Aerogel nanocomposites of ZnO–SnO2 as efficient photocatalysts for the degradation of rhodamine B†
Marauo
Davis
a,
Walid M.
Hikal
b,
Cenk
Gümeci
a and
Louisa J.
Hope-Weeks
*a
aDepartment of Chemistry and Biochemistry, Texas Tech University, USA. E-mail: louisa.hope-weeks@ttu.edu; Fax: +1-806-742-1289; Tel: +1-806-742-4487
bDepartment of Chemical Engineering, Texas Tech University, USA. Fax: +1-806-742-1289; Tel: +1-806-742-3552
First published on 23rd February 2012
Abstract
For the first time, aerogel nanocomposites containing ZnO and SnO2 were successfully prepared through a facile, sol–gel method without the use of a template or supporting matrix. These nanoparticles exhibit high potential for application as a photocatalyst for wastewater remediation.
In recent years, vast research has been carried out to explore the novel properties of 2D and 3D nanostructures.1–4 Among these, are a unique group called aerogels. Aerogels are highly porous 3D networks of nanoparticles that typically have large surface areas. The solid content of aerogel materials is usually 1–15% of the total volume resulting in very low-density structures. These interesting and unusual properties have lead researchers to investigate the use of aerogel materials for various applications such as catalysts, sensors, cosmic dust collectors, and as capacitors.5–7
Metal oxide semiconductors have received vast attention and have been investigated for incorporation into solar cells,8 photocatalysts,9–13 electrodes,14,15 as well as gas and chemical sensors.16,17 Moreover, particular interest has been given to ZnO and SnO2 due to their transparency in the visible light region.18–20
ZnO–SnO2 has been prepared by dip coating,21 solvothermal,22 and hydrothermal methods.23 Yet, to the best of our knowledge there has been no mention in the literature on the preparation of ZnO–SnO2nanocomposite aerogels by the epoxide addition sol–gel method. This is a facile, efficient approach for the preparation of complex metal oxides, which employs inorganic metal salt precursors and uses an epoxide for gel polymerization without the use of a template or polymer matrix.1
Herein, we report the fast, facile preparation of ZnO–SnO2 nanocomposites. Through this method, we are able to obtain highly stable gel networks in ∼1 min (ESI, Fig. S1†). After washing and drying, we obtain an intact, stable monolith (ESI, Fig. S2†). This monolith is then annealed in a programmable furnace to 500 °C, under static air, to induce the desired metal oxide phases. Photocatalytic activity of the mixed metal oxide aerogel shows to be highly efficient in the degradation of rhodamine B (RhB). The illustrated superior photocatalytic activity of these nanostructures makes them promising candidates for future photocatalytic applications.
The crystalline nature of the as-synthesized and annealed ZnO–SnO2 aerogels was investigated using powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), and high-resolution transmission electron microscopy (HRTEM). Fig. 1 illustrates the amphorous character of the as-synthesized aerogel. Due to the nature of the as-synthesized sample, it is very difficult to definitively assign phases to the broad, weak reflections. We can, nonetheless, given the transformation of the olation and oxolation reactions that occur, assume that the most prevalent phases at this point might be due to the hydroxide form(s) of the starting Zn and Sn sources. However, both ZnO and SnO2 are known to form under room temperature conditions.24,25 Conversely, upon annealing at 500 °C, the major reflections can best be assigned to tetragonal SnO2 (PDF 99-000-0607) and hexagonal ZnO (PDF 97-015-4488). Additionally, minor contribution from ZnSnO3 cannot be excluded due to overlapping reflections. To further investigate the stability of the formed ZnO and SnO2 nanoparticles, the sample was annealed to 800 °C, and the diffraction pattern collected again. As expected, the resulting pattern was more crystalline with all reflections being easily assigned to ZnO and/or SnO2 (Fig. 1). The average particle size as indexed for the (110) reflection of SnO2 was found to be ∼7 nm from Scherrer's equation for the 500 °C annealed sample, and this value correlates to the particle size observed via HRTEM.
 | ||
| Fig. 1 Powder X-ray diffraction patterns for the as-synthesized (lower pattern) and the corresponding patterns upon annealing at 500 °C and 800 °C, respectively (shown above). (Circles denote SnO2and triangles indicate ZnO). | ||
Fig. 2A and B illustrate the fluffy, sponge-like nanoporous nature of the as-synthesized and annealed aerogel. It should also be noted that the pore size of the as-synthesized aerogel sample greatly increases upon annealing which is also illustrated by the N2 adsorption/desorption analyses. Fig. 2C shows the as-synthesized aerogel to be highly amphorous, as illustrated by X-ray diffraction (Fig. 1), with a lack of visible particle shape. In contrast, upon annealing, Fig. 2D shows interconnected, highly crystalline cubic nanoparticles, which are about 5–6 nm in diameter.
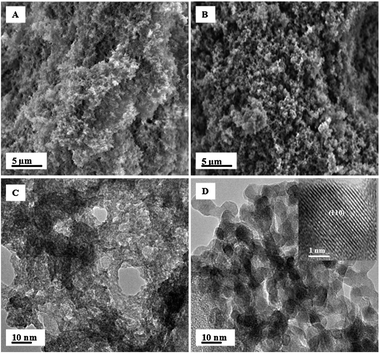 | ||
| Fig. 2 (A) SEM image of as-synthesized aerogel (B) SEM image of the annealed sample (C) TEM image of as-synthesized aerogel (D) TEM image of the annealed sample with corresponding high resolution TEM image inset. | ||
The crystalline nature of the material can be attributed to the formation of SnO2 and ZnO from the amphorous as-synthesized material. Fig. 2D inset further demonstrates the crystallinity of the annealed sample and highlights the lattice fringes as indexed for the (110) plane of SnO2.
The effective change in surface area, pore volume and pore size as a function of annealing the as-synthesized aerogel was investigated using BET (Brunauer-Emmett-Teller) and BJH (Barrett-Joyner-Halenda). The produced isotherms are typical of type IV and have a slight hysteresis in the range of 0.5–1.0 P/P0, which confirms the mesoporous character of these materials.
From the N2 adsorption-desorption isotherms (ESI, Fig. S3†), the surface area for the as-synthesized and annealed aerogels were determined to be 475 m2 g−1 and 92 m2 g−1, respectively. The average size of the pores based on desorption data shows a significant increase from the as-synthesized (3.2 nm) to annealed (8.4 nm). This increase in pore size is most probably an indication of vapor transport,26 which allows the materials to undergo sintering without densification. Such a process would cause evaporation of atoms (amphorous, as-synthesized material) and condensation on to a different surface. Typically, sintering would result in the reduction of average pore size due to the repacking of the material. However, here the result of sintering, essentially causes stretching of the pores and results in the observed increase. The mesoporous character of the as-synthesized aerogel is observed in the narrow pore size distribution plot indicating most of the pores to be around 3 nm in diameter (ESI, Fig. S4†). However, upon annealing, a broad shift occurs in the pore size distribution and yields a more porous material. Such findings may account for the excellent catalytic activity observed from the annealed sample.
The photocatalytic activity of the annealed ZnO–SnO2 nanocomposites was evaluated for RhB degradation. The photocatalytic activity of the nanocomposites in the dark was performed and reveals essentially no degradation (less than 2%) even after 1h of continuous stirring in RhB (ESI, Fig. S5†). Moreover, it should also be noted that, under ultraviolet (UV) illumination, RhB undergoes less than 20% degradation after 1h in the absence of ZnO–SnO2nanocomposites.
Fig. 3 shows the absorbance spectra of a 1.2 × 10−5 M aqueous solution of RhB in presence of ZnO–SnO2nanocomposites (5.0 mg) at different illumination times under UV illumination. This plot indicates an exposure time-dependent decrease of the absorbance spectra of RhB with nearly a full disappearance/degradation of the concentrated RhB after only 25 min of illumination. Additionally, a blue shift observed in the absorbance peak of RhB indicates the destruction of the RhB structure including both chromophores and aromatic rings.27,28
 | ||
| Fig. 3 UV-vis absorbance spectra of 1.2 × 10−5 M aqueous solution of RhB in presence of ZnO-SnO2 nanoparticles showing the photodegradation of RhB upon illumination with UV light. | ||
These findings indicate excellent photocatalytic activity of the nanoparticles. In comparison with other metal oxide semiconductors, these nanocomposites show better degradation (>20% better activity) of RhB than Degussa P25 TiO2 and ZnSnO3 powders.1,29,30 We accredit the activity of the aerogel nanoarchitectures to the high internal surface area, small crystallite size, and porous network which is preserved even after annealing. Moreover, the originality of this work is encompassed in the preparation technique in that this is the first ever report of ZnO–SnO2 nanocomposites prepared from the facile, epoxide addition method, sol–gel method. We are able to obtain highly photoactive, 3D monolithic networks without the use of a matrix or templating route. This work further capitalizes the viability of the cost efficient, epoxide addition method and expands the collection of very promising, inexpensive materials for photocatalytic applications.
This work was supported by the Texas Tech University Provost Fellowship program for, which the authors are exceedingly grateful.
Notes and references
- C. H. Fang, B. Y. Geng, J. Liu and F. M. Zhan, Chem. Commun., 2009, 2350 RSC.
- M. Li, H. Schnablegger and S. Mann, Nature, 1999, 402, 393 CrossRef CAS.
- N. I. Kovtyukhova and T. E. Mallouk, Chem.–Eur. J., 2002, 8, 4354 CrossRef CAS.
- Y. Y. Wu, H. Q. Yan, M. Huang, B. Messer, J. H. Song and P. D. Yang, Chem.–Eur. J., 2002, 8, 1260 CrossRef CAS.
- D. R. Rolison and B. J. Dunn, J. Mater. Chem., 2001, 11, 963 RSC.
- C.-C. Lin, T.-Y. Wei, K.-T. Lee and S.-Y. Lu, J. Mater. Chem., 2011, 21, 12688 Search PubMed.
- H.-C. Chien, W.-Y. Cheng, Y.-H. Wang, T.-Y. Wei and S.-Y. Lu, J. Mater. Chem., 2011, 21, 18180 RSC.
- R. Jose, V. Thavasi and S. Ramakrishna, J. Am. Ceram. Soc., 2009, 92, 289 CrossRef CAS.
- A. Hameed, T. Montini, V. Gombac and P. Fornasiero, Photochem. Photobiol. Sci., 2009, 8, 677 CAS.
- H. Ma, K. Teng, Y. Fu, Y. Song, Y. Wang and X. Dong, Energy Environ. Sci., 2011, 4, 3067 CAS.
- Q. Wu, D. Li, Z. Chen and X. Fu, Photochem. Photobiol. Sci., 2006, 5, 653 CAS.
- H. Yu, H. Irie and K. Hashimoto, J. Am. Chem. Soc., 2010, 132, 6898 CrossRef CAS.
- S. Ouyan and J. Ye, J. Am. Chem. Soc., 2011, 133, 7757 CrossRef.
- A. A. Tahir, K. G. U. Wijayantha, S. Saremi-Yarahamadi, M. Mazhar and V. McKee, Chem. Mater., 2009, 21, 3763 CrossRef CAS.
- R. Dharmadasa, A. A. Tahir and K. G. U. Wijayantha, J. Am. Ceram. Soc., 2011, 94, 3540 CrossRef CAS.
- G. Shen, P.-C. Chen, K. Ryu and C. Zhou, J. Mater. Chem., 2009, 19, 828 RSC.
- C. Wang, L. Yin, L. Zhang, D. Xiang and R. Gao, Sensors, 2010, 10, 2088 CrossRef CAS.
- T. Oekermann, T. Yoshida, H. Minoura, K. G. U. Wijayantha and L. M. Peter, J. Phys. Chem. B, 2004, 108, 8364 CrossRef CAS.
- H. J. Snaith and C. Ducati, Nano Lett., 2010, 10, 1259 CrossRef CAS.
- M. Batzill and U. Diebold, Prog. Surf. Sci., 2005, 79, 47 CrossRef CAS.
- C. Cheng, B. Liu, H. Yang, W. Zhou, L. Sun, R. Chen, S. F. Yu, J. Zhang, H. Gong, H. Sun and H. J. Fan, ACS Nano, 2009, 3, 3069 CrossRef CAS.
- X. Liu, J. Iqbal, Z. Wu, B. He and R. Yu, J. Phys. Chem. C, 2010, 114, 4790 CAS.
- W.-W. Wang, Y.-J. Zhu and L.-X. Yang, Adv. Funct. Mater., 2007, 17, 59 CrossRef CAS.
- Y. P. Gao, C. N. Sisk and L. J. Hope-Weeks, Chem. Mater., 2007, 19, 6007 CrossRef CAS.
- S. O. Kucheyev, A. E. Gash, J. H. Satcher and T. F. Baumann, Adv. Mater., 2005, 17, 1546 CrossRef.
- F. A. Dullien, Porous Media: Fluid Transport and Pore Structure, Academic Press, San Diego, CA, 2nd edn, 1991 Search PubMed.
- X. Li, N. Kikugawa and J. Ye, Chem.–Eur. J., 2009, 15, 3538 CrossRef CAS.
- X. Li, N. Kikugawa and J. Ye, Adv. Mater., 2008, 20, 3816 CrossRef CAS.
- C. Tian, W. Li, K. Pan, Q. Zhang, G. Tian, W. Zhou and H. Fu, J. Solid State Chem., 2010, 183, 2720 CrossRef CAS.
- D. Lin, H. Wu, R. Zhang and W. Pan, Chem. Mater., 2009, 21, 3479 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Images of gel formation and free standing aerogel. Additional porosity data including pore size distribution plots and absorbance spectra of RhB with and without catalyst in dark. See DOI: 10.1039/c2cy20020a |
| This journal is © The Royal Society of Chemistry 2012 |
