts.4 Pt is able to react with molecular H2 at a lower temperature than Ni and the resultant adsorbed H+ reacts with Ni particles at significantly lower temperatures. In the absence of Pt particles much higher temperatures are required for Ni particles reduction.
Ni and Pt are known hydrogenation catalysts, with Ni showing slightly better activity than Pt in a gas phase reaction.23 Under hydrothermal conditions at low temperature however, Ni is not very active as shown in our previous results with aqueous phase reforming of oxygenated hydrocarbons.4,25 With a Pt promoter the spillover hydrogen adsorbed on the Ni site is able to hydrogenate the intermediate glucose. Therefore conversion of glucose into sorbitol happens rapidly. This is indicated from the fact that we did not detect any glucose in the product mixture in the HPLC analysis. The results in this paper show some improvement over the the reported literature in terms of the residence time of the reactants. Each of our experiments was carried out over 6 hour duration, while previous reports have used a 24 hour run.8,10,13,26
3.3 Cellulose conversion over beta zeolite supported catalysts
Fig. 7 shows sorbitol and mannitol yields and cellulose conversion using the beta zeolite supported catalysts. The reaction conditions were identical to those of the alumina supported catalysts; however, for HPLC analysis the ELSDII temperature was reduced to 30 °C to detect volatile species produced during the reaction. As a result we were able to detect glycerol in the product which was not detected earlier when ELSDII was operated at 40 °C. The “C” conversion values in Fig. 7 obtained using the elemental carbon analysis were validated against those obtained using liquid TOC analysis and were found to be similar within the range of errors in the instrument precision and accuracy of manually handling the samples. | ||
| Fig. 7 Sorbitol, mannitol and glycerol yields and cellulose ‘C’ conversion (circles) and TOC conversion (triangles) from the aqueous phase hydrolysis–hydrogenation of cellulose at 200 °C and 50 bar H2 pressure (at room temperature) for 6 hours. ELSD temperature was lowered to 30 °C to detect glycerol during HPLC analysis. | ||
The results show similar trends as obtained with alumina catalysts. The beta zeolite support also showed highest cellulose conversion and hexitol yield with the bi-metallic Ni–Pt catalyst. The metal dispersion and support acidity, which were a result of the different Si/Al ratios in the samples, both affected the conversion. The cellulose “C” conversion and hexitol yield were in the following increasing order – Ni–Pt/Beta_38 (46.5% and 25.5%) < Ni–Pt/Beta_150 (48.2% and 30.3%) < Ni–Pt/Beta_75 (51.3% and 36.6%), respectively. The metal dispersion of the catalysts is in the following increasing order – Beta_38 < Beta_150 < Beta_75. This suggests that cellulose conversion is directly associated with the number of active metal sites present on the catalyst. These metal sites assist in formation of protonic acid sites via dissociation of molecular hydrogen by spillover.
These acid sites increase the rate of cellulose hydrolysis, increasing the overall conversion. It is also important to note that an optimum level of support acidity is required for higher yield of hexitols. If the acidity is too low the rate of hydrolysis is low. Whereas, higher acidity leads to degradation of glucose by dehydration reaction, lowering the hexitol yield. Therefore we find that the hexitol selectivity is in the following increasing order – Ni–Pt/Beta_38 (54.8%) < Ni–Pt/Beta_150 (62.9%) < Ni–Pt/Beta_75 (71.3%).
To confirm the synergistic effect of bi-metallic Ni–Pt catalysts, where Ni and Pt particles are in close proximity due to co-impregnation, a control run was performed where a physical mixture of Pt/Beta_75 and Ni/Beta_75 was put into the reaction mixture under identical reaction conditions. The metal loading of the mono-metallic catalysts in the control reaction mixture was chosen carefully to match the mass composition of the metals and supports used in the reaction mixture with bi-metallic Ni–Pt/Beta_75. From the results, as shown in Fig. 7, it is clear that the yield of hexitols and glycerol is significantly lower with a physical mixture of the mono-metallic catalysts compared to all the bi-metallic catalysts studied. This is not surprising since we know from our previous reports4,18,25 that Pt promotes the Ni sites during reduction of the catalysts thereby increasing the active metal surface area and dispersion. Therefore, we can conclude that the active sites of Ni and Pt have to be in close proximity to enhance the catalytic activity due to the synergistic effect of Ni–Pt interaction.
Glycerol is a product of sorbitol hydrogenolysis which takes place in the presence of an H2 rich atmosphere on a metal site (Scheme 1). Other products of sorbitol hydrogenolysis are propylene glycol, ethylene glycol, erythritol and xylitol (in decreasing order of their selectivity27). The reaction conditions in our study favour glycerol production through hydrogenolysis of sorbitol.27,28 A study by Clark27 showed that the rate of sorbitol hydrogenolysis is inversely proportional to the reaction pressure. Clark reported that the reaction follows first order kinetics and at 200 °C and 138 bar (2000 psi) the rate constant is 0.0112 min−1. Since the reaction pressure in our study at 200 °C is ∼80 bar we expect the glycerol production rate will be higher than that reported by Clark.
 | ||
| Scheme 1 Hydrogenolysis of sorbitol to produce glycerol, propylene glycol and ethylene glycol. | ||
Since propylene glycol and ethylene glycol are more volatile than glycerol they could not be detected in HPLC analysis. The presence of erythritol and xylitol in the product could not be confirmed due to their concentration being under the detection limit. Inclusion of the glycerol yield with that of hexitol does not change the trend in Fig. 7. The maximum polyol yield (glycerol + hexitol) in the product mixture was achieved by Ni–Pt/Beta_75 (46%) followed by Ni–Pt/Beta_150 (44.8%) and Ni–Pt/Beta_38 (40.1%).
Confirmation of the extent of cellulose conversion is provided by the XRD patterns of the used catalysts (Fig. 8). All the catalysts show only traces of cellulose microcrystalline peaks. The absolute intensity of the cellulose peaks in Fig. 6 is lower than that in Fig. 4, but since the scale of Fig. 6 is smaller, the apparent height of the peaks appears to be the same. Additionally, the cellulose peak at 26.1° overlaps with the beta zeolite peak at 26.25° making it undiscernible from each other and may give a false impression of high cellulose content. Overall, we can conclude that all the beta zeolite catalysts showed higher cellulose conversion by looking at the lower intensity of the XRD peaks.
 | ||
| Fig. 8 XRD patterns of the used Pt and Ni–Pt catalysts supported on beta zeolites with Si/Al ratios of 38 (Beta_38), 75 (Beta_75) and 150 (Beta_150). After the reaction was complete the used catalysts were filtered with a 0.45 μm filter and then washed with deionised water to remove soluble products. | ||
Recycling experiments were performed using Ni–Pt/Beta_75 to study the loss of catalytic activity. The used catalyst was calcined to remove unreacted cellulose, which was then reduced and tested again under the same reaction conditions by adding fresh cellulose feed. The results show reduction in catalytic activity after the 1st run. Cellulose conversion of 36.97% was obtained which is lower than the initial result of 51.3%. The yield of hexitols also reduced to 16.7%. Selectivity towards hexitols reduced from 71.3% to 45.17%. This reduction in activity and selectivity of catalyst is attributed to the loss of metal from the catalyst after the 1st run. Metal content of Beta_75 reduced to 0.28% Pt and 6.34% Ni. A significant decrease in platinum from the support surface resulted in reduction of the promoter effect in the bimetallic catalyst which caused a loss in activity. It is important to note that the catalyst support structure itself is not changed after the reaction. The XRD patterns of fresh and used catalysts are very similar. Therefore, it may be possible to reuse the catalysts in multiple runs by reducing the loss of metals via improved catalyst preparation techniques.
4. Conclusions
Hydrolysis of cellulose is one of the key steps in converting biomass to fuels and chemicals. However a cheap and reliable catalyst is required to utilise this technology on a large commercial scale. In this regard this report shows that Ni based catalysts can be used with high efficiency on non-functionalised supports, which are often used in current industries. Beta zeolite supports used in this study were modified by dealuminating the original Si/Al = 38 support, which increased the mesopores in the resulting samples. Supported bimetallic Ni–Pt catalysts on the modified beta zeolites showed a maximum conversion of 51.3% and a maximum polyol yield of 46%, which gives a selectivity of 90%. Moreover, this result was achieved in only 6 hours of the catalytic run. The bimetallic Ni–Pt catalysts, supported on the alumina nanofibre, mesoporous alumina and beta zeolites, all provided slightly better yield of hexitols when compared to the corresponding Pt catalysts with significant reduction in the Pt loading on these supports. Ni monometallic catalysts did not show good activity for cellulose conversion and hexitol yield. We believe this is due to the lack of in situ acid sites created in the absence of noble metal active sites. In the case of the bi-metallic catalysts the presence of a small amount of Pt promotes the protonation of water and molecular hydrogen, which then spills over to Ni sites creating a large amount of in situ acid sites. With the development of a much cheaper catalyst, the challenge now is to develop a continuous heterogeneous catalytic process for the conversion of cellulose to sorbitol to make this process viable for commercial application.References
-
T. Werpy and G. Petersen, Top Value Added Chemicals from Biomass Volume I-Results of Screening for Potential Candidates from Sugars and Synthesis Gas, National Renewable Energy Laboratory (NREL), NREL/TP-510-35523, 2004 Search PubMed
.
- M. Gu, D. Yu, H. Zhang, P. Sun and H. Huang, Catal. Lett., 2009, 133, 214–220 CrossRef CAS
.
-
R. Williamson, J. Holladay, M. Jaffe and D. Brunelle, Continuous Isosorbide Production from Sorbitol using Solid Acid Catalysis, Pacific Northwest National Laboratory, Richland, WA, 2006 Search PubMed
.
- A. Tanksale, J. N. Beltramini, J. A. Dumesic and G. Q. Lu, J. Catal., 2008, 258, 366–377 CrossRef CAS
.
-
C. E. Wyman, S. R. Decker, M. E. Himmel, J. W. Brady, C. E. Skopec and L. Viikari, in Polysaccharides, ed. S. Dumitriu, Marcel Dekker, New York, 2nd edn, 2005, pp. 995–1033 Search PubMed
.
- R. W. Torget, J. S. Kim and Y. Y. Lee, Ind. Eng. Chem. Res., 2000, 39, 2817–2825 CrossRef CAS
.
-
Organic chemicals from biomass, ed. I. S. Goldstein, CRC Press, Boca Raton, FL, 1981 Search PubMed
.
- A. Fukuoka and P. L. Dhepe, Angew. Chem., Int. Ed., 2006, 45, 5161–5163 CrossRef CAS
.
- P. L. Dhepe, M. Ohashi, S. Inagaki, M. Ichikawa and A. Fukuoka, Catal. Lett., 2005, 102, 163–169 CrossRef CAS
.
- d. V. S. Van, L. Peng, J. Geboers, H. Schepers, C. F. de, C. J. Gommes, B. Goderis, P. A. Jacobs and B. F. Sels, Green Chem., 2010, 12, 1560–1563 RSC
.
- D. Brunel, A. C. Blanc, A. Galarneau and F. Fajula, Catal. Today, 2002, 73, 139–152 CrossRef CAS
.
- D.-H. Lee, M. Choi, B.-W. Yu and R. Ryoo, Chem. Commun., 2009, 74–76 RSC
.
- W. Deng, X. Tan, W. Fang, Q. Zhang and Y. Wang, Catal. Lett., 2009, 133, 167–174 CrossRef CAS
.
- P. L. Dhepe and A. Fukuoka, ChemSusChem, 2008, 1, 969–975 CrossRef CAS
.
- H. Hattori and T. Shishido, Catal. Surv. Jpn., 1997, 1, 205–213 CrossRef CAS
.
- A. Zhang, I. Nakamura and K. Fujimoto, J. Catal., 1997, 168, 328–333 CrossRef CAS
.
- G. M. Pajonk, Appl. Catal., A, 2000, 202, 157–169 CrossRef CAS
.
- A. Tanksale, H. Z. Chunhui, J. N. Beltramini and G. Q. Lu, J. Inclusion Phenom. Macrocyclic Chem., 2009, 65, 83–88 CrossRef CAS
.
- J. M. Rynkowski, T. Paryjczak, M. Lenik, M. Farbotko and J. Goralski, J. Chem. Soc., Faraday Trans., 1995, 91, 3481–3484 RSC
.
- S. Chen, Y. Yang, K. Zhang and J. Wang, Catal. Today, 2006, 116, 2–5 CrossRef CAS
.
- H. Wang and Y. Zou, Catal. Lett., 2003, 86, 163–167 CrossRef CAS
.
- M. Arai, T. Ebina and M. Shirai, Appl. Surf. Sci., 1999, 148, 155–163 CrossRef CAS
.
- B. Coq and F. Figueras, J. Mol. Catal. A: Chem., 2001, 173, 117–134 CrossRef CAS
.
-
V. Ponec and G. C. Bond, in Studies in Surface Science and Catalysis, Elsevier, 1995, pp. 143–174 Search PubMed
.
- A. Tanksale, Y. Wong, J. N. Beltramini and G. Q. Lu, Int. J. Hydrogen Energy, 2007, 32, 717–724 CrossRef CAS
.
- J. Geboers, d. V. S. Van, K. Carpentier, B. K. de, P. Jacobs and B. Sels, Chem. Commun., 2010, 46, 3577–3579 RSC
.
- I. Clark, Ind. Eng. Chem., 1958, 50, 1125–1126 CrossRef CAS
.
- L. Zhao, J. H. Zhou, Z. J. Sui and X. G. Zhou, Chem. Eng. Sci., 2010, 65, 30–35 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cy20119d |
| ‡ Equal contribution from AS and AT. |
| This journal is © The Royal Society of Chemistry 2012 |