Aromatic transformations over aluminosilicate micro/mesoporous composite materials
T.
Odedairo
,
R. J.
Balasamy
and
S.
Al-Khattaf
*
Center of Excellence in Petroleum Refining and Petrochemicals, King Fahd University of Petroleum & Minerals, Dhahran 31261, Saudi Arabia. E-mail: skhattaf@kfupm.edu.sa; Fax: +966-3-860-4234; Tel: +966-3-860-1429
First published on 20th March 2012
Abstract
Catalytic behavior of micro/mesoporous ZSM-5/MCM-41 composites were investigated in the transformation of 1,2,4-trimethylbenzene (TMB), meta-xylene transformation and in the cracking of 1,3,5-triisopropylbenzene (TIPB). The samples were characterized by XRD, TGA, SEM, nitrogen sorption and FTIR of pyridine adsorption. The composite materials exhibited exceptional catalytic performance compared with the microporous ZSM-5 in the transformation of 1,2,4-trimethylbenzene and m-xylene. In the cracking of 1,3,5-triisopropylbenzene, the composite materials showed higher activity as compared with the conventional Y-zeolite. The distinctive catalytic performance of these micro/mesoporous composite materials in the reactions studied was attributed to the excellent accessibility of the active sites provided by the mesopores for both reactant and product molecules. In the transformation of m-xylene, selectivity towards para-xylene over all catalysts under study follows the order: conventional ZSM-5 ≈ ZM41A1 < ZM41A2 < ZM41A3.
1. Introduction
Zeolites and zeolite-like materials have been extensively used in various chemical transformations related to petrochemical and refining processes due to several catalytic properties.1–6 However, due to their limited pore size range (maximum pore size is typically <1.5 nm), much effort has been devoted to the development of new zeolite-type materials with larger (>1.5 nm) pores.7 The synthesis of a new family of mesoporous M41S materials is one of such achievement.8 However, due to the amorphous nature of their frameworks, M41S-type mesoporous aluminosilicates generally exhibit low stability and acidity compared to zeolites, which limits their potential applications.9,10Several synthesis strategies have been developed to obtain materials which combine the advantages of mesoporous materials and those of zeolites in terms of stability and acidity to improve the conversion and selectivity. The significant progress toward the zeolitisation of mesoporous aluminosilicates was pioneered by the van Bekkum group via partially recrystallizing mesoporous framework walls into nanosized ZSM-5 zeolite.11 Karlsson et al.12 prepared composite materials by simultaneous synthesis of MFI/MCM-41 phases using a two-template approach at optimized template concentrations and reaction temperatures. A composite micro/mesoporous ZSM-5/MCM-48 material, prepared using a simple two step crystallization process, was recently reported by Xia and Mokaya.10 Huang et al.13 also reported the synthesis of MCM-41/ZSM-5 composite materials containing an interconnected mesopore and micropore structure using a dual template method via two-step crystallization.
Production of xylenes via the disproportionation of toluene or the transalkylation of heavy aromatics offers refiners an excellent opportunity to add value to their product streams.14–16 Xylenes are important raw materials for a wide variety of petrochemical intermediates that are used in the production of synthetic fibers, plasticizers and resins. The major sources of this aromatic hydrocarbon are the reforming and steam cracking of naphtha, along with benzene and toluene. However, because of thermodynamic constraints, the product yields from these sources give lower amounts of the more valuable xylenes compared to the less desirable toluene.17 1,2,4-Trimethylbenzene is usually used as a model compound in most transalkylation studies since it represents more than 40 wt% of C9 aromatics in heavy reformate. Transformation of toluene and trimethylbenzene over zeolite beta was also reported by Das et al.18 The authors noticed the highest yield of xylenes at a reaction temperature of 400 °C and at a toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TMB molar ratio of 1
TMB molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Cejka et al.19 reported that the diffusion of trimethylbenzenes into the ZSM-5 zeolite channel system is rather slow as compared to large pore zeolites, because of significant steric constraints for the large trimethylbenzene molecules. Lately, catalytic transformations of toluene, TMB and an equimolar mixture of the two compounds were investigated over ZSM-5 and mordenite catalysts.20
1. Cejka et al.19 reported that the diffusion of trimethylbenzenes into the ZSM-5 zeolite channel system is rather slow as compared to large pore zeolites, because of significant steric constraints for the large trimethylbenzene molecules. Lately, catalytic transformations of toluene, TMB and an equimolar mixture of the two compounds were investigated over ZSM-5 and mordenite catalysts.20
m-Xylene isomerization and disproportionation have been used by a number of groups for the characterization of acidic zeolites.21–23 With shape selective zeolites, in particular those with average pore size such as HMFI, direct p-xylene to ortho-xylene isomerization can be observed and m-xylene is preferably transformed into p-xylene, the smallest isomer.23 The bimolecular isomerization mechanism proposed by Corma and Sastre24 involves xylene disproportionation followed by transalkylation between trimethylbenzenes and the xylene reactant. A novel shape-selective H-MCM-22/MCM-41 composite, synthesized by overgrowing MCM-41 over the external surface of H-MCM-22, was reported to exhibit significant p-xylene selectivity.25 Llopis et al.26 investigated the isomerization and disproportionation of xylene over NU-87 as compared with 12-membered ring channels (β), 10- and 12-MR channels (SSZ-33), and 10-MR channels (ZSM-5). Recent publications present studies on the transformation of m-xylene on ZSM-5, TNU-9, SSZ-33 and mordenite at a more fundamental level.27,28
Fluid catalytic cracking of hydrocarbons continues to remain a novel process in the petroleum refining industry for upgrading vacuum distillates and residues to valuable gasoline and diesel fuels.29,30 The cracking of heavier feedstocks tends to be more diffusion controlled, since the majority of the active sites of the zeolite are within its pores. Thus, great interest has been generated in the synthesis of composite materials for cracking of these heavy feedstocks. Morales-Pacheco et al.31 investigated the correlation between the mean crystallite size and catalytic performance of nanometric FAU and MFI in the cracking of 1,3,5-TIPB. The catalytic cracking of 1,3,5-TIPB was also studied over a novel mesoporous beta catalyst.32 The mesoporous beta showed high catalytic activity as compared with the conventional microporous beta zeolites. Catalytic cracking of 1,3,5-TIPB has also been reported over ZSM-5/MCM-48 composite material.33 The authors attributed the exceptional catalytic performance of the catalyst in the cracking of 1,3,5-TIPB to the easy access of active sites provided by the mesopores for both reactant and larger product molecules.
Herein we present composite micro/mesoporous materials in order to overcome the limitations of single micro- or mesoporous materials and to combine the advantages of these two types of molecular sieves. The catalytic activity of the materials was tested in the transformation of 1,2,4-TMB and in the transformation of m-xylene. The catalytic properties of the composite micro/mesoporous materials were also investigated in the catalytic cracking of 1,3,5-TIPB, and its activities compared with the conventional microporous Y-zeolite. There is no report available to our knowledge on the use of ZSM-5/MCM-41 composite materials for the transformation of 1,2,4-trimethylbenzene.
2. Experimental
2.1. Synthesis of materials
The ZSM-5 zeolite used in this work was obtained from Tosoh Company, Japan. The synthesized Na zeolite was ion-exchanged with NH4NO3 to replace the Na cation with NH4+. Following this, NH3 was removed and the H form of the zeolite was obtained.The micro/mesoporous composite materials were synthesized from the gathering of cetyltrimethylammonium bromide (CTAB) with a preformed ZSM-5 solution. The synthesis procedure involved two steps; first the zeolite precursor (ZSM-5) was disintegrated and then assembled into the mesostructure (MCM-41). 2 g of the precursor zeolite species (ZSM-5) with an Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio of 13.5 was disintegrated using 0.2 M and 0.7 M sodium hydroxide solution at a pH condition of 12.1 and 13, respectively, by gradually heating without stirring at 100 °C for 24 h in the presence of 4.5 wt% cetyltrimethylammonium bromide. The mixture was cooled down and the pH was adjusted to 9.0 through the addition of dilute sulfuric acid. The mixture was then aged at 100 °C for 24 h to form a composite material. The resulting synthesized solid product was recovered by filtration and dried in air. The template and organic additives were removed by calcination at 550 °C for 6 h with ramp of 3 °C min−1. The calcined sodium containing sample was then subjected to three-times repeated ion-exchange with 0.05 M NH4NO3 solution, followed by calcination at 500 °C for 2 h. By varying the pH of the composite materials at 12.1 and 13, two samples denoted as ZM41A1 (pH 12) and ZM41A2 (pH 13) were prepared. The third sample, designated as ZM41A3, was synthesized in a similar fashion to the procedure describe above, but during the stage of zeolite disintegration at pH 13, the solution was heated and vigorously stirred at 100 °C for 24 h in the presence of 4.5 wt% cetyltrimethylammonium bromide.
Al ratio of 13.5 was disintegrated using 0.2 M and 0.7 M sodium hydroxide solution at a pH condition of 12.1 and 13, respectively, by gradually heating without stirring at 100 °C for 24 h in the presence of 4.5 wt% cetyltrimethylammonium bromide. The mixture was cooled down and the pH was adjusted to 9.0 through the addition of dilute sulfuric acid. The mixture was then aged at 100 °C for 24 h to form a composite material. The resulting synthesized solid product was recovered by filtration and dried in air. The template and organic additives were removed by calcination at 550 °C for 6 h with ramp of 3 °C min−1. The calcined sodium containing sample was then subjected to three-times repeated ion-exchange with 0.05 M NH4NO3 solution, followed by calcination at 500 °C for 2 h. By varying the pH of the composite materials at 12.1 and 13, two samples denoted as ZM41A1 (pH 12) and ZM41A2 (pH 13) were prepared. The third sample, designated as ZM41A3, was synthesized in a similar fashion to the procedure describe above, but during the stage of zeolite disintegration at pH 13, the solution was heated and vigorously stirred at 100 °C for 24 h in the presence of 4.5 wt% cetyltrimethylammonium bromide.
The commercial Y-zeolite having an Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio of 3.2 used in this work was obtained from Tosoh Company in the Na form. The zeolite was ion exchanged with NH4NO3 to replace the sodium cation with NH4+. The process of sodium removal was repeated for the pelletized catalyst. Following this, the catalyst was calcined for 2 h at 600 °C.
Al ratio of 3.2 used in this work was obtained from Tosoh Company in the Na form. The zeolite was ion exchanged with NH4NO3 to replace the sodium cation with NH4+. The process of sodium removal was repeated for the pelletized catalyst. Following this, the catalyst was calcined for 2 h at 600 °C.
2.2. Characterization
The catalysts were characterized by X-ray power diffraction (XRD), thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR) of pyridine adsorption and nitrogen adsorption–desorption to understand the textural and chemical properties of micro/mesoporous composite materials.Powder X-ray diffraction (XRD) was recorded on a Mac Science MX18XHF-SRA powder diffractometer with monochromatized Cu-Kα radiation (λ = 0.154 nm) at 40 kV and 30 mA. Thermogravimetric analysis (TGA) was performed using a TA Instrument SDT Q 600 TGA analyzer with a heating rate of 10 °C min−1 under nitrogen flow.
Concentration of Lewis and Brønsted acid sites were determined after adsorption of pyridine by FTIR spectroscopy (Nicolet 6700 FTIR). Samples were pressed into self-supporting wafers with a density of 8.0–12 mg cm−2 and activated in situ at 430 °C overnight. Pyridine adsorption was carried out at 150 °C for 20 min at partial pressure 800–1000 Pa, followed by desorption for 15 min.
Nitrogen sorption isotherms were performed at liquid nitrogen temperature (−196 °C) on a Quantachrome AUTOSORB-1 (model ASI-CT-8). Prior to the sorption measurements, all samples were degassed at 250 °C for at least 24 h until a pressure of 10−3 Pa was attained. The surface area was calculated using the Brunauer–Emmett–Teller (BET) method. The total pore volume was determined from the amount of nitrogen adsorbed at P/P0 = ca. 0.99. The Barrett–Joyner–Halenda (BJN) method and t-plot analysis were used to determine the micropore surface area and pore volume. A scanning electron microscopy (SEM) image was recorded using a JEOL, JSM-5500LV scanning electron microscope.
2.3. Reaction apparatus and procedures
The catalytic experiments were carried out in the riser simulator (Fig. 1) and were operated under atmospheric pressure. Catalytic experiments were performed at a catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) reactant ratio of 3.75 (weight of catalyst = 0.60 g) for different residence times of 5, 10, 15 and 20s and at a reaction temperature of 400 °C. Analytical grade (99% purity) 1,2,4-trimethylbenzene, m-xylene and 1,3,5-triisopropylbenzene were obtained from Sigma-Aldrich. All chemicals were used as received, and no attempt was made to further purify the samples. A four-port valve enables the connection and isolation of the 45 cm3 reactor and the vacuum box, and a six-port valve allows for the collection of a sample of reaction products in a sampling loop. The products were analyzed in an Agilent model 6890N gas chromatograph with a flame ionization detector and a capillary column INNOWAX, 60 m cross-linked methyl silicone with an internal diameter of 0.32 mm. During the course of the investigation, a number of runs were repeated to check for reproducibility in the experiment results, which was found to be excellent. Typical errors were in the range of ±2%.
reactant ratio of 3.75 (weight of catalyst = 0.60 g) for different residence times of 5, 10, 15 and 20s and at a reaction temperature of 400 °C. Analytical grade (99% purity) 1,2,4-trimethylbenzene, m-xylene and 1,3,5-triisopropylbenzene were obtained from Sigma-Aldrich. All chemicals were used as received, and no attempt was made to further purify the samples. A four-port valve enables the connection and isolation of the 45 cm3 reactor and the vacuum box, and a six-port valve allows for the collection of a sample of reaction products in a sampling loop. The products were analyzed in an Agilent model 6890N gas chromatograph with a flame ionization detector and a capillary column INNOWAX, 60 m cross-linked methyl silicone with an internal diameter of 0.32 mm. During the course of the investigation, a number of runs were repeated to check for reproducibility in the experiment results, which was found to be excellent. Typical errors were in the range of ±2%.
 | ||
| Fig. 1 Schematic diagram of the riser simulator. | ||
3. Results and discussion
X-Ray powder diffraction, scanning electron microscopy, nitrogen adsorption isotherms and FTIR spectroscopy were utilized to understand the textual and chemical properties of micro/mesoporous composite materials. These catalysts were further investigated in the transformation of 1,2,4-trimethylbenzene, m-xylene transformation and cracking of 1,3,5-triisopropylbenzene.3.1. Textural characterization: powder X-ray diffraction and nitrogen sorption studies
The X-ray powder diffraction patterns of the composite materials and the conventional ZSM-5 are shown in Fig. 2, while the physicochemical properties are presented in Table 1. XRD diffraction patterns of the conventional ZSM-5 show high crystallinity and phase purity. The XRD pattern of sample ZM41A1 shows a material containing both microporous and mesoporous phases, but predominantly showing a zeolitic character. In the low angle region, an intense (100) peak along with less intense higher order diffraction peaks indexed to (110) and (200), corresponding to MCM-41, were observed. The presence of intense peaks of ZSM-5 diffraction patterns shows the dominant zeolitic character in the composite material. The ZM41A2 composite material shows enhanced diffraction peaks in the low angle region, indicating an improved mesostructure formation. The XRD pattern indicates a composite material with both microporous and mesoporous phases in approximately equal proportion. Fig. 2d shows the XRD pattern of a material with a pure mesoporous phase, but containing fragments of ZSM-5 particles (ZM41A3). In the case of ZM41A3, the ZSM-5 phase disappears completely due to complete dissolution of ZSM-5 by stirring in 0.7M NaOH solution at 100 °C for 2 h. The wide-angle XRD pattern of ZM41A3 composite material presented in Fig. 2 indicates that the zeolite seeds formed during the crystallization process were used up as structural building units for the construction of the mesoporous materials. The presence of three well resolved peaks in the lower angle region is characteristic of long range ordered hexagonal symmetry.34 | ||
| Fig. 2 Powder X-ray diffraction for calcined (a) ZSM-5, (b) ZM41A1, (c) ZM41A2, (d) ZM41A3. | ||
| Catalyst | Surface area (m2 g−1) | Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio Al ratio |
Pore volume (cm3 g−1) | Lewis sites (mmol g−1) | Brønsted sites (mmol g−1) | Lewis sites (%) | Brønsted sites (%) |
|---|---|---|---|---|---|---|---|
| The values in parentheses are the micropore surface area or pore volume. | |||||||
| ZSM-5 | 0 (364) | 14 | 0 (0.17) | 0.21 | 0.45 | 32 | 68 |
| ZM41A1 | 405 (341) | 8.5 | 0 (0.18) | 0.27 | 0.39 | 41 | 59 |
| ZM41A2 | 468 (359) | 12 | 0 (0.19) | 0.24 | 0.35 | 41 | 59 |
| ZM41A3 | 577 (237) | 13 | 0.68 (0.21) | 0.73 | 0.07 | 91 | 9 |
| Y-zeolite | 549 (499) | 3.2 | 0.34 (0.24) | 2.39 | 2.58 | 48 | 52 |
The nitrogen sorption isotherms for all materials are shown in Fig. 3 and the corresponding textural properties are summarized in Table 1. The nitrogen adsorption–desorption isotherm of the conventional ZSM-5 shows typical features of microporous material, namely very fast adsorption of nitrogen at low pressures. On the other hand, the micro/mesoporous materials show a hysteresis loop at relative pressure 0.4–1.0 (P/P0). ZM41A1 and ZM41A2 did not exhibit well defined mesoporous characteristics, i.e. their nitrogen sorption isotherm does not have a clearly defined mesopore filling step. The sorption isotherms of samples (ZM41A1 and ZM41A2) are consistent with the presence of zeolitic material as the major phase. The isotherm of ZM41A3 composite material gives typical type-IV isotherms with a sharp inflection at relative pressure P/P0 = 0.3–0.4, characteristic of capillary condensation, which indicates the uniformity of the mesopore-size distribution.
 | ||
| Fig. 3 Nitrogen adsorption isotherms for calcined (a) ZSM-5, (b) ZM41A1, (c) ZM41A2, (d) ZM41A3. | ||
3.2. Infrared spectroscopy
In order to confirm the presence of the ZSM-5 structure in the composite materials, infrared spectra of the calcined samples are presented in Fig. 4. It is evident from Fig. 4 that a broad but distinct vibration band at 550–600 cm−1 is noticed in all composite materials. This relatively well developed band at 550–600 cm−1 has been assigned to the asymmetric stretching mode of five-membered ring blocks present in the ZSM-5 structure.13,35,36 The band corresponding to the five-membered ring of zeolites is higher in samples ZM41A1 and ZM41A2, as compared with the intensity of the band noticed in sample ZM41A3. The intensity of the band indicates that the extent of zeolitisation in samples ZM41A1 and ZM41A2 is rather high. | ||
| Fig. 4 IR spectra for calcined (a) ZM41A1, (b) ZM41A2 and (c) ZM41A3. | ||
3.3. Acidic properties of the samples and electron microscopy
To determine the acidic properties of all catalysts under study, pyridine was used as a probe molecule to evaluate possible differences in type, concentration, strength of acid sites and their location. The amounts of Lewis and Brønsted acid sites for ZSM-5, Y-zeolite and the composite materials after pyridine desorption are presented in Table 1. Fig. 5 presents the FTIR spectra of the hydroxyl region of all catalysts after pyridine adsorption and the spectrum area of vibrations of pyridine interaction with different acid sites. The hydroxyl region consists of an absorption band centered around 3745 cm−1, which is assigned to silanol groups, and a band at 3610 cm−1 due to the presence of acidic bridging OH groups (Fig. 5A). Adsorption of pyridine over all catalysts under study resulted in the formation of bands at 1545 cm−1, 1455 cm−1 and 1446 cm−1. The absorption band at 1545 cm−1 stems from the interaction of pyridine with Brønsted acid sites, while the absorption bands at 1446 and 1455 cm−1 are typical for pyridine interacting with non-acidic OH groups of silica or alumina and with typical adsorbed Lewis acid sites, respectively. At all temperatures studied, the intensity of the band related to the Brønsted acid sites over all catalysts under study was found to decrease in the following order: Y-zeolite > ZSM-5 > ZM41A1 > ZM141A2 > ZM41A3. On the other hand, the amount of Lewis acid sites over all samples under study decreases as follows: Y-zeolite > ZM41A3 > ZM41A1 > ZM41A2 > ZSM-5.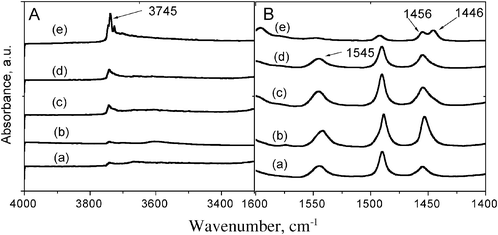 | ||
| Fig. 5 Infrared spectra of the hydroxyl region after pyridine desorption (A) and area of pyridine interacting with Brønsted and Lewis acid sites (B), (a) ZSM-5, (b) Y-zeolite, (c) ZM41A1, (d) ZM41A2 and (e) ZM41A3. | ||
The particle morphology of the materials was investigated using scanning electron microscopy. Fig. 6 shows the different morphological structures of the calcined ZSM-5, ZM41A1, ZM41A2 and ZM41A3 catalysts. Scanning electron images of the composite materials show unique aggregated crystal morphology, while regular cubic particles were noticed for the conventional ZSM-5 (Fig. 6a). Large solid shell-like particles reported for similar mesoporous aluminosilicates33 were also observed in these composite materials.
 | ||
| Fig. 6 Scanning electron microscopy (SEM) image of (a) ZSM-5, (b) ZM41A1, (c) ZM41A2 and (d) ZM41A3. | ||
3.4. Thermal analysis
Thermogravimetric analysis (TGA) of synthesized samples may be used to probe the presence of zeolite building units in the mesostructured materials.37 The thermogravimetric analysis of samples under study is presented in Fig. 7. All samples show a weight loss below 120 °C, which is due to desorption of water. Three weight loss events centered at about 150, 280 and 420 °C were observed over samples ZM41A2 and ZM41A3. Over all composite materials, weight loss in the 150–300 °C temperature range is attributed to decomposition and removal of occluded organics. Sample ZM41A1, which is predominantly zeolitic in character, shows two weight loss events (apart from desorption of water) centered at 275 and 447 °C. The weight losses above 350 °C noticed over all samples are due to water losses resulting from dehydroxylation reactions. The decrease in the amount of organic additives/templates (CTAB) retained in the synthesized composite materials is consistent with the gradual shift from a mesostructured (ZM41A3) to a zeolitic material (ZM41A1).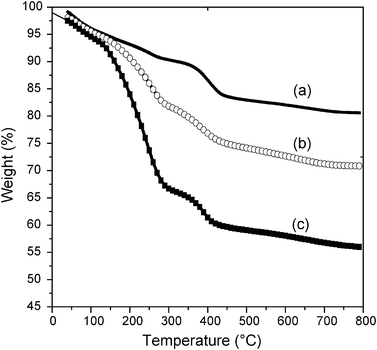 | ||
| Fig. 7 Thermogravimetric analysis curves for synthesized ZM41A1 (a), ZM41A2 (b) and ZM41A3 (c). | ||
3.5. Catalytic behavior of the composite materials
The catalytic behaviors of all micro/mesoporous composite materials were evaluated in different reactions. Transformations of aromatic hydrocarbons have been known to be sensitive to textural/structural and acidity changes as reported by several researchers in the literature.38–403.6. Transformation of 1,2,4-trimethylbenzene
The transformation of 1,2,4-trimethylbenzene was carried out at 400 °C over the conventional microporous ZSM-5 and ZSM-5/MCM-41 composite materials for reaction times of 5, 10, 15 and 20 s. Isomers of 1,2,4-trimethylbenzene were the main products noticed in the transformation of 1,2,4-TMB, while xylenes, tetramethylbenzenes (TeMB), benzene and toluene are also produced in significant amounts. Conversions and complete product distribution for the transformation of 1,2,4-trimethylbenzene over the ZSM-5 and the composite materials are presented in Table 2.| Sample | Time (s) | 1,2,4-TMB Conv (%) | Gases | Tol | Ben | Xy | TeMB | 1,3,5-TMB | 1,2,3-TMB |
|---|---|---|---|---|---|---|---|---|---|
| TMB–trimethylbenzene, Tol–toluene, Ben–benzene, Xy–xylene, TeMB–tetra methylbenzene, reaction T = 400 °C and catalyst/feed = 3.8. | |||||||||
| ZSM-5 | |||||||||
| 5 | 15.39 | 0.15 | 0.39 | 0.05 | 2.02 | 2.39 | 6.21 | 4.03 | |
| 10 | 29.51 | 0.27 | 0.87 | 0.07 | 4.61 | 4.96 | 11.40 | 5.97 | |
| 15 | 31.60 | 0.32 | 1.03 | 0.12 | 4.94 | 5.12 | 13.10 | 6.85 | |
| 20 | 34.27 | 0.33 | 1.05 | 0.12 | 5.37 | 6.01 | 13.80 | 7.10 | |
| ZM41A1 | |||||||||
| 5 | 15.45 | 0.09 | 0.36 | 0.05 | 2.60 | 3.26 | 5.18 | 3.52 | |
| 10 | 24.40 | 0.18 | 0.66 | 0.07 | 3.53 | 5.19 | 8.47 | 5.24 | |
| 15 | 29.17 | 0.21 | 0.90 | 0.10 | 5.64 | 6.45 | 9.84 | 5.71 | |
| 20 | 32.31 | 0.27 | 0.92 | 0.11 | 6.14 | 8.02 | 10.50 | 6.07 | |
| ZM41A2 | |||||||||
| 5 | 17.39 | 0.07 | 0.40 | — | 4.71 | 5.35 | 4.24 | 2.42 | |
| 10 | 25.41 | 0.14 | 0.59 | 0.07 | 6.19 | 6.51 | 7.32 | 3.93 | |
| 15 | 30.14 | 0.18 | 0.61 | 0.08 | 6.30 | 6.53 | 10.80 | 5.57 | |
| 20 | 36.40 | 0.30 | 0.81 | 0.14 | 7.76 | 8.57 | 12.40 | 6.04 | |
| ZM41A3 | |||||||||
| 5 | 13.61 | — | 0.17 | — | 4.44 | 5.16 | 2.04 | 1.57 | |
| 10 | 21.26 | — | 0.26 | — | 6.88 | 7.96 | 3.46 | 2.49 | |
| 15 | 25.46 | — | 0.31 | — | 7.54 | 8.62 | 5.19 | 3.51 | |
| 20 | 29.80 | — | 0.45 | — | 9.28 | 10.70 | 5.47 | 3.61 | |
Transformation of 1,2,4-trimethylbenzene was also investigated over the conventional microporous ZSM-5. The conversion of 1,2,4-TMB over the catalyst based on ZSM-5 increased with increasing contact time. A maximum 1,2,4-TMB conversion of ∼34.3% was observed at 400 °C for a reaction time of 20 s. The main products in the transformation of 1,2,4-TMB over the ZSM-5 based catalyst are 1,3,5-TMB and 1,2,3-TMB. Significant amounts of TeMB (6.0%) and xylenes (5.4%) were also noticed over the conventional microporous ZSM-5 at a reaction temperature of 400 °C. Formation of a significant amount of TeMB over the catalyst based on ZSM-5 occurred on the external surface of ZSM-5 crystals, since TeMB can hardly diffuse out of the ZSM-5 channels. The dealkylation reaction was found to be inconsequential, due to the amount of benzene and toluene observed in the transformation of 1,2,4-TMB over the catalyst based on ZSM-5.
 | ||
Fig. 8 Product distribution in the transformation of 1,2,4-TMB over the ZSM-5 catalyst and the composite materials (1,2,4-TMB conversion = ∼30%, reaction temperature = 400 °C, catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) feed = ∼3.8). feed = ∼3.8). | ||
The effect of 1,2,4-TMB conversion on xylene selectivity at 400 °C over all catalysts under study is shown in Fig. 9. Xylene selectivity over ZSM-5 and ZM41A3 shows a moderate dependence on 1,2,4-TMB conversion, while ZM41A1 and ZM41A2 shows a high dependence on the conversion of 1,2,4-TMB.
 | ||
Fig. 9 Effect of 1,2,4-TMB conversion on xylene selectivity in the transformation of 1,2,4-TMB over ZSM-5 (□), ZM41A1 (●), ZM41A2 (△) and ZM41A3 (▼) (catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) feed = 3.8, reaction temperature = 400 °C). feed = 3.8, reaction temperature = 400 °C). | ||
3.7. Transformation of m-xylene
The transformation of m-xylene has been used by several researchers as a test reaction to differentiate between 10- and 12-MR pore zeolites, to identify if there are lobes, cages, or crossing channels.43–45 The catalytic activities of conventional ZSM-5 and the composite materials were investigated in the transformation of m-xylene. The reaction was carried out at reaction temperatures of 350 and 400 °C for residence times of 5, 10, 15 and 20 s using a catalyst to reactant ratio of 3.8. | ||
Fig. 10 Reaction time dependence of m-xylene conversion and xylene selectivity in m-xylene transformation over ZSM-5 (■), ZM41A1 (○), ZM41A2 (△) and ZM4141A3 (▼); (reaction temperature = 350 °C (A, C) and 400 °C (B, D), catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) feed = ∼3.8). feed = ∼3.8). | ||
Over the ZSM-5 based catalyst, p-xylene and o-xylene were formed via the isomerization reaction, while toluene and TMB are produced through the disproportionation reaction. Traces of benzene and tetramethylbenzenes were also observed in the transformation of m-xylene over the conventional microporous ZSM-5. At reaction temperatures of 350 and 400 °C, the para to ortho (P/O) ratio noticed over the catalyst based on ZSM-5 was higher than the equilibrium value. The high para to ortho ratio can be attributed to the high diffusivity of p-xylene that is approximately 100 times higher than that of o-xylene.46 The ratio of the rates of isomerization to disproportionation (I/D) provides important information relating to the reaction space available in zeolite channel systems. The ratios of isomerization to disproportionation for m-xylene over ZSM-5 at a reaction temperature of 400 °C were found to range between 1.7 and 2.0.
The transformation of m-xylene over all the micro/mesoporous composite materials used in this study follows the bimolecular isomerization mechanism proposed by Corma et al.,24,47 which involves the disproportionation of xylene, followed by one or several successive transalkylation reactions. Toluene, trimethylbenzenes and isomers of xylenes were the major products noticed in the transformation of m-xylene over all composite materials. Insignificant amounts of benzene and tetramethylbenzenes were observed over ZM41A1 and ZM41A2, while no traces of these products were noticed over ZM41A3. Over the catalyst based on ZSM-5, the disproportionation to isomerization ratio (D/I) is the rate of bimolecular and monomolecular reactions. The differences between the D/I ratios in all composite materials would be due to differences in the number of transalkylation steps undergone by trimethylbenzene molecules.48 Thus, an increase in the D/I ratio would be related to a reduction in the number of transalkylation steps. The selectivities towards xylenes in the transformation of m-xylene over all catalysts under study at 350 °C follow the order: ZSM-5 < ZM41A1 < ZM41A3 < ZM41A2 (Fig. 10C). It was observed that increasing the temperature from 350 to 400 °C changed the order of xylene selectivity, in which the highest xylene selectivity was noticed over ZM41A3 (Fig. 10D).
 | ||
Fig. 11 Product selectivity of m-xylene transformation at ∼18% m-xylene conversion (A) and reaction time dependence of D/I ratio in m-xylene transformation (B) over ZM41A1 (■, □), ZM41A2 (●, ○) and ZM41A3 (▲, Δ) (reaction temperature = 350 °C (■, ●, ▲) and 400 °C (□, ○, Δ), catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) feed = ∼3.8). feed = ∼3.8). | ||
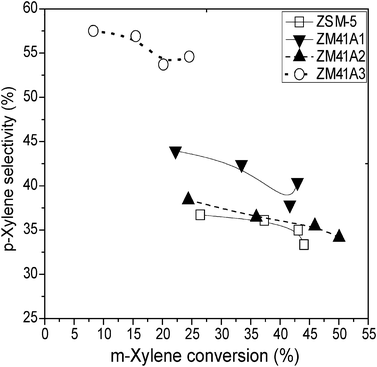
The selectivity towards p-xylene over the different catalysts under study follows the order: ZM41A1 ≈ conventional ZSM-5 < ZM41A2 < ZM41A3. Higher p-xylene selectivity was observed over ZM41A2 and ZM41A3 as compared with the conventional microporous ZSM-5. The co-existence which occurs between the mesoporous materials and the ZSM-5 zeolite unit accounts for the unique para-selectivity noticed over the composite materials. Over the composite materials, p- and o-xylene were formed through successive reactions of disproportionation and transalkylation via the mesoporous molecular sieves. The ZSM-5 zeolite units present in the composite materials were responsible for the screening of these products (p- and o-xylene) according to the ease of diffusivity of the molecules.
The T/TMB molar ratio over ZSM-5 and the various ZSM-5/MCM-41 composites at 350 °C, for a contact time of 20 s, follows the order: ZM41A1 > ZSM-5 > ZM41A2 > ZM41A3. The highest toluene to trimethylbenzene ratio was observed over ZM41A1, the composite material with a predominantly zeolitic character. Comparing the T/TMB ratio at the same conversion level of ∼24% showed a similar order: ZM41A1 > ZSM-5 > ZM41A2 > ZM41A3. At this same conversion level, the xylene production (p- and o-xylene) was found to follow the order: ZM41A3 > ZM41A2 > ZSM-5 > ZM41A1. This trend shows that sample ZM41A3 allowed the formation of more trimethylbenzene molecules, which is needed for the formation of xylene isomers, via the transalkylation reaction. The high ratio observed of T/TMB over the conventional ZSM-5 can be related to secondary transalkylation or dealkylation reactions taking place over the catalyst. It can also be attributed to the small reaction volume in the channel intersections of the ZSM-5 based catalyst. In the absence of secondary transalkylation or dealkylation, the molar ratio of T/TMBs over the conventional microporous ZSM-5 should be unity. The dealkylation reaction was found to be insignificant due to the absence of gases. The effect of the conversion of m-xylene on p-xylene selectivity at 400 °C over ZSM-5 and the composite materials is presented in Fig. 12. p-Xylene selectivities over the ZSM-5 based catalyst, ZM41A1, ZM41A2 and ZM41A3 show high dependence on m-xylene conversion and were observed to decrease with increasing conversion.
3.8. Catalytic activity for 1,3,5-triisopropylbenzene cracking
Table 3 presents the reaction products noticed from the catalytic cracking of 1,3,5-triisopropylbenzene over Y-zeolite and the micro/mesoporous composite materials at 400 °C for residence times of 5, 10, 15 and 20 s. The experimental results showed that the conversion occurred through cleavage of the propyl group from the benzene ring, with the benzene ring remaining unaltered.49 It has also been reported that the catalytic cracking of 1,3,5-triisopropylbenzene is a reaction network with three prevailing series reactions.50| Sample | Time (s) | TIPB Conv (%) | Gases | Tol | Ben | EB | Xy | Cum | TMB's | m-DIPB | p-DIPB | o-DIPB | T. DIPBs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
1,3,5-TIPB–triispropylbenzene, Tol–toluene, Ben–benzene, EB–ethylbenzene, Xy–xylene, Cum–cumene, TMB's–trimethylbenzenes, DIPB–diisopropylbenzene, reaction T = 400 °C and catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) feed = 3.8. feed = 3.8. |
|||||||||||||
| Y-zeolite | |||||||||||||
| 5 | 31.64 | 21.02 | 2.17 | 7.06 | 0.54 | 0.39 | 0.35 | — | 0.08 | — | — | 0.08 | |
| 10 | 52.89 | 31.60 | 4.34 | 13.09 | 1.20 | 0.91 | 1.41 | 0.06 | 0.21 | — | — | 0.21 | |
| 15 | 65.54 | 38.01 | 5.61 | 16.26 | 1.60 | 1.25 | 2.35 | 0.09 | 0.23 | 0.07 | — | 0.30 | |
| 20 | 72.71 | 40.70 | 6.79 | 18.63 | 1.75 | 1.67 | 2.39 | 0.34 | 0.25 | 0.08 | — | 0.33 | |
| ZM41A1 | |||||||||||||
| 5 | 43.08 | 13.55 | 1.57 | 8.38 | 1.72 | 0.75 | 1.91 | 0.65 | 9.61 | 1.99 | 0.08 | 11.68 | |
| 10 | 62.01 | 21.12 | 2.41 | 12.70 | 2.94 | 1.55 | 1.94 | 0.68 | 14.01 | 2.95 | 0.12 | 17.07 | |
| 15 | 71.02 | 23.35 | 3.10 | 14.74 | 3.75 | 2.10 | 2.47 | 0.82 | 14.59 | 3.08 | 0.11 | 17.78 | |
| 20 | 78.30 | 24.30 | 4.35 | 16.22 | 4.60 | 2.60 | 2.55 | 0.83 | 17.30 | 3.55 | 0.13 | 20.98 | |
| ZM41A2 | |||||||||||||
| 5 | 44.05 | 16.87 | 0.64 | 8.09 | 1.05 | 0.58 | 2.30 | 0.23 | 10.30 | 1.87 | 0.10 | 12.27 | |
| 10 | 63.59 | 22.15 | 0.97 | 11.74 | 1.64 | 1.06 | 3.13 | 0.38 | 17.13 | 2.98 | 0.18 | 20.29 | |
| 15 | 74.16 | 25.74 | 1.38 | 14.46 | 2.12 | 1.51 | 3.41 | 0.52 | 17.84 | 3.13 | 0.21 | 21.11 | |
| 20 | 83.16 | 28.77 | 2.27 | 17.43 | 3.11 | 2.23 | 3.52 | 0.62 | 19.26 | 3.34 | 0.21 | 22.81 | |
| ZM41A3 | |||||||||||||
| 5 | 45.73 | 17.51 | 0.19 | 4.93 | 0.39 | 0.42 | 8.95 | 0.63 | 9.65 | 2.04 | 0.18 | 11.87 | |
| 10 | 68.06 | 24.03 | 0.23 | 7.02 | 0.62 | 0.70 | 12.92 | 1.08 | 16.01 | 3.40 | 0.34 | 19.75 | |
| 15 | 79.30 | 27.75 | 0.28 | 8.81 | 0.79 | 0.94 | 14.65 | 1.39 | 17.51 | 3.48 | 0.40 | 21.39 | |
| 20 | 86.13 | 31.66 | 0.45 | 11.93 | 1.05 | 0.98 | 14.73 | 1.64 | 18.27 | 3.76 | 0.41 | 22.44 | |
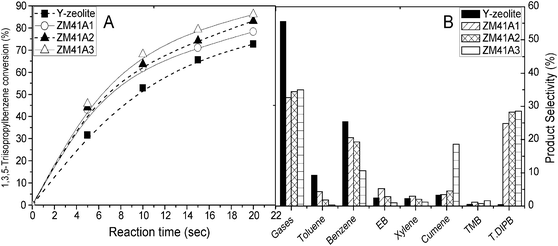 | ||
Fig. 13 Variation of 1,3,5-TIPB conversion with reaction time (A) and product selectivity of the catalytic cracking of 1,3,5-TIPB (B) achieved over Y-zeolite (■), ZM41A1 (○), ZM41A2 (▲) and ZM41A3 (△) at ∼73% 1,3,5-TIPB conversion (reaction temperature = 400 °C, catalyst![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) feed = ∼3.8). feed = ∼3.8). | ||
Similar to the reaction products noticed over the composite materials, propylene, benzene and cumene were the main products noticed in the catalytic cracking of 1,3,5-TIPB over the catalyst based on Y-zeolite. Over Y-zeolite, insignificant amounts of ethylbenzene (1.8%), TMBs (0.3%) and DIPBs (0.3%) were detected in the reaction products at 400 °C for a reaction time of 20 s. The low yield of DIPBs observed over Y-zeolite may be attributed to pre-cracking at the surface and/or near surface acid sites of Y-zeolite, leading to some products diffusing into openings and further cracking to lighter products. Several researchers have reported the possibility of 1,3,5-TIPB dealkylating on weak surface acid sites.51,52 It has also been reported that 1,3,5-TIPB, with a critical diameter of 9.5 Å, can rarely diffuse into the Y-zeolite structure where most of the acid sites are located.53 The yield of benzene increased with contact time to a maximum of ∼18.6%, while only ∼2.4% cumene yield was noticed at 400 °C for a reaction time of 20 s. The high yield of benzene observed in the catalytic cracking of 1,3,5-TIPB over the catalyst based on Y-zeolite can also be attributed to the pre-cracking taking place at the surface of the catalyst.
Fig. 13B presents the product selectivity during the cracking of 1,3,5-TIPB over Y-zeolite, ZM41A1, ZM41A2 and ZM41A3 at a constant conversion level of ∼73%. Over all catalysts, gaseous products (mainly propylene) were observed as the major product in the catalytic cracking reaction. The selectivity of benzene over Y-zeolite and the composite materials follows the order: ZM41A3 < ZM41A2 < ZM41A1 < Y-zeolite. Similarly, the DIPB selectivity was found in the order: Y-zeolite < ZM41A1 < ZM41A2 ≈ ZM41A3. The lower selectivity of DIPBs noticed over Y-zeolite as compared with the composite materials is a result of the pre-cracking of 1,3,5-TIPB taking place at the surface of the Y-zeolite catalyst.
4. Conclusions
In this study, composite micro/mesoporous materials were prepared by assembly of precursor zeolite species into a mesostructured material. The catalytic activity of these composite materials was investigated in m-xylene transformation, transformation of 1,2,4-TMB and in the cracking of 1,3,5-TIPB. The following conclusions can be drawn from the reactions:• All micro/mesoporous composite materials exhibited enhanced mass transfer properties and reduced diffusional limitations both in cracking (compared to Y-zeolite), transformation of 1,2,4-TMB and in m-xylene transformation (compared to ZSM-5).
• In the transformation of 1,2,4-TMB, the selectivity towards xylenes over all catalysts follows the order: ZM41A3 > ZM41A2 > ZM41A1 > ZSM-5.
• The co-existence that occurs between the mesopores and the zeolite units led to the unique p-xylene selectivity noticed over the composite materials in m-xylene transformation.
• Catalyst acidity as well as the pore size of the catalysts plays a major role in the cracking of 1,3,5-triisopropylbenzene.
• Presence of mesopores in the micro/mesoporous composite materials led to higher 1,3,5-TIPB conversions compared with the Y-zeolite catalyst.
Acknowledgements
We are grateful for the support from the Ministry of Higher Education, Saudi Arabia for the establishment of the Center of Research Excellence in Petroleum Refining and Petrochemicals at King Fahd University of Petroleum and Minerals (KFUPM). Mr Mariano Gica is also acknowledged for his help during the experimental work.References
- A. A. Avidan, in Fluid catalytic cracking: science and technology, ed. J. S. Magee and M. M. Mitchell, Elsevier, Amsterdam, 1993 Search PubMed.
- M. E. Davis, Nature, 2002, 417, 813–821 CrossRef CAS.
- W. O. Haag, R. M. Lago and P. B. Weisz, Nature, 1984, 309, 589–591 CrossRef CAS.
- R. H. Jensen, in Zeolites for cleaner technologies, ed. M. Guisnet and J.-P. Gilson, Imperial College Press, London, 2005 Search PubMed.
- V. Ducarme and J. C. Vedrine, Appl. Catal., 1985, 17, 175–184 CrossRef CAS.
- M. Csicsery, Pure Appl. Chem., 1986, 58, 841–856 CrossRef.
- A. Corma, Chem. Rev., 1997, 97, 2373–2419 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- L. Wang, A. Wang, X. Li, F. Zhou and Y. Hu, J. Mater. Chem., 2010, 20, 2232–2234 RSC.
- Y. Xia and R. Mokaya, J. Mater. Chem., 2004, 14, 863–870 RSC.
- K. R. Kloetstra, H. van Bekkum and J. C. Jansen, Chem. Commun., 1997, 23, 2281–2282 RSC.
- A. Karlsson, M. Stocker and R. Schmidt, Microporous Mesoporous Mater., 1999, 27, 181–192 CrossRef CAS.
- L. Huang, W. Guo, P. Deng, Z. Xue and Q. Li, J. Phys. Chem. B, 2000, 104, 2817–2823 CrossRef CAS.
- A. Bhavani, D. Karthekayen, A. Rao and N. Lingappan, Catal. Lett., 2005, 103, 89–100 CrossRef CAS.
- M. A. Ugina, J. L. Sotelo and D. P. Serrano, Appl. Catal., 1991, 76, 183–195 CrossRef.
- A. Baduraig, T. Odedairo and S. Al-Khattaf, Top. Catal., 2010, 53, 1446–1456 CrossRef CAS.
- R. Roldan, F. J. Romero, C. Jimenez, V. Borau and J. M. Marinas, Appl. Catal., A, 2004, 266, 203–210 CrossRef CAS.
- J. Das, Y. S. Bhat and A. B. Halgeri, Catal. Lett., 1994, 23, 161–168 CrossRef CAS.
- J. Cejka, J. Kotrla and A. Krejci, Appl. Catal., A, 2004, 277, 191–199 CrossRef CAS.
- S. M. Waziri, A. M. Aitani and S. Al-Khattaf, Ind. Eng. Chem. Res., 2010, 49, 6376–6387 CrossRef CAS.
- J. Weitkamp and S. Ernst, Catal. Today, 1994, 19, 107–150 CrossRef CAS.
- A. Corma, C. Corell, F. Llopis, A. Martinez and J. Perez-Pariente, Appl. Catal., A, 1994, 115, 121–134 CrossRef CAS.
- J. A. Martens, J. Perez-Pariente, E. Sastre, A. Corma and P. A. Jacobs, Appl. Catal., 1988, 45, 85–101 CrossRef CAS.
- A. Corma and E. Sastre, J. Catal., 1991, 129, 177–185 CrossRef CAS.
- B. Xue, J. Xu, C. Xu, R. Wu, Y. Li and K. Zhang, Catal. Commun., 2010, 12, 95–99 CrossRef CAS.
- F. J. Llopis, G. Sastre and A. Corma, J. Catal., 2004, 227, 227–241 CrossRef CAS.
- N. M. Tukur and S. Al-Khattaf, Chem. Eng. J., 2011, 166, 348–357 CrossRef CAS.
- S. Al-Khattaf, M. N. Akhtar, T. Odedairo, A. Aitani, N. M. Tukur, M. Kubu, Z. Musilova-Pavlackova and J. Cejka, Appl. Catal., A, 2011, 394, 176–190 CrossRef CAS.
- N. Hosseinpour, A. A. Khodadadi, Y. Mortazavi and A. Bazyari, Appl. Catal., A, 2009, 353, 271–281 CrossRef CAS.
- N. Hosseinpour, Y. Mortazavi, A. Bazyari and A. A. Khodadadi, Fuel Process. Technol., 2009, 90, 171–179 CrossRef CAS.
- P. Morales-Pacheco, J. M. Dominguez, L. Bucio, F. Alvarez, U. Sedran and M. Falco, Catal. Today, 2010, 166, 25–38 CrossRef.
- J. Qi, T. Zhao, X. Xu, F. Li, G. Sun, C. Miao and H. Wang, Catal. Today, 2009, 10, 1523–1528 CAS.
- T. Odedairo, R. J. Balasamy and S. Al-Khattaf, J. Mol. Catal. A: Chem., 2011, 345, 21–36 CrossRef CAS.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. U. Chu, D. H. Olsen, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- Y. Liu, W. Zhang and T. J. Pinnavaia, Angew. Chem., Int. Ed., 2001, 40, 1255–1258 CrossRef CAS.
- D. Lesthaeghe, P. Vansteenkiste, T. Vertraelen, A. Ghysels, C. E. A. Kirschhock, J. A. Martens, V. V. Speybroeck and M. Waroquier, J. Phys. Chem. C, 2008, 112, 9186–9191 CAS.
- P. Prokesova, S. Mintova, J. Cejka and T. Bein, Microporous Mesoporous Mater., 2003, 64, 165–174 CrossRef CAS.
- T. Odedairo and S. Al-Khattaf, Appl. Catal., A, 2010, 381, 31–45 CrossRef.
- J. Cejka and B. Wichterlova, Catal. Rev. Sci. Eng., 2002, 44, 375–421 CAS.
- T. C. Tsai, S. B. Liu and I. Wang, Appl. Catal., A, 1999, 181, 355–398 CrossRef CAS.
- S. M. Csicsery, J. Catal., 1971, 23, 124–130 CrossRef CAS.
- S. M. Csicsery, Zeolites, 1984, 4, 202–213 CrossRef CAS.
- L. B. Young, S. A. Butter and W. W. Kaeding, J. Catal., 1982, 76, 418–432 CrossRef CAS.
- M. Guisnet, N. S. Gnep and S. Morin, Microporous Mesoporous Mater., 1998, 22, 343–356 CrossRef.
- H. Du and D. H. Olson, J. Phys. Chem. B, 2002, 106, 395–400 CrossRef CAS.
- G. Mirth, J. Cejka and J. A. Lercher, J. Catal., 1993, 139, 24–33 CrossRef CAS.
- A. Corma, F. Llopis, J. B. Monton, in: L. Guczi, F. Solymosi and P. Telenyi, Studies in Surface Science and Catalysis, 1983, vol. 75, pp. 1145–1157 Search PubMed.
- S. Morin, P. Ayrault, S. El Mouahid, N. S. Gnep and M. Guisnet, Appl. Catal., A, 1997, 159, 317–331 CrossRef CAS.
- A. Corma and B. W. Wojciechowski, Catal. Rev. Sci. Eng., 1982, 24, 1–65 CAS.
- S. Al-Khattaf and H. de Lasa, Appl. Catal., A, 2002, 226, 139–153 CrossRef CAS.
- N. Katada, Y. Kageyamaa, K. Takahara, T. Kanai, H. A. Beguma and M. Niwa, J. Mol. Catal. A: Chem., 2004, 211, 119–130 CrossRef CAS.
- L. Zhu, S. Xiao, Z. Zhang, Y. Sun, Y. Han and S. Qiu, Catal. Today, 2001, 68, 209–216 CrossRef CAS.
- A. Bazyari, A. A. Khodadadi, N. Hosseinpour and Y. Mortazavi, Fuel Process. Technol., 2009, 90, 1226–1233 CrossRef CAS.
- Q. Tan, X. Bao, T. Song, Y. Fan, G. Shi, B. S. C. Liu and X. Gao, J. Catal., 2007, 251, 69–79 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |