Low-cost and highly efficient composite visible light-driven Ag–AgBr/γ-Al2O3 plasmonic photocatalyst for degrading organic pollutants†
Yanyan
Zhao
,
Long
Kuai
and
Baoyou
Geng
*
College of Chemistry and Materials Science, Anhui Key Laboratory of Functional Molecular Solids, Anhui Laboratory of Molecular-Based Materials, Anhui Normal University, Wuhu, 241000, P. R. China. E-mail: bygeng@mail.ahnu.edu.cn; Fax: (+86)-553-3869303
First published on 20th March 2012
Abstract
Visible light-driven photocatalysts have attracted a great deal of attention for their direct use of solar energy. In this paper, an efficient and low-cost visible light-driven composite photocatalyst, comprising of Ag–AgBr/γ-Al2O3 nanostructures, has been facilely prepared by a surfactant-controlled precipitation–deposition and subsequent light-driven route. Hollow flower-like γ-Al2O3 nanostructures was chosen as the support. CTAB controlled the size of the deposited nanoparticles in both the Br source and the surfactant. The microstructures, surface chemical composition, visible light-driven photocatalytic performance and mechanism have been investigated systematically by SEM, TEM, XPS and so forth. The Ag–AgBr nanoparticles were distributed uniformly on the surface of the hollow flower-like γ-Al2O3 nanostructures, whose specific surface area value reached 116.5 m2 g−1. The Ag–AgBr/γ-Al2O3 composite nanostructures exhibit photocatalytic advantages over pure Ag–AgBr nanoparticles, such as decomposing aromatic compounds and organic dyes within several minutes of visible light irradiation. They also have good stabilities meaning the photocatalytic activity seldom decreases even when used 5 times under visible light irradiation. Meanwhile, the amount of Ag–AgBr needed and the cost decreases significantly. Thus, the as-prepared composite photocatalyst, consisting of Ag–AgBr/γ-Al2O3 nanostructures, will be an efficient and low-cost material for the removal of organic pollutants under visible light irradiation.
1. Introduction
Many kinds of aromatic compounds and organic dyes, produced from industry, agriculture and waste processes, have had a negative impact on humans and the environment. A photocatalytic route towards removing these pollutants is widely accepted because it is an economic and clear approach.1–4 Many studies concentrated on TiO2 due to its stability, non-toxicity and low-cost.5–7 However, the low utilization of visible light limits its practical applications although tremendous attention has been paid towards extending its absorption into the visible light region.8–11 Hence, it is important and exigent to find new materials with a lower band energy that can be directly and efficiently used under sunlight irradiation.Silver halides, as new efficient visible light photocatalysts, have drawn the attention of many researchers recently.12–18 Their excellent visible light photocatalytic performance was a surprise, especially as their high stability seems inconsistent with the fact that silver halides are very unstable under sunlight irradiation. Almost all the superior photocatalytic activity and stability is contributed to the surface plasmonic resonance (SPR) of the silver nanoparticles produced on the surface of the silver halide.19–21 Photoelectrons can be quickly brought out through the silver nanoparticles to prevent them from combining with the photogenerated hole, which markedly enhances the stability of the catalyst. However, silver is one of the noble metals and as such the cost of silver halide, as a material which removes pollutants, is much higher than that of TiO2-based photocatalytic materials. Hence, efforts are increasingly focused on decreasing the cost of silver halide materials to make them viable photocatalysts.
Based on the points discussed above, subsequent work has been carried out to improve their performance and reduce the cost. Examples include controlling the morphology,22 building composite materials based on silver halides23 and looking for good supports for loading silver halides.24–26 Interestingly, a cheap and stable support is a direct approach to both improving their performance and reducing the cost. On the one hand, the catalysts could be distributed uniformly on the surface which significantly reduces the conglomeration of the nanoparticles. On the other hand, the amount needed and the cost of the catalysts can also be decreased significantly. Hence, it is desirable to load silver halide nanoparticles onto a suitable support.
Herein, we chose hollow flower-like γ-Al2O3 nanostructures as the support for AgBr, which has been recognized as a highly active visible light photocatalyst. Firstly, γ-Al2O3 is nontoxic to the environment and very stable under natural conditions. Secondly, it is much cheaper than Ag-based photocatalysts. Finally, the hollow flower-like nanostructures have a higher specific surface area than other nanostructures. The selective hollow flower-like γ-Al2O3 nanostructures are synthesized according to a previous report,25 and the composite Ag–AgBr/γ-Al2O3 photocatalysts are prepared by a facile surfactant-controlled precipitation–deposition and subsequent light-driven method. Typically, CTAB and AgNO3 are introduced into the γ-Al2O3 colloids in turn to prepare the AgBr/γ-Al2O3 nanostructures. Then the Ag–AgBr/γ-Al2O3 can be obtained after light irradiation. CTAB can control the size of the deposited nanoparticles in both the Br source and the surfactant. As a result, the photocatalytic nanoparticles are distributed uniformly onto the surface of the hollow flower-like γ-Al2O3 nanostructures. Moreover, the small size (about 20 nm) of the Ag–AgBr nanoparticles is very beneficial for SPR absorption in the visible light region. In addition, the as-designed route is suitable for the synthesis of other silver halide/γ-Al2O3 composite nanostructures.
In this work, the photocatalytic activity and stability of the as-prepared catalysts is evaluated by degrading the organic pollutants of p-nitrophenol (PNP) and methyl orange (MO) under visible light irradiation. PNP is a typical aromatic compound and can be difficult to decompose under sunlight irradiation. Therefore, the removal of PNP is very effective for evaluating a photocatalyst and is important for humans and the environment. The photocatalytic performance is in good agreement with the design. The organic pollutants can be decomposed within several minutes under visible light irradiation, even with very low amounts of Ag–AgBr. Moreover, the as-prepared Ag–AgBr/γ-Al2O3 photocatalysts exhibit good stabilities meaning that the activity seldom decreases even after it has been used 5 times. The catalytic activity of the supported composite catalyst is much higher than that of the unsupported pure Ag–AgBr nanoparticles, meaning the performance is significantly enhanced and the cost is decreased. Therefore, the as-prepared composite Ag–AgBr/γ-Al2O3 photocatalyst will be a practical, low-cost and highly active visible light-driven material for degrading organic pollutants.
2. Experimental
All the chemicals were analytic grade except for AgNO3, which was chemical grade. They were obtained from Shanghai Chemicals Company (China) without any purification before being used. Additionally, the Xe lamp (PLS-SXE300) was bought from Beijing Perfect Light Limited Corporation (China) with a UV cut-off filter (λ > 400 nm).2.1 Preparation of Ag–AgBr/γ-Al2O3
Generally, the photocatalyst was prepared using the precipitation–deposition and subsequent photoreduction method. The details are described as follows.2.2 Characterization of catalysts
The as-prepared samples were characterized by scanning electron microscopy (SEM, Hitachi S-4800), transmission electron microscopy (TEM; Tecnai G2 20 S-TWIN, Holland), X-ray diffraction (XRD, Philips X'Pert with Cu–Kα1 radiation (λ = 0.154056 nm), X-ray photoelectron spectroscopy (XPS, ESCALAB 250 with monochromatized Al–Kα X-ray as the source), and room temperature UV-visible diffuse reflectance spectroscopy (UV-4100, SHIMADZU). Specific surface areas were computed from the results of the N2 physisorption at 77 K (model: BECKMAN SA3100 COULTER) using the BET (Brunauer–Emmet–Teller) formalism.2.3 The degradation of p-nitrophenol (PNP)
2.4 The degradation of methyl orange (MO) dye
The degradation of MO (0.05 mmol L−1) was nearly the same as the procedure for the degradation of PNP. The difference between these two experiments is the sampling. About 3 mL of the MO solution was obtained at 30 s intervals under visible light irradiation.3. Results and discussion
Fig. 1a and b are typical SEM and TEM images of the as-prepared hollow γ-Al2O3 nanostructures, prepared according to a previous report.25 As is shown, the supported γ-Al2O3 material had a flower-like morphology with a diameter of 2–3 μm. We found they were typical hollow nanostructures according to the TEM image. As shown in the high-magnification SEM and TEM images (Fig. 1c and d), the final products were assembled by many lamellas, which contributed to an increase in the specific surface. Fig. 1e and f are the SEM images of the as-prepared Ag–AgBr/γ-Al2O3 nanostructures. As is clearly shown, the hollow flower-like nanostructures were not destroyed after loading. Compared with the SEM images of pure γ-Al2O3, we can see that the Ag–AgBr catalysts were successfully deposited onto the surface of the γ-Al2O3 nanostructures with a uniform distribution (as shown in the high-magnification SEM image (Fig. 1f). The size of Ag–AgBr nanoparticles was 10–20 nm. | ||
| Fig. 1 The low (a) and high (c) magnification SEM image, low (b) and high (d) magnification TEM images of pure γ-Al2O3, and low (e) and high (f) magnification SEM images of the as-prepared Ag–AgBr/γ-Al2O3 catalyst. | ||
Fig. 2a shows the N2 adsorption–desorption isotherm of the obtained γ-Al2O3 hollow flower-like microspheres. The specific surface area value of 116.5 m2 g−1 was determined according to the computer calculations. Such a high specific surface area supplies sufficient space for the CTAB molecule to be adsorbed onto the surface and into the pore, resulting in the small mean particle size (about 20 nm) of the supported materials and uniform distribution. The procedure is shown in Scheme 1. Firstly, CTAB was adsorbed onto the surface and into the pore of γ-Al2O3. CTAB acts as the source of both Br− and Ag+, which can easily react with CTAB to form the AgBr nanoparticles because of the electrostatic attraction. Finally, the composite Ag–AgBr/γ-Al2O3 nanostructures were obtained after the photo-induced production of Ag0. This method prevents conglomeration among the AgBr nanoparticles, leading to a uniform distribution on the surface and in the pores. These nanostructures ensure the Ag–AgBr nanoparticle surfaces are sufficiently exposed, increasing the active area and efficiency.
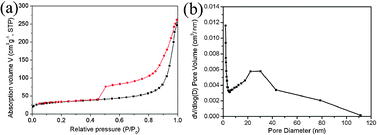 | ||
| Fig. 2 Nitrogen adsorption–desorption isotherm (a) and the corresponding pore-size distribution of the γ-Al2O3 hollow microspheres (b). | ||
 | ||
| Scheme 1 The proposed formation mechanism of the as-prepared Ag–AgBr/γ-Al2O3 nanostructures. | ||
Fig. 3a shows the XRD patterns of pure γ-Al2O3 and the Ag–AgBr/γ-Al2O3 nanostructures, further confirming the composition of the nanostructures. Compared with γ-Al2O3 (red line), we found that the six additional peaks (shown in Fig. 3a, black line), could be indexed to AgBr. Because the Ag0 content is a fraction of the composite, the weak peaks are covered by the impure peaks. However, the existence of Ag0 can be clearly demonstrated by the XPS spectrum (as shown in Fig. 3d). It shows peaks located at 368.2 eV and 374.2 eV, which belong to the Ag 3d orbital. As shown in Table 1, the content of Br is less than that of Ag, indicating Ag0 is produced. This result makes up for the deficiency in the XRD measurement. In addition, the overview XPS spectrum is displayed in Fig. 3b. The O and Al are from Al2O3, and the Ag and Br are from Ag–AgBr. Furthermore, the C is from the adsorbed CTAB or CO2. Fig. 3c shows the peaks of Al and Br in the XPS spectrum. Thus, all the characterizations indicate that the obtained hollow flower-like nanostructures were Ag–AgBr/γ-Al2O3.
 | ||
| Fig. 3 (a) XRD patterns of γ-Al2O3 (red line) and the as-prepared Ag–AgBr/γ-Al2O3 nanostructures (black line). The whole (b), Al 3p, Br 3d (c) and Ag 3d (d) XPS spectra. | ||
| Sample | Binding energy of Ag0 3d peak | The atomic number ratio of Ag | The atomic number ratio of Br | The atomic number ratio of Al |
|---|---|---|---|---|
| before photocatalysis | 368.2, 374.2 | 1.47 | 1.32 | 53.03 |
| after photocatalysis | 368.3, 374.3 | 1.03 | 0.86 | 48.98 |
Fig. 4 shows the UV-vis diffuse reflectance spectra of pure γ-Al2O3 and the as-prepared Ag–AgBr/γ-Al2O3 composite plasmonic photocatalysts. As is clearly shown, the Ag–AgBr/γ-Al2O3 catalyst has a strong absorption throughout the whole UV-vis light region (200–800 nm) while pure γ-Al2O3 has almost no absorption at all. The strong absorption in the visible light region is indexed to the strong surface plasmonic resonance (SPR) (see Fig. S1, ESI†) of the light-induced formation of small Ag nanoparticles.19–21 As a consequence, the composite materials can make use of the whole visible light region, making them a practical material under visible light irradiation.
 | ||
| Fig. 4 UV-vis diffuse reflectance spectra of γ-Al2O3 (red line) and the as-prepared Ag–AgBr/γ-Al2O3 nanostructures (black line). | ||
In this work, we investigated the visible light-driven photocatalytic decomposition of p-nitrophenol (PNP) as a way of evaluating the advantages of the as-prepared Ag–AgBr/γ-Al2O3 nanostructures. As is known, PNP is a toxic organic pollutant to both human beings and the environment and can be difficult to decompose under solar light due to its chemical and biological stability.28,29 As shown in the UV-vis absorption spectra (see Fig. S2, ESI†), the peak located at 400 nm, belongs to the nitro group and declines quickly under visible light irradiation, revealing that PNP is quickly removed. As is shown in Fig. 5a, PNP can be decomposed by up to 80% within 3 min of visible light irradiation with the as-prepared catalysts, and can be almost completely decomposed after 12 min of irradiation. However, pure Ag–AgBr nanoparticles exhibit much lower photocatalytic activity, resulting in only about 50% of the PNP being decomposed after 12 min of irradiation. Furthermore, the PNP kept almost the original concentration when no photocatalysts were present. In addition, in the presence of pure Al2O3, the concentration of PNP declined significantly as a result of the adsorption of the hollow flower-like γ-Al2O3 structure with a high specific surface area. Interestingly, we found that the concentration of PNP increased when the system was under visible light irradiation, which is due to the desorption of PNP with increasing temperature during visible light irradiation.
 | ||
| Fig. 5 (a) The time-dependent photodecomposition curve of PNP. C0 is the original concentration (10 mg L−1) and C is the concentration of the remaining PNP at time t. (b) Histogram of the cycling photodecomposition of PNP. C is the concentration of PNP after 6 min of irradiation, and C0 is the original concentration of PNP. | ||
The photocatalytic stability is also an important factor. Herein, the cycling degradation experiments (after 6 min of visible light irradiation) were carried out to evaluate the photocatalytic stability under the same conditions. As shown in Fig. 5b, it can be clearly seen that the Ag–AgBr/γ-Al2O3 structures have a high stability, resulting in a high decomposition ratio even after 5 cycles with 6 min of visible light irradiation every time. Furthermore, based on the XPS data (Table 1), the atomic ratio of silver and bromide was 1.11 and 1.20 before and after the photocatalytic reactions, respectively. This means Ag (I) is seldom reduced into Ag0 during the photocatalysis. In addition, according to Table 1, the Ag, Br, and Al content was reduced while the O content was increased during the photocatalytic process. One reason may be due to the adsorption of organic O onto the photocatalyst. Another may be that some Ag+ ions are transformed into Ag2O, leading to instability in the Ag–AgBr/γ-Al2O3 photocatalyst, which can be seen in Fig. 5b and 6b in which a slight reduction in the photocatalytic activity appears.
The degradation of PNP has been extensively studied.30–34 Based on previous reports and our photocatalytic experiments, we proposed the possible degradation processes of PNP and the corresponding products from the as-prepared Ag–AgBr/γ-Al2O3 photocatalysis, which is illustrated in Scheme 2. The photocatalytic mechanism for AgX (X = Cl, Br and I)-based plasmonic photocatalysts has been confirmed by Hu et al.16,17 In their work, an ESR spin-trap technique (with DMPO) confirmed the existence of OH˙ and O2˙ radicals during the visible light irradiation, and the effect of various radical scavengers further revealed that the OH˙ and O2˙ radicals were the main active species. Generally, the combination of photo-induced OH˙/O2˙ radicals (the formation mechanism will be discussed later in detail) with PNP decides the degradation products. If OH˙ attacks the 3 position of PNP, 4-nitrocatechol (product I) can be obtained. If OH˙ attacks the 1 position of PNP, p-benzenediol (product II) is produced. In addition, p-benzenediol can further react with photo-induced O2˙ or OH˙ and so benzoquinone (product III) and hydroxyquinol (product IV) appear, respectively. In summary, four main products may be produced during the photodecomposition of PNP. Furthermore, PNP can also be reduced to p-amino phenol (product V) by electrons.29
 | ||
| Scheme 2 The possible degradation process of PNP and the corresponding products from the as-prepared Ag–AgBr/γ-Al2O3 photocatalysis. | ||
To further investigate the advantages of the as-prepared Ag–AgBr/γ-Al2O3 photocatalysts, we carried out photodegradation reactions for MO under visible light irradiation. As shown in Fig. S3 (ESI†), the peak located at 463 nm, belongs to the azo group, which quickly declines under visible light irradiation with the as-prepared catalysts. According to Fig. 6a, we can see that the Ag–AgBr/γ-Al2O3 nanostructures display excellent photocatalytic activities. The MO dye can be decomposed by 82% within 1 min of visible light irradiation, and can be almost completely decomposed within 3 min. Similar to the results for the degradation of PNP, the pure Ag–AgBr nanoparticles display much less activity, meaning that less than 50% is decomposed after 3 min irradiation. In addition, we evaluated the photocatalytic stability of the structures by cycling degradation experiments under the same conditions. As shown in Fig. 6b, the stability of the photocatalyst is very good. The photocatalytic activity of the Ag–AgBr/γ-Al2O3 photocatalysts was seldom reduced even after 5 cycles. Therefore, the stability of the photocatalysts, comprised of Ag–AgBr/γ-Al2O3 nanostructures, is significantly enhanced and they are potential visible light-driven catalysts for the degradation of organic pollutants.
 | ||
| Fig. 6 (a) The photodecomposition curve of MO. C0 is the original concentration of MO (0.05 mmol L−1), and C is the concentration of the remaining MO at time t. (b) Histogram of the cycling photodecomposition of MO. C is the final concentration (3 min) of degraded MO, and C0 is the original concentration of MO. | ||
Based on these results, we can see that the prepared Ag–AgBr/γ-Al2O3 photocatalyst shows a faster degradation rate for the MO dye than that of PNP. To further investigate the photocatalytic performance, we investigated the kinetic rate constant value. As shown in Fig. 7, the normalized concentration of PNP (a) and MO (b) show a linear relationship. In other words, the photocatalytic reactions have first-order kinetics (−dc/dt = kt). In accordance with Fig. 7a and b, we can calculate the kinetic rate constant value for PNP and MO and kPNP is 0.1515 min−1 and kMO is 0.1972 min−1, respectively. Based on the kinetic rate constant, the degradation rate for MO is faster. On the one hand, MO is a dye, so MO may produce a sensitizer effect under visible light irradiation. On the other hand, the structure of MO may be destroyed more easily than that of PNP.
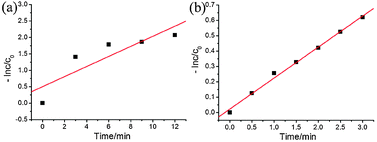 | ||
| Fig. 7 The normalized concentration of PNP (a) and MO (b) as a function of reaction time in a logarithmic scale. C0 is the concentration after adsorption, and C is concentration at time t. | ||
According to our results, the excellent visible light-driven photocatalytic performance of the as-prepared Ag–AgBr/γ-Al2O3 nanostructures may be due to the following 3 reasons. Firstly, the high visible light catalytic performance of Ag–AgBr ensures the basic photocatalytic ability of the Ag–AgBr/γ-Al2O3 composite nanostructures. The proposed photocatalytic mechanism is illustrated in Scheme 3. It has been confirmed that the Ag nanoparticles contribute to the high visible light photocatalytic activity and stability due to their SPR produced by the collective oscillations of the surface electrons.19–21 In addition, Ag is one of the best conductive metals meaning light-induced electrons can be quickly transferred outside the structure and therefore avoid recombination with the light-induced hole (h+). As a result, the electrons can combine with O2 to form active O2˙ radicals. H+ can also be captured by H2O and produce OH˙ radicals.35–37 OH˙/O2˙ act on the pollutants and decompose them efficiently. Secondly, the electrons can be translated quickly from AgBr because of the excellent conductivity of the Ag nanoparticles, resulting in Ag+ seldom capturing the electrons and the stability ensured.12,13 Finally, but no less importantly, the hollow flower-like γ-Al2O3 support ensures that the Ag–AgBr photocatalyst can be distributed well, which optimizes the photocatalytic performance of the Ag–AgBr nanoparticles. As shown in the BET measurements (Fig. 2), the hollow flower-like γ-Al2O3 nanostructures have a large specific surface area of 116.5 m2 g−1, which supplies a sufficient surface and pores for the loading of the Ag–AgBr nanoparticles. In addition, with the help of CTAB, the size of the deposited Ag–AgBr nanoparticles are so small and uniform that the utilization of the Ag–AgBr nanoparticles is significantly increased, which has been revealed by the photocatalytic results. In other words, the photocatalytic performance is enhanced significantly while the cost greatly decreased.
 | ||
| Scheme 3 The proposed photocatalytic mechanism of the Ag–AgBr/γ-Al2O3 nanostructures. | ||
4. Conclusions
In summary, a very efficient and low-cost visible light-driven composite photocatalyst, comprised of Ag–AgBr/γ-Al2O3 nanostructures, has been facilely prepared. Their microstructures, surface chemical composition, visible light-driven photocatalytic performance and mechanism have been investigated systematically. The Ag–AgBr/γ-Al2O3 composite nanostructures exhibit photocatalytic advantages over pure Ag–AgBr nanoparticles as they can decompose aromatic compounds and organic dyes within several minutes of visible light irradiation. Meanwhile, the amount of Ag–AgBr needed and the cost is decreased significantly. Thus, the as-prepared composite Ag–AgBr/γ-Al2O3 nanostructures will be an efficient and low-cost material for the removal of organic pollutants under visible light irradiation.Acknowledgements
This work was supported by the National Natural Science Foundation of China (20671003 and 20971003), the Key Project of Chinese Ministry of Education (209060), the Program for New Century Excellent Talents in University (NCET 11-0888), the Science and Technological Fund of Anhui Province for Outstanding Youth (10040606Y32), the Foundation of Key Project of Natural Science of Anhui Education Committee (KJ2012A143) and the Program for Innovative Research Team at Anhui Normal University.Notes and references
- T. Arai, M. Yanagida, Y. Konishi, Y. Iwasaki, H. Sugihara and K. Sayama, J. Phys. Chem. C, 2007, 111, 7574 CAS.
- J. H. Huang, Y. J. Cui and X. C. Wang, Environ. Sci. Technol., 2010, 44, 3500 CrossRef CAS.
- Q. Wang, B. Y. Geng and S. Z. Wang, Environ. Sci. Technol., 2009, 43, 8968 CrossRef CAS.
- H. C. Ma, K. Teng, Y. H. Fu, Y. Song, Y. W. Wang and X. L. Dong, Energy Environ. Sci., 2011, 4, 3067 CAS.
- J. M. Li and D. S. Xu, Chem. Commun., 2010, 46, 2301 RSC.
- J. B. Joo, Q. Zhang, M. Dahl, I. Lee, J. Goebl, F. Zaera and Y. D. Yin, Energy Environ. Sci., 2012, 5, 6321 CAS.
- B. S. Liu, K. Nakata, M. Sakai, H. Saito, T. Ochiai, T. Murakami, K. Takagi and A. Fujishima, Langmuir, 2011, 27, 8500 CrossRef CAS.
- X. Shu, Z. An, L. Y. Wang and J. He, Chem. Commun., 2009, 5901 RSC.
- A. Pearson, S. K. Bhargava and V. Bansal, Langmuir, 2011, 27, 9245 CAS.
- X. G. Liu, D. Y. Geng, X. L. Wang, S. Ma, H. Wang, D. Li, B. Q. Li, W. Liu and Z. D. Zhang, Chem. Commun., 2010, 46, 6956 RSC.
- H. Zhu, J. Tao and X. Dong, J. Phys. Chem. C, 2010, 114, 2873 CAS.
- P. Wang, B. B. Huang, X. Y. Qin, X. Y. Zhang, Y. Dai, J. Y. Wei and M. H. Whangbo, Angew. Chem., Int. Ed., 2008, 47, 7931 CrossRef CAS.
- P. Wang, B. B. Huang, X. Y. Zhang, X. Y. Qin, H. Jin, Y. Dai, Z. Y. Wang, J. Y. Wei, J. Zhan, S. Y. Wang, J. P. Wang and M. H. Whangbo, Chem. Eur. J., 2009, 15, 1821 CrossRef CAS.
- P. Wang, B. B. Huang, Z. Z. Lou, X. Y. Zhang, X. Y. Qin, Y. Dai, Z. K. Zheng and X. N. Wang, Chem. Eur. J., 2010, 16, 538 CrossRef CAS.
- Y. P. Bi and J. H. Ye, Chem. Eur. J., 2010, 16, 10327 CrossRef CAS.
- C. Hu, T. W. Peng, X. X. Hu, Y. L. Nie, X. F. Zhou, J. H. Qu and H. He, J. Am. Chem. Soc., 2010, 132, 857 CrossRef CAS.
- C. Hu, Y. Q. Lan, J. H. Qu, X. X. Hu and A. M. Wang, J. Phys. Chem. B, 2006, 110, 4066 CrossRef CAS.
- L. Kuai, B. Y. Geng, X. T. Chen, Y. Y. Zhao and Y. C. Luo, Langmuir, 2010, 26, 18723 CrossRef CAS.
- S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 8410 CrossRef CAS.
- R. K. Roy, S. Bandyopadhyaya and A. K. Pal, Eur. Phys. J. B, 2004, 39, 491 CrossRef CAS.
- T. A. El-Brolossy, T. Abdallah, M. B. Mohamed, S. Abdallah, K. Easawi, S. Negm and H. Talaat, Eur. Phys. J. Spec. Top., 2008, 153, 361 CrossRef.
- C. H. An, S. Peng and Y. G. Sun, Adv. Mater., 2010, 22, 2570 CrossRef CAS.
- H. F. Cheng, B. B. Huang, Y. Dai, X. Y. Qin and X. Y. Zhang, Langmuir, 2010, 26, 6618 CrossRef CAS.
- X. F. Zhou, C. Hu, X. X. Hu, T. W. Peng and J. H. Qu, J. Phys. Chem. C, 2010, 114, 2746 CAS.
- H. F. Cheng, B. B. Huang, P. Wang, Z. Y. Wang, Z. Z. Lou, J. P. Wang, X. Y. Qin, X. Y. Zhang and Y. Dai, Chem. Commun., 2011, 47, 7054 RSC.
- P. Wang, B. B. Huang, X. Y. Qin, X. Y. Zhang, Y. Dai and M. H. Whangbo, Inorg. Chem., 2009, 48, 10697 CrossRef CAS.
- W. Q. Cai, J. G. Yu and S. Mann, Microporous Mesoporous Mater., 2009, 122, 42 CrossRef CAS.
- L. X. Yang, S. L. Luo, Y. Li, Y. Xiao, Q. Kang and Q. Y. Cai, Environ. Sci. Technol., 2010, 44, 7641 CrossRef CAS.
- S. Gazi and R. Ananthakrishnan, Appl. Catal., B, 2011, 105, 317 CrossRef CAS.
- S. Q. Yu, J. Hu and J. L. Wang, Radiat. Phys. Chem., 2010, 79, 1039 CrossRef CAS.
- L. Gu, X. W. Zhang and L. C. Lei, Ind. Eng. Chem. Res., 2008, 47, 6809 CrossRef CAS.
- P. Cañizares, C. Sáez, J. Lobato and M. A. Rodrigo, Ind. Eng. Chem. Res., 2004, 43, 1944 CrossRef.
- A. Kotronarou, G. Mills and M. R. Hoffmann, J. Phys. Chem., 1991, 95, 3630 CrossRef CAS.
- M. H. Zhou and L. C. Lei, Chemosphere, 2006, 63, 1032 CrossRef CAS.
- Z. K. Zheng, B. B. Huang, X. Y. Qin, X. Y. Zhang, Y. Dai and M. H. Whangbo, J. Mater. Chem., 2011, 21, 9079 RSC.
- Y. D. Xie, F. Chen, J. J. He, J. C. Zhao and H. Wang, J. Photochem. Photobiol., A, 2000, 136, 235 CrossRef CAS.
- S. Gazi, R. Ananthakrishnan and N. D. P. Singh, J. Hazard. Mater., 2010, 183, 894 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures and figure captions. See DOI: 10.1039/c2cy20074k |
| This journal is © The Royal Society of Chemistry 2012 |
