Development of a visible light active photocatalytic portable water purification unit using ZnO nanorods
Sunandan
Baruah
*ab,
Mayuree
Jaisai
a and
Joydeep
Dutta
ac
aCenter of Excellence in Nanotechnology, Asian Institute of Technology, Klong Luang, Pathumthani 12120, Thailand. Fax: +66 2 524 5617; Tel: +66 2 524 5680
bElektronmikroskopi och Nanoteknologi, The Ångström Laboratory, Box 534, SE-751 21 Uppsala, Sweden. E-mail: Sunandan.Baruah@angstrom.uu.se
cSultan Qaboos University, P.O. Box 50, Muscat 12, Sultanate of Oman
First published on 16th February 2012
Abstract
A ZnO nanorods based water purification unit was designed which operates with solar energy as the source of activation. The purifier was tested on two model bacteria Escherichia coli and Staphylococcus aureus with concentration as high as 1010 colony forming units (CFU) per litre, which is about 105 times higher than the bacterial concentration in tap water. Up to 99% (0.99 × 1010 CFU L−1) removal of viable bacterial cells was achieved under sunlight activation.
Waterborne epidemics have almost been eradicated in developed countries due to the extensive use of chemical disinfection methods like chlorination and ozonation. However, water borne diseases are still alarmingly high in developing countries1,2 and as such water purification is an area of utmost importance. The currently employed chemical disinfection methods can effectively control microbial pathogens but result in the formation of harmful disinfection byproducts (DBPs).3 The notion of decentralized or dispersed water treatment systems has been seriously considered owing to concerns on water quality deterioration associated with old distribution networks. The ever-increasing transportation cost is another reason for the urgent need of point of use water purification systems. ZnO is a wide bandgap semiconductor and is a good photocatalyst. Nanostructures of ZnO can be grown following simple hydrothermal methods at very low temperatures (<100 °C).4 We have already reported the growth of ZnO nanorods on different substrates that can have possible applications as membranes for water purification.5–8 Our studies on the antibacterial activity of ZnO nanorods revealed that growth of both gram-positive and gram-negative bacteria is inhibited in the presence of ZnO and the inhibition improves drastically upon photo irradiation.9 Based on these studies, we designed a portable antimicrobial water purifier using ZnO nanorods grown on a polyethylene membrane that utilizes solar radiation to remove disease-causing bacteria.10 This filter does not require any other energy sources and is ideal for use in rural or disaster-hit regions where there is maximum possibility of drinking1 water related epidemics. The importance of membranes in drinking water and wastewater treatment systems is gaining importance.11 Integration of antimicrobial or photocatalytic nanomaterials makes the membranes reactive instead of a simple physical barrier, achieving multiple treatment goals in a single process while minimizing fouling.3 This work is focused on visible light photocatalysis using ZnO nanorods containing mid-band defect states or quasi-stable energy states in the form of zinc interstitials and oxygen vacancies that result from the hydrothermal crystal growth.12 These energy states allow electron–hole pair generation and separation through multiple absorptions of low energy photons in the visible part of the electromagnetic spectrum. The ZnO nanorods used in this work are therefore photoactive even upon excitation with visible light, which forms a major part of the solar spectrum. We present here an antimicrobial water filter that is inexpensive and highly efficient in using solar energy, which is available in abundance.
Fig. 1 shows a schematic representation of the water purifier comprising of nonwoven polyethylene fibers with ZnO nanorods enclosed in a glass tube. ZnO nanorods were grown on polyethylene fibers (procured from Honeywell, USA) with a base weight of 110 g m−2 following a procedure detailed in one of our previous publications.7 A thin layer of n-dodecane thiol was deposited on the polyethylene fibers by dipping in a 1% dodecane thiol solution in ethanol and then heated at 100 °C for 15 minutes prior to the ZnO nanowire growth. The fibers were then dipped in a colloidal solution of ZnO nanoparticles in ethanol for 15 minutes, the synthesis process of which has been reported in previous publications.13–15 The nanorods were grown in a chemical bath containing a 10 mM solution of zinc nitrate hexahydrate [Zn(NO3)2·6H2O, Aldrich, 99% purity] and hexamethylenetetramine [C6H12N4, Carlo Erba, 99.5% purity] maintained at 90 °C for 10 hours.7 The antimicrobial water filter was fabricated by enclosing polyethylene fibers with ZnO nanorods in a cylindrical glass tube of diameter 1.5 cm, length 30 cm and 0.5 cm openings as inlet and outlet for the water. Filter media (polyethylene fiber with ZnO nanorods) of three different sizes were studied for optimization.
 | ||
| Fig. 1 Schematic representations of (a) water purifier comprising of ZnO nanorods on polyethylene fibers enclosed in a glass tube, (b) mechanism of bacterial immobilization by ZnO nanorods. Free electrons interact with oxygen forming superoxides while the holes convert hydroxyl ions into highly reactive hydroxyl radicals. | ||
A major advantage of the use of antimicrobial nanomaterial like ZnO in water purification is the possibility of point-of-use treatment systems.3 Another advantage of ZnO as a nanostructured photocatalyst material is that it can be engineered to absorb visible light thereby enhancing solar photocatalysis.13,16 ZnO is also reported to be strongly antibacterial on a variety of targets like Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Bacillus subtilis (B. subtilis), to name a few.17–19 The antibacterial activity of ZnO can be attributed to (i) the binding of Zn2+ ions, released through dissolution, to the pili of bacteria thereby prolonging the lag phase of the bacterial growth cycle20 and (ii) photocatalytic activity upon light illumination.17 Photogenerated electrons and holes can create highly reactive radicals that can adversely affect the cell walls of microbes (schematically explained in Fig. 1(b)).
E. coli and S. aureus cells were cultured in 150 mL nutrient broth after incubation for 48 h in an incubator shaker at a speed of 120 rpm at 37 °C. Cultures of the bacteria were centrifuged at 4000 rpm for 10 minutes. The cell pellets were re-dispersed in sterile distilled water for sequential experiments. The initial concentration of the bacterial suspension was set at 107 CFU mL−1. Spiked water containing bacterial cells of E. coli or S. aureus was flowed through the filter under continuous white light irradiation. Two different light sources were used for the study: white light from a tungsten halogen lamp (385 W m−2 on the filter wall) under lab conditions (25 °C) and solar light in the open (1532 W m−2 to 2407 W m−2 on the filter wall). The light intensity was measured using a Kipp and Zonen pyranometer. The temperature on the filter tube surface after continuous irradiation for a few minutes was noted to be around 35 °C in both the cases. The flow rate was maintained at 15 ml per minute. 100 mL of spiked water was allowed to pass through the filter, which needed about 7 min to complete the passage through the filter media. Bacterial cell concentrations in the water before and after passing through the filter were determined and compared.
Inductively coupled plasma optical emission spectrometry (ICP-OES) was used to measure the amount of Zn2+ ions in the water collected after the filtration process. Standard solutions were prepared by dissolving metallic Zn powder in 18.5% HCl after which was diluted with deionized (DI) water to obtain different Zn2+ ion concentrations: (1) 500 μg L−1, (2) 1000 μg L−1, (3) 3000 μg L−1 and (4) 5000 μg L−1. For preparing the calibration blank, 2 mL of 1+1 HNO3 and 10 mL of 1+1 HCl were mixed and then diluted to 100 mL with DI water.
Thiol creates an irreversible bonding of the ZnO nanoparticles with the polyethylene fiber and as such the seed particles get firmly attached to the surface.7 These seeds grow into the nanorods, which are therefore also firmly attached to the fibers. Firm attachment of the ZnO nanorods to the polyethylene fibers is essential to avoid dislocation of the nanorods during the process of water circulation thereby ending up in the purified water. The antibacterial activity has been represented as the amount of viable cells present expressed as a percentage of the initial cell concentration before passing through the filter. To optimize the surface area available for adsorption of the bacterial cells, three filters were designed using different amounts of polyethylene fibers as catalyst supports for growing ZnO nanorods: (1) Filter 1 with 3 g of nonwoven polyethylene fibers, (2) Filter 2 with 6 g of nonwoven polyethylene fibers and (3) Filter 3 with 9 g of nonwoven polyethylene fibers. The results under dark conditions, halogen light illumination and under sunlight are summarized in Fig. 2(a) (b) and (c) respectively.
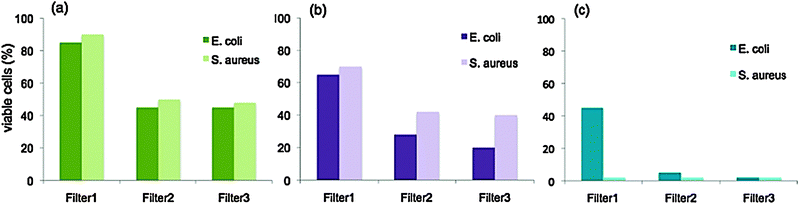 | ||
| Fig. 2 Concentration of viable cells expressed as a percentage of the concentration of bacterial cells in the water before passing through the filter (a) in the dark, (b) under halogen light illumination at 8.8 klux and (c) under sunlight at 53 klux. | ||
In both the cases, gram negative E. coli and gram positive S. aureus, a decrease in cell concentration could be observed when the spiked water was allowed to pass through the filter even under dark conditions. This can be attributed to the slow release of Zn2+ ions due to dissolution of ZnO.21 The effect of n-dodecane thiol on the antimicrobial activity can be considered insignificant as the thiol molecules are firmly attached on to the polyethylene fibers on one hand and the ZnO nanoparticles on the other. Upon photoexcitation of the ZnO nanorods, reactive oxygen species (ROS) such as hydroxyl radicals, hydrogen peroxide and superoxide anions are generated and enhanced Zn2+ ion release due to photocorrosion leads to the damage of bacterial cells. ROS can remove a hydrogen atom from one of the carbon (C) atoms of the fatty acid chain, forming a water molecule and thereby leaving the C atom with an unpaired electron. The fatty acid chain can then be termed as a free radical, which may interact with other molecules to get stable configuration, which again leads to the formation of another free radical. This chain reaction continues till two radicals share the unpaired electrons to form a covalent bond. These reactions distort the fatty acid side chains of the membrane lipids resulting in permanent damage to the membranes.9 This route of cell damage through ROS corroborates well with our results where we observed increased antimicrobial activity under sunlight with higher intensity leading to increased electron–hole pair generation and subsequently higher ROS formation. Filter 3 gave the best results owing to higher surface area available for adsorption of the target bacterial cells. Better antibacterial activity could be observed on E. coli under low lighting (halogen lighting at 385 W m−2) as compared to S. aureus, which is evident as gram negative bacteria are known to have thinner cell walls.22 However under sunlight illumination at intensities above 1600 W m−2, both the bacteria could be immobilized up to comparable levels (99% immobilization using Filter 3 as shown in Fig. 2(c)).
The pH of naturally available water is not constant but normally varies within a range of 6–7. The efficiency of the antimicrobial filter was tested at different pH values by adjusting the pH of the initial bacterial cell solutions from 5 to 9. The results of the antimicrobial effect at varying pH are shown in Fig. 3. The best result for E. coli was obtained at a pH of 7 with a 99% reduction in viable cells. A similar result was observed for S. aureus between pH 5 and 6. The filter was found to work best at the pH level of naturally occurring water and as such can be ideal for on the spot water purification especially in rural or disaster stricken areas.
 | ||
| Fig. 3 Viable bacteria (cells cultured at different pH values) after passing through the antimicrobial filter under sunlight at 44 klux. | ||
A section of the antimicrobial filter (nonwoven polyethylene fibers with ZnO nanorods) was removed and studied under a scanning electron microscope. The bacterial cells could be clearly observed to be immobilized onto the ZnO nanorods as shown in a representative SEM image in Fig. 4. The release of Zn2+ ions through the slow dissolution of ZnO is evident from the observed antibacterial activity in the dark. This may raise a concern about the quality of drinking water purified using the ZnO nanorod based water purifier as acute toxicity is reported to arise from the ingestion of excessive amounts of zinc salts. Excessive intake of Zn can cause nausea, vomiting, and diarrhoea, sometimes accompanied by bleeding and abdominal cramp.23 However, Zn also has nutritional values and should be a part of a healthy diet.
 | ||
| Fig. 4 Scanning electron micrograph showing Escherichia coli cells immobilized on ZnO nanorods after water containing the bacteria was allowed to pass through the filter under illumination with sunlight at 44 klux. | ||
Nutritional zinc deficiency in humans has been reported in a number of countries.24–27 The amounts of Zn present in the water before and after single passage through the antimicrobial filter were measured from samples through inductively coupled plasma mass spectroscopy (ICP-MS) and the results are summarized in Table 1. The concentration of Zn in the filtered water using the designed filter was found to be between 0.819 mg L−1 and 2.138 mg L−1. Considering an average adult intake of 2 litres of water per day, the amount of Zn consumed would be within 1.638 to 4.276 g day−1, which is well within the minimum zinc necessary for a healthy person.
In conclusion, an antibacterial water purifier was designed using ZnO nanorods grown on polyethylene fibers. The water purifier was shown to successfully immobilize two model bacteria E. coli and S. aureus. The antibacterial activity of the ZnO nanorods was attributed to arise due to the combined effects of two mechanisms, release of zinc ions through slow dissolution of ZnO and the formation of reactive oxygen species through photocatalysis. Up to 99% (0.99 × 1010 CFU L−1) of the E. coli and S. aureus cells could be immobilized upon exposure to sunlight, while under room lighting conditions, 80% (0.8 × 1010 CFU L−1) of E. coli and 59% (0.59 × 1010 CFU L−1) of S. aureus could be inactivated. Apart from fit to tap applications, the water purifier can be used in disaster areas to alleviate the scarcity of pure drinking water.
References
- M. F. Craun, G. F. Craun, R. L. Calderon and M. J. Beach, J. Water Health, 2006, 4, 19 CrossRef.
- http://www.who.int/water_sanitation_health/en/factsfigures04.pdf downloaded on 12. 12. 11.
- Q. Li, S. Mahendra, D. Y. Lyon, L. Brunet, M. V. Liga, D. Li and P. J. J. Alvarez, Water Res., 2008, 42, 4591 CrossRef CAS.
- S. Baruah and J. Dutta, Sci. Technol. Adv. Mater., 2009, 10, 013001 CrossRef.
- S. Baruah and J. Dutta, J. Sol-Gel Sci. Technol., 2009, 50, 456–464 CrossRef CAS.
- S. Baruah, M. Jaisai, R. Imani, M. M. Nazhad and J. Dutta, Sci. Technol. Adv. Mater., 2010, 11, 055002 CrossRef.
- S. Baruah, C. Thanachayanont and J. Dutta, Sci. Technol. Adv. Mater., 2008, 9, 025009 CrossRef.
- R. Kitsomboonloha, S. Baruah, M. T. Z. Myint, V. Subramanian and J. Dutta, J. Cryst. Growth, 2009, 311, 2352–2358 CrossRef CAS.
- A. Sapkota, A. J. Anceno, S. Baruah, O. V. Shipin and J. Dutta, Nanotechnology, 2011, 22, 215703 CrossRef.
- S. Baruah and J. Dutta, Indian patent appl., 2458/MUM/2011 Search PubMed.
- M. Marcucci, I. Ciabatti, A. Matteucci and G. Vernaglione, Ann. N. Y. Acad. Sci., 2003, 984, 53 CrossRef CAS.
- S. Baruah, M. A. Mahmood, M. T. Z. Myint, T. Bora and J. Dutta, Beilstein J. Nanotechnol., 2010, 1, 14 CrossRef CAS.
- S. Baruah, R. F. Rafique and J. Dutta, Nano, 2008, 3, 399 CrossRef CAS.
- M. A. Mahmood, S. Baruah and J. Dutta, Mater. Chem. Phys., 2011, 30, 531 CrossRef.
- R. Ullah and J. Dutta, J. Hazard. Mater., 2008, 156, 194 CrossRef CAS.
- S. Baruah, S. S. Sinha, B. Ghosh, S. K. Pal, A. K. Raychaudhuri and J. Dutta, J. Appl. Phys., 2009, 105, 074308 CrossRef.
- J. Sawai, J. Microbiol. Methods, 2003, 54, 177 CrossRef CAS.
- N. Jones, B. Ray, K. T. Ranjit and A. C. Manna, FEMS Microbiol. Lett., 2008, 279, 71 CrossRef CAS.
- Z. Huang, X. Zheng, D. Yan, G. Yin, X. Liao, Y. Kang, Y. Yao, D. Huang and B. Hao, Langmuir, 2008, 24, 4140 CrossRef CAS.
- S. Atmaca, K. Gul and R. Clcek, Turk. J. Med. Sci., 1998, 28, 595 CAS.
- J. Han, W. Qiu and W. Gao, J. Hazard. Mater., 2010, 178, 115 CrossRef CAS.
- A. Erkan, U. Bakir and G. Karakas, J. Photochem. Photobiol., A, 2006, 184, 313 CrossRef CAS.
- Handbook on the toxicology of metals, ed. L. Friberg and G. F. Nordberg, Elsevier Science Publishers, Amsterdam, 2nd edn, 1986 Search PubMed.
- M. J. Jackson, R. Giugliano, L. G. Giugliano, E. F. Oliveira, R. Shrimpton and I. G. Swainbank, Br. J. Nutr., 1988, 59, 193 CrossRef CAS.
- A. O. Cavdar, A. Arcasoy, S. Cin and H. Gumus, Haematologica, 1980, 65, 403 CAS.
- R. M. Smith, R. A. King and R. M. Spargo, Lancet, 1985, 1, 923 CrossRef CAS.
- C. Xue-Cun, Y. Tai-An and H. Jin-Sheng, Am. J. Clin. Nutr., 1985, 42, 694 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
