A novel magnetic Fe–Ti–V spinel catalyst for the selective catalytic reduction of NO with NH3 in a broad temperature range†
Shijian
Yang
ab,
Chizhong
Wang
a,
Jinghuan
Chen
a,
Yue
Peng
a,
Lei
Ma
a,
Huazhen
Chang
a,
Liang
Chen
a,
Caixia
Liu
a,
Jiayu
Xu
a,
Junhua
Li
*a and
Naiqiang
Yan
*b
aSchool of Environment, Tsinghua University, Haidian District, Beijing, 100084, P. R. China. E-mail: lijunhua@tsinghua.edu.cn; Fax: +86-10-62771093
bSchool of Environmental Science and Engineering, Shanghai Jiao Tong University, 800 Dong Chuan Road, Shanghai, 200240, P. R. China. E-mail: nqyan@sjtu.edu.cn; Fax: +86-21-54745591
First published on 1st February 2012
Abstract
Fe–Ti–V spinel showed excellent SCR activity, N2 selectivity and H2O/SO2 durability at 250–400 °C, and an external magnetic field can effectively prevent the emission of a vanadium based catalyst to the environment due to its magnetization.
Nitrogen oxides (NOx), which result from automobile exhaust gas and industrial combustion of fossil fuels, are major pollutants for air pollution.1 Selective catalytic reduction (SCR) of NO with NH3 has been an efficient and economic technique for the control of NOx emission. V2O5–WO3(MoO3)/TiO2 has been widely used as an SCR catalyst to control the emission of NOx.2 However, there are still some problems with the application of this system, for example the relatively narrow temperature window of 300–400 °C, the low N2 selectivity in the high temperature range, and the toxicity of V2O5 to the environment.3
The temperatures of the flue gases from some industrial furnaces, for example cement kilns, are often less than 300 °C. Therefore, great efforts have been made to develop new NH3-SCR catalysts with high SCR activity and N2 selectivity in a broad temperature range. In our previous research, Fe–Ti spinel was developed as an environmentally friendly SCR catalyst.4 Fe–Ti spinel showed excellent activity and selectivity at 300–400 °C. However, the presence of SO2 and H2O showed an obvious interference with NO conversion over Fe–Ti spinel below 300 °C. A vanadium based catalyst is famous for its SCR activity and SO2 durability,5 so vanadium was incorporated into Fe–Ti spinel to improve its SCR activity and SO2 durability. Furthermore, nanosized Fe–Ti–V spinel has the super-paramagnetization,6 which may be helpful for the control of vanadium based catalyst emission.
In this study, magnetic Fe–Ti–V spinel was synthesized using a co-precipitation method. Meanwhile, Fe–Ti spinel, Fe–Ti–Mn spinel and conventional vanadium based catalysts (V2O5–WO3/TiO2) were prepared for comparison.
As the loading of V2O5 increased from 1% to 10%, the SCR activity of V2O5–WO3/TiO2 obviously increased at 150–300 °C (shown in Fig. 1a). However, the SCR activity of 10%V2O5–WO3/TiO2 gradually decreased at 350–400 °C. N2 selectivity of V2O5–WO3/TiO2 gradually decreased above 300 °C, and N2 selectivity of 10%V2O5–WO3/TiO2 was much worse than that of 1%V2O5–WO3/TiO2 (shown in Fig. 1b). Fe–Ti spinel showed a moderate SCR activity at 250–300 °C, and an excellent SCR activity above 300 °C. Meanwhile, little N2O can be observed during the SCR reaction over Fe–Ti spinel (shown in Fig. 1b). After the incorporation of V and Mn into Fe–Ti spinel, NO conversion obviously increased especially at 200–300 °C (shown in Fig. 1a). Fig. 1a also shows that the effect of the incorporation of V on the SCR reaction over Fe–Ti spinel was much more noticeable than that of Mn. The SCR activity of Fe–Ti–V spinel was close to that of 10%V2O5–WO3/TiO2 at 150–350 °C, but it did not decrease at 350–400 °C. Meanwhile, N2 selectivity of Fe–Ti–V spinel only slightly decreased to about 90% as the reaction temperature increased to 400 °C, which was much better than those of 10%V2O5–WO3/TiO2 and 1%V2O5–WO3/TiO2 (shown in Fig. 1b).
![SCR performances of synthesized catalysts: (a) NO conversion; (b) N2 selectivity. ■, Fe–Ti spinel; ●, Fe–Ti–Mn spinel; ▲, Fe–Ti–V spinel; ◀, 1%V2O5–WO3/TiO2; ▼, 10%V2O5–WO3/TiO2. Reaction conditions: [NO] = [NH3] = 500 ppm, [O2] = 2 vol%, catalyst mass = 100 mg, total flow rate = 200 mL min−1, GHSV = 150 000 h−1.](/image/article/2012/CY/c2cy00459c/c2cy00459c-f1.gif) | ||
Fig. 1 SCR performances of synthesized catalysts: (a) NO conversion; (b) N2 selectivity. ■, Fe–Ti spinel; ●, Fe–Ti–Mn spinel; ▲, Fe–Ti–V spinel; ◀, 1%V2O5–WO3/TiO2; ▼, 10%V2O5–WO3/TiO2. Reaction conditions: [NO] = [NH3] = 500 ppm, [O2] = 2 vol%, catalyst mass = 100 mg, total flow rate = 200 mL min−1, GHSV = 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1. 000 h−1. | ||
Then, the mechanism of the SCR reaction over Fe–Ti–V spinel was studied using in situ DRIFTS (shown in Fig. 2). After Fe–Ti–V spinel was treated with NH3/N2 at 150 °C, both ionic NH4+ bound to the Brønsted acid sites (at 1665, 1453 and 1413 cm−1) and coordinated NH3 bound to the Lewis acid sites (at 1613, 1270 and 1238 cm−1) appeared. Meanwhile, amide (−NH2), which resulted from the activation of adsorbed NH3, can be observed at 1511 cm−1.7 After NO + O2/N2 passed over NH3 pretreated Fe–Ti–V spinel at 150 oC, both ionic NH4+ and coordinated NH3 diminished (shown in Fig. 2a). Meanwhile, adsorbed H2O, which is a product of the SCR reaction, can be clearly observed at 1630 cm−1.7 They both suggest that adsorbed ammonia species on Fe–Ti–V spinel can react with NO. However, the bands corresponding to adsorbed nitrogen oxides can hardly be detected after NO + O2/N2 passed over NH3 pretreated Fe–Ti–V spinel.
 | ||
| Fig. 2 (a) DRIFT spectra taken at 150 °C upon passing NO + O2 over NH3 pretreated Fe–Ti–V spinel; (b) DRIFT spectra taken at 150 °C upon passing NH3 over NO + O2 pretreated Fe–Ti–V spinel. | ||
After Fe–Ti–V spinel was treated with NO + O2/N2 at 150 °C, only a small amount of monodentate nitrite8 can be detected at 1640, 1553, 1500 and 1436 cm−1 (shown in Fig. 2b). After NH3/N2 passed over NO + O2 pretreated Fe–Ti–V spinel, adsorbed H2O, which is the product of the SCR reaction, can hardly be detected at 1630 cm−1. This suggests that the reaction between ammonia and adsorbed nitrogen oxide species on Fe–Ti–V spinel can be approximately neglected.
An in situ DRIFTS study demonstrated that the SCR reaction over Fe–Ti–V spinel mainly followed the Eley–Rideal mechanism (i.e. the reaction between activated ammonia species and gaseous NO), which was similar to those over Fe–Ti spinel4 and conventional vanadium based catalysts.2,9
Therefore, the possible routes of the SCR reaction over Fe–Ti–V spinel can be approximately described as follows:
| NH3(g) ⇌ NH3(ad) | (1) |
NH3(ad) + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ → −NH2 + Fe3+ → −NH2 + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + H+ Fe2+ + H+ | (2) |
NH3(ad) + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) V5+ → −NH2 + V5+ → −NH2 + ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) V4+ + H+ V4+ + H+ | (3) |
| −NH2 + NO(g) → N2 + H2O | (4) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe2+ + ¼O2 → Fe2+ + ¼O2 →![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe3+ + ½ Fe3+ + ½ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O O | (5) |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) V4+ + ¼O2 → V4+ + ¼O2 →![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) V5+ + ½ V5+ + ½ ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O O | (6) |
Reaction (1) is the adsorption of gaseous ammonia on the acid sites to form adsorbed ammonia species. There is general agreement that the SCR reaction starts with the adsorption of NH3, which is very strong compared to the adsorption of NO + O2 and the reaction products. On Fe–Ti–V spinel, both Fe3+ and V5+ can activate adsorbed NH3. Reactions (2) and (3) are the activation of adsorbed ammonia species to form –NH2 by Fe3+ and V5+ cations on the surface, respectively. Then, gaseous NO was reduced by –NH2 on the surface to form N2 and H2O (reaction (4)). Reactions (5) and (6) are the re-oxidization of formed Fe2+ and V4+, respectively.
As shown in Fig. 1a, only a small amount of NO can be reduced over Fe–Ti spinel at 150–200 °C. This indicates that reaction (2) can be approximately neglected at 150–200 °C. However, a lot of NO can be reduced over V2O5–WO3/TiO2 at 150–200 °C, and the ratio of NO conversion increased with the increase of V2O5 loading. This indicates that adsorbed NH3 can be activated by V5+ on the surface at 150–200 °C. As shown in Fig. 1a, a lot of NO was reduced over Fe–Ti–V spinel at 150–200 °C. Therefore, the activation of adsorbed NH3 over Fe–Ti–V spinel at 150–200 °C mainly resulted from reaction (3). This suggests that the oxidative ability of the V5+ cation was much better than that of the Fe3+ cation. As a result, the SCR activity of Fe–Ti spinel was promoted due to the incorporation of V (shown in Fig. 1a).
Some adsorbed NH3 could be over-oxidized by V5+ cations on the surface to form –NH or −N above 300 °C.9 Then, –NH or –N reacted with gaseous NO to form N2O.10 Therefore, N2 selectivity of V2O5–WO3/TiO2 obviously decreased above 300 °C (shown in Fig. 1b). If a large amount of NH3 adsorbed was activated by V5+ on Fe–Ti–V spinel above 300 °C, a large amount of N2O should form. Only a little N2O formed over Fe–Ti–V spinel (shown in Fig. 1b), although the content of V in Fe–Ti–V spinel was much higher than that in 10%V2O5–WO3/TiO2. This suggests that NH3 adsorbed on Fe–Ti–V spinel was mainly activated by Fe3+ above 300 °C.
NO conversion over Fe–Ti spinel obviously increased as the reaction temperature increased from 200 to 400 °C (shown in Fig. 1a). This indicates that reaction (2) was obviously promoted due to the increase of reaction temperature. Therefore, both reactions (2) and (3) may account for the activation of adsorbed NH3 over Fe–Ti–V spinel at 200–400 °C. Fig. 1a also shows that most NO can be reduced over Fe–Ti spinel above 350 °C. This suggests that most of NH3 adsorbed on Fe–Ti–V spinel should be activated through reaction (2) above 350 °C even if V5+ cations did not take part in the activation of adsorbed NH3. Therefore, reaction (2) may compete with reaction (3) for the activation of adsorbed NH3 during the SCR reaction over Fe–Ti–V spinel at 350–400 °C. In/on Fe–Ti–V spinel, the concentration of Fe3+ cation was about four times that of the V cation (including V5+ and V4+ cations). Furthermore, the ratio of V5+ to V4+ on Fe–Ti–V spinel decreased from 1.77 to 1.27 with the increase of reaction temperature from 300 to 400 °C to sustain the spinel structure, which was demonstrated by the XPS analysis (shown in the ESI†). Therefore, reaction (2) predominated over the activation of adsorbed NH3 during the SCR reaction over Fe–Ti–V spinel at 350–400 °C, and only a small amount of activated NH3 resulted from reaction (3). As a result, Fe–Ti–V spinel showed an excellent N2 selectivity at 350–400 °C.
Then, the effect of 10% of H2O and/or 100 ppm of SO2 on the SCR reaction over Fe–Ti–V spinel was investigated. As shown in Fig. 3, the presence of SO2 showed an obvious interference with the SCR reaction over Fe–Ti–V spinel at 150–200 °C due to the deposition of ammonium bisulfate.1 As the reaction temperature increased to 250 °C, the effect of SO2 on the SCR reaction can hardly be observed, and NO conversion did not gradually decrease during the 24 h test (shown in Fig. 4). Because H2O can compete with gaseous NH3 for the active sites,11 the presence of H2O showed a severe interference with the SCR reaction at 150–250 °C. However, the effect gradually decreased with the increase of reaction temperature. As shown in Fig. 3, the effect of H2O + SO2 on the SCR reaction was slightly higher than that of H2O. This suggests that H2O and SO2 only showed a slight synergy effect on the SCR reaction. Fig. 4 shows that the ratio of NO conversion over Fe–Ti–V spinel in the presence of H2O and/or SO2 at 250 °C did not gradually decrease during the 24 h test. As the GHSV decreased to 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 h−1, NO conversion over Fe–Ti–V spinel reached about 90% at 250 °C and near 100% at 300–400 °C in the presence of 10% of H2O and 100 ppm of SO2 (shown in Fig. 3).
500 h−1, NO conversion over Fe–Ti–V spinel reached about 90% at 250 °C and near 100% at 300–400 °C in the presence of 10% of H2O and 100 ppm of SO2 (shown in Fig. 3).
 | ||
Fig. 3 Effect of 10% of H2O and/or 100 ppm of SO2 on the SCR reaction over Fe–Ti–V spinel: ■, in the absence of H2O and SO2, GHSV = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1; ●, in the presence of SO2, GHSV = 75 000 h−1; ●, in the presence of SO2, GHSV = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1; ▲, in the presence of H2O, GHSV = 75 000 h−1; ▲, in the presence of H2O, GHSV = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1; ▼, in the presence of H2O and SO2, GHSV = 75 000 h−1; ▼, in the presence of H2O and SO2, GHSV = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1; ◀, in the presence of H2O and SO2, GHSV = 37 000 h−1; ◀, in the presence of H2O and SO2, GHSV = 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 h−1. 500 h−1. | ||
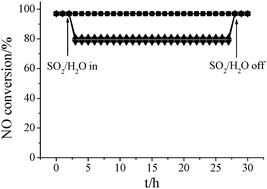 | ||
Fig. 4 Effect of 10% of H2O and/or 100 ppm of SO2 on the SCR reaction over Fe–Ti–V spinel at 250 °C (GHSV = 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1): ■, in the absence of H2O and SO2; ●, in the presence of SO2; ▲, in the presence of H2O; ▼, in the presence of H2O and SO2. 000 h−1): ■, in the absence of H2O and SO2; ●, in the presence of SO2; ▲, in the presence of H2O; ▼, in the presence of H2O and SO2. | ||
An SCR catalyst can be abraded by the fly ash, and it then emits to the fly ash. Because of the toxicity of V2O5, the emission of a vanadium based catalyst to the environment during the SCR reaction is a serious concern. Fe–Ti–V spinel is a super-paramagnetic catalyst with a minimized coercivity and a negligible magnetization hysteresis (shown in the ESI†), and its saturated magnetization is about 30 emu g−1. The magnetization characteristics ensure that the emission of Fe–Ti–V spinel to the environment during the SCR reaction can be effectively prevented by being exposed to an external magnetic field.
In conclusion, Fe–Ti–V spinel showed excellent SCR activity, N2 selectivity and H2O/SO2 durability at 250–400 °C. Meanwhile, an external magnetic field can prevent the emission of Fe–Ti–V spinel to the environment due to its inherent magnetization. Therefore, Fe–Ti–V spinel could be a promising catalyst to substitute the conventional vanadium based catalyst for the control of NOx emission especially from industrial furnace.
This study was financially supported by the National Natural Science Fund of China (Grant No. 51078203), the National High-Tech Research and Development (863) Program of China (Grant No. 2010AA065002 and 2009AA06Z301) and the Scholarship Award for Excellent Doctoral Student granted by Ministry of Education of China.
Notes and references
- G. S. Qi and R. T. Yang, Appl. Catal., B, 2003, 44, 217–225 CrossRef CAS.
- N. Y. Topsoe, Science, 1994, 265, 1217–1219 CAS.
- G. S. Qi, R. T. Yang and R. Chang, Appl. Catal., B, 2004, 51, 93–106 CrossRef CAS.
- S. Yang, J. Li, C. Wang, J. Chen, L. Ma, H. Chang, L. Chen, Y. Peng and N. Yan, Appl. Catal., B, 2012, 117–118, 73–80 CrossRef.
- Z. P. Zhu, Z. Y. Liu, H. X. Niu and S. J. Liu, J. Catal., 1999, 187, 245–248 CrossRef CAS.
- S. Yang, Y. Guo, N. Yan, D. Wu, H. He, J. Xie, Z. Qu, C. Yang and J. Jia, Chem. Commun., 2010, 46, 8377–8379 RSC.
- G. S. Qi and R. T. Yang, J. Phys. Chem. B, 2004, 108, 15738–15747 CrossRef CAS.
- K. I. Hadjiivanov, Catal. Rev. Sci. Eng., 2000, 42, 71–144 CAS.
- G. Busca, L. Lietti, G. Ramis and F. Berti, Appl. Catal., B, 1998, 18, 1–36 CrossRef CAS.
- X. F. Tang, J. H. Li, L. A. Sun and J. M. Hao, Appl. Catal., B, 2010, 99, 156–162 CrossRef CAS.
- S. Yang, C. Wang, J. Li, N. Yan, L. Ma and H. Chang, Appl. Catal., B, 2011, 110, 71–80 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation and characterization of synthesized catalysts. See DOI: 10.1039/c2cy00459c |
| This journal is © The Royal Society of Chemistry 2012 |
