The crystal-facet-dependent effect of polyhedral Cu2O microcrystals on photocatalytic activity†
Shaodong
Sun
,
Xiaoping
Song
,
Yuexia
Sun
,
Dongchu
Deng
and
Zhimao
Yang
*
School of Science, MOE Key Laboratory for Non-Equilibrium Synthesis and Modulation of Condensed Matter, State Key Laboratory for Mechanical Behavior of Materials, Xi'an Jiaotong University, Xi'an 710049, ShaanXi, People's Republic of China. E-mail: zmyang@mail.xjtu.edu.cn
First published on 2nd February 2012
Abstract
We have systematically investigated the crystal-facet-dependent effect of polyhedral Cu2O microcrystals exposed with different-index facets on photodegradation of methyl orange, which provides the convincing evidence that the performance of catalysts can be enhanced by high-index facets tailoring.
The shape- and crystal-facet-tailored syntheses of materials have recently attracted considerable attention because their photoelectric and photocatalytic properties can be effectively improved or optimized by controlling the surface atomic structures, such as {001} facets of anatase TiO2 showed higher activity than that of {101} facets,1 {001} facets of ZnO displayed high activity than that of other facets,2 {110} facets of Ag3PO4 exhibited higher surface energy than that of {100} planes,3 and tetrahexahedral Pt and Au nanocrystals enclosed by 24 {037} or {122} facets possessed excellent electro-oxidation activity.4,5 Hence, the study of the properties of definite crystal facets does not only help us to precisely control their structures and shapes, but also offers a new opportunity for discovering multifunctional materials with potentially exciting and unique properties.
Cuprous oxide (Cu2O), a nonstoichiometric p-type semiconductor (direct band gap ≈ 2.17eV), is a perspective material with applications in solar energy conversion,6 catalysts,7 gas sensors,8 negative electrode material for lithium-ion batteries,9 templates,10 antibacterial activity,11 solar-driven water splitting,12 CO oxidation,13 and metal–insulator–metal resistive switching memory.14 In the past decade, shape-controlled synthesis of Cu2O crystals has been attracting much interest, and Cu2O crystals with various morphologies have been successfully synthesized, including nanowires,15 spheres,16 multipods,17 hierarchical structures,18 hollow cages,19 and different polyhedra (such as cubes,20 octahedra,21 cuboctahedra,21 truncated octahedra,21 dodecahedra,22 26-facet polyhedra,23 50-facet polyhedra,24,25 74-facet polyhedra24 and complex multi-facet polyhedral structures26,27). Among these complex morphologies of Cu2O crystals, well-defined polyhedral Cu2O crystals with high-index planes are interesting by virtue of their high-activity in chemical reactions. Although these highly reactive facets are more interesting and significant for enhancing catalytic reactivity, they usually disappear rapidly during the growth process because of their high surface energies.24 Hence, the fabrication of Cu2O catalysts exposed with highly active facets and exploration of their potential application is still a great challenge.
In our previous work,23–27 highly symmetric multi-facet polyhedral Cu2O microcrystals partially enclosed with controllable-index facets (including low-index {110}, {100} and {111} facets and high-index {544}, {522} and {211} facets) have been artificially synthesized via a complex-precursor solution route. A schematic illustration of the proposed particle reaction pathways that lead to the formation of polyhedral Cu2O crystals with various shapes is shown in Fig. 1a.23–27 As the essence of our synthesis, the precursor ([Cu(OH)4]2−) is reduced by C6H12O6 to form Cu2O atoms, which subsequently aggregate to form nuclei by an oriented-attachment process.22,24 Once these nuclei have grown past the critical size, they will become seeds with varied shapes under different conditions.23 The seeds will grow into various intermediate structures by a ripening process, then these intermediate structures would evolve into different polyhedral architectures via surface reconstruction and preferential adsorption under different reaction conditions,22 which can modify the ratio (R) between the growth rates along the 〈100〉 and 〈111〉 directions, finally resulting in the shape-controlled synthesis of polyhedral architectures with controllable-index planes (see Fig. 1b).24 The appearance of these novel polyhedral architectures further enriches the current morphologies of Cu2O crystals, and might become useful for the fundamental study of crystal designs. More recently, we have also reported the synthesis of novel polyhedral 50-facet Cu2O semiconductors with high-index {211} facets via a homogeneous seed-mediated solution route and investigated their new use in photocatalytic applications, where the photocatalytic degradation of methyl orange (MO) demonstrated that the as-prepared polyhedral 50-facet Cu2O crystals, attributed to their highly active high-index facets, exhibited catalytic activity higher than that of the products with low-index facets.25 However, the detailed study of the facet-dependent effect of polyhedral Cu2O microcrystals with multi-index facets on catalytic activity has remained unclear to date. Herein, we focus the discussion on the significance of high-index facets for improving photocatalytic degradation of MO through comparative investigations of different well-defined Cu2O polyhedral architectures.
 | ||
| Fig. 1 A schematic illustration of the proposed particle (a) reaction pathways and (b) growth mechanism24 that lead to the formation of polyhedral Cu2O crystals. | ||
To the best of our knowledge, two main strategies have been employed for chemical syntheses of crystals with high-index planes: (i) the use of external energy (such as square-wave potential method),4 which can continually confine the shapes of nanoparticles to evolve into high-index planes; and (ii) the use of capping agents (molecules or ions) during nanoparticles growth to tailor the ratio (R) between the growth rates along the 〈100〉 and 〈111〉 directions to selectively control the formation of high-index planes.28 As previously reported, the morphologies of Cu2O crystals are determined by the R values.29 The relation between R values and morphologies of crystals with different index planes can be described as shown in Fig. 2, and it can be found that high-index planes could be synthesized by modifying the R values appropriately.
 | ||
| Fig. 2 The relation between R values and morphologies of crystals with different index facets.24 (a) Only low-index facets; (b) both low-index and high-index facets; (c) the detailed calculations of R values and morphologies of crystals with different index planes. | ||
The detailed calculations of R values and morphologies of crystals with different index planes are shown in Fig. 2a, where the polyhedral architecture is made up of only low-index {100} and {111} facets. The red and blue lines represent the projection of {100} and {111} facets perpendicular to the [110] zone axis, respectively. The theoretical values of angle α = 35.25° between {111} vs. {111} facets, and θ is the variable, R is the function (Fig. 2c, eqn (I)).24 For example, when θ = 0°, R = 1.73 (octahedron), while θ = 90°, R = 0.58 (cube). It is in good agreement with the previous report by Wang.30 Moreover, when the polyhedral architecture contains {100}, {111} and high-index {hkl} planes, the projection of {100}, {111} and {hkl} facets perpendicular to the [110] zone axis is shown in Fig. 2b. The red, blue and cyan lines represent the {111}, {100} and {hkl} planes, respectively. The R value can be calculated based on Fig. 2b, where α = 35.25°, and β = 54.7° (the acute angle between {111} vs. {100}). In this case, the R value can be described by the equations given in Fig. 2c (eqn (II)).24 Based on the above discussion, it can be seen that the controllable high-index planes can be formed via changing the R values under appropriate reaction conditions.24
In a solution-phase system, the reaction conditions (such as capping agents, anions of investigated metal salts and concentration, etc.) can strongly affect the R values,24 and determine the appearance of high-index planes in the final products. The R values would be altered because of facet-selective adsorption of anions or additives on their intermediate structures, and the formation of high-index facets can be attributed to the synergistic effect of aggregation and ripening mechanism.22,24 The synthesis of Cu2O polyhedral architectures with controllable-index facets can be successfully achieved via a template-free complex-precursor solution route, which has been studied in detail in our previous reports.23,24 In the present work, different types of Cu2O polyhedral architectures were chosen for investigating the facet-dependent effect. Fig. 3 shows the typical FESEM images of the six types of polyhedral Cu2O microcrystals. Structural analysis of these Cu2O products was performed by X-ray diffraction (XRD), and the result is shown in Fig. S1 (see ESI†). All of the diffraction peaks are well indexed to the peaks of standard cubic structure Cu2O (space group: Pn![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, lattice constant a = 0.4252 nm, JCPDS file No. 05-0667). No peaks of impurities such as cupric oxide or copper were detected, suggesting the high purity of the as-prepared products. According to Steno's law, the angles between two corresponding facets on the crystals are constant.21,23,24 The Miller indices of exposed facets of the as-obtained polyhedron can be identified by a conjunction of the angle between the facets.21,23,24Fig. 3a displays the octahedral architectures with four pairs of {111} facets (R = 1.73).30 Typical truncated octahedral (14-facet) Cu2O microcrystals are shown in Fig. 3b, where the formation of four pairs of {111} facets and three pairs of {100} facets (1 ≤ R < 1.15) is obviously observed.30 Two different kinds of 26-facet Cu2O architectures with three pairs of {100} facets, four pairs of {111} facets and six pairs of {110} facets are shown in Fig. 3c and Fig. 3d, respectively. Fig. 3c shows the representative 26-facet polyhedral Cu2O cubes can be viewed as a result of cutting the 6 vertices and 12 edges. The 26-facet polyhedral Cu2O octahedra as shown in Fig. 3d can be generated by “cutting” the corners and edges of well-defined octahedra. Fig. 3e shows the 50-facet polyhedral Cu2O microcrystals enclosed by 24 high-index {522} facets, 8 low-index {111} facets, 6 low-index {100} facets, and 12 low-index {110} facets, and it can be described as further cutting the joints of square, triangular and rectangular facets of the truncated edge cubes as shown in Fig. 3c.24 Similarly, another 50-facet polyhedral architecture (Fig. 3f) with 24 high-index {211} facets, 8 low-index {111} facets, 6 low-index {100} facets, and 12 low-index {110} facets can be formed by cutting the edges between {100} and {110} facets of the truncated edge octahedra as shown in Fig. 3d.24 Based on the above results, the R values can be changed by adjusting the [Cu(OH)4]2− species in our experiment. As shown in Fig. 1, the different [Cu(OH)4]2− precursors formed under different reaction conditions can change the reduction process (Fig. 1a), which affects the competition between thermodynamics and kinetics during the reduction of precursors, nucleation and growth of Cu2O crystals (Fig. 1b).24 So the formation of [Cu(OH)4]2− species strongly depended on the ratio between sodium hydroxide and copper salt (Fig. 1a). The predominant OH− ions play a crucial role in controlling the growth of the different crystalline facets due to the formation of the different [Cu(OH)4]2− precursors.24 The negatively charged [Cu(OH)4]2− complexes might preferably adsorb on the surface of Cu2O seeds because the electrically neutral state of {100} facets is susceptible to modification,24 which improves the growth of Cu2O intermediate-products along different growth directions (Fig. 1b). Therefore, it has been found that the tailoring of the concentration of sodium hydroxide in our synthetic method would yield a wide variety of other shapes of the Cu2O crystals. The corresponding FESEM images of these products are shown in Fig. S2 (see ESI†).
m, lattice constant a = 0.4252 nm, JCPDS file No. 05-0667). No peaks of impurities such as cupric oxide or copper were detected, suggesting the high purity of the as-prepared products. According to Steno's law, the angles between two corresponding facets on the crystals are constant.21,23,24 The Miller indices of exposed facets of the as-obtained polyhedron can be identified by a conjunction of the angle between the facets.21,23,24Fig. 3a displays the octahedral architectures with four pairs of {111} facets (R = 1.73).30 Typical truncated octahedral (14-facet) Cu2O microcrystals are shown in Fig. 3b, where the formation of four pairs of {111} facets and three pairs of {100} facets (1 ≤ R < 1.15) is obviously observed.30 Two different kinds of 26-facet Cu2O architectures with three pairs of {100} facets, four pairs of {111} facets and six pairs of {110} facets are shown in Fig. 3c and Fig. 3d, respectively. Fig. 3c shows the representative 26-facet polyhedral Cu2O cubes can be viewed as a result of cutting the 6 vertices and 12 edges. The 26-facet polyhedral Cu2O octahedra as shown in Fig. 3d can be generated by “cutting” the corners and edges of well-defined octahedra. Fig. 3e shows the 50-facet polyhedral Cu2O microcrystals enclosed by 24 high-index {522} facets, 8 low-index {111} facets, 6 low-index {100} facets, and 12 low-index {110} facets, and it can be described as further cutting the joints of square, triangular and rectangular facets of the truncated edge cubes as shown in Fig. 3c.24 Similarly, another 50-facet polyhedral architecture (Fig. 3f) with 24 high-index {211} facets, 8 low-index {111} facets, 6 low-index {100} facets, and 12 low-index {110} facets can be formed by cutting the edges between {100} and {110} facets of the truncated edge octahedra as shown in Fig. 3d.24 Based on the above results, the R values can be changed by adjusting the [Cu(OH)4]2− species in our experiment. As shown in Fig. 1, the different [Cu(OH)4]2− precursors formed under different reaction conditions can change the reduction process (Fig. 1a), which affects the competition between thermodynamics and kinetics during the reduction of precursors, nucleation and growth of Cu2O crystals (Fig. 1b).24 So the formation of [Cu(OH)4]2− species strongly depended on the ratio between sodium hydroxide and copper salt (Fig. 1a). The predominant OH− ions play a crucial role in controlling the growth of the different crystalline facets due to the formation of the different [Cu(OH)4]2− precursors.24 The negatively charged [Cu(OH)4]2− complexes might preferably adsorb on the surface of Cu2O seeds because the electrically neutral state of {100} facets is susceptible to modification,24 which improves the growth of Cu2O intermediate-products along different growth directions (Fig. 1b). Therefore, it has been found that the tailoring of the concentration of sodium hydroxide in our synthetic method would yield a wide variety of other shapes of the Cu2O crystals. The corresponding FESEM images of these products are shown in Fig. S2 (see ESI†).
 | ||
| Fig. 3 Typical FESEM images of the six types of polyhedral Cu2O microcrystals (scale bar = 1 μm). (a) 8-facet; (b) 14-facet; (c) 26-facet polyhedral Cu2O cubes; (d) 26-facet polyhedral Cu2O octahedra; (e) 50-facet polyhedral with high-index {522} facets; (f) 50-facet polyhedral with high-index {211} facets. The insets denote the corresponding geometrical illustrations. The red, blue, yellow, cyan, green and black colors represent the {100}, {111}, {110}, {522}, and {211} planes, respectively. | ||
To study the crystal-facet-dependent effect of polyhedral Cu2O microcrystals on degradation of organic pollutes, we have investigated their photocatalytic activities by choosing the photocatalytic degradation of methyl orange (MO) dyes (see ESI†). UV–Vis spectra were used to demonstrate the photocatalytic degradation activity of MO. The characteristic absorption peak at 465 nm of MO was used as a monitored parameter during the photocatalytic degradation process. The previous reference has reported that the octahedral Cu2O crystals show a higher activity in the photodegradation of MO molecules than the cubic Cu2O crystals,31 and the results reveal that the different photodegradation activities of octahedral and cubic Cu2O crystals arise from their intrinsic difference in the crystallographic structures, in which the polar {111} facet has coordination unsaturated “Cu”, but the nonpolar {100} facet does not.31 However, in a cuprite structured Cu2O crystal lattice, the distance between two “Cu” atoms of {110} facets is about half of that in {111} facets, implying that the number and the density of “Cu” dangling bonds in the {110} facets are higher than those of the {111} facets, so the polar {110} facets possess more dangling bonds and higher surface energy than that of {111} facets. The results indicate that the photocatalytic superiority of low-index facets follows such a sequence: {110} > {111} > {100}.31
High-index facets, as one type of perspective building blocks with high surface energy, can provide more chemically active sites for Cu2O architectures.4 Herein, to demonstrate the excellent superiority of the as-synthesized polyhedral Cu2O microcrystals with high-index facets in the degradation of organic contaminants, a control experiment was first carried out to compare the catalytic activity of the 50-facet polyhedral Cu2O microcrystals with high-index {211} facets (Fig. 3f). Fig. 4a shows the corresponding photodegradation results. The intensity absorption peak at 465 nm of MO decreased rapidly with an increase in reaction time, about 82.3% of the MO was degraded after 5 h (see Fig. 4e, panel C). Fig. 4b shows the optical absorption spectra of MO tested at different durations with 26-facet polyhedral Cu2O octahedra without high-index {211} facets (Fig. 3d), the intensity absorption peak at 465 nm of MO decreased relatively slowly with an increase in reaction time, and about 72.1% of the MO was degraded after 5 h (see Fig. 4e, panel A). Therefore, the 50-facet polyhedral Cu2O with high-index {211} facets (Fig. 3f) shows much better photocatalytic degradation of MO than that of the original 26-facet Cu2O architectures without high-index {211} facets (Fig. 3d), which suggests that the presence of {211} facets in the 26-facet polyhedral Cu2O crystals can enhance the degradation of MO. However, as shown in Fig. 3c and e, other two different types of 26-facet and 50-facet polyhedral Cu2O crystals were still interesting for investigating the facet-dependent effect of polyhedral Cu2O microcrystals. Fig. 4c shows the optical absorption spectra of MO tested at different durations with 26-facet polyhedral Cu2O cubes (Fig. 3c), and about 78.9% of the MO was degraded after 5 h (see Fig. 4e, panel B). Compared to the abovementioned example of 26-facet polyhedral Cu2O octahedra with smaller areas of {110} facets, it is observed that the degradation of MO using 26-facet polyhedral Cu2O cubes with larger proportion of {110} facets is obviously better than the 26-facet polyhedral Cu2O octahedra, which suggests that the exposure of a greater proportion of polar {110} facets leads to greater photocatalytic activity. Fig. 4d shows the optical absorption spectra of MO tested at different durations using the 50-facet Cu2O microcrystals with high-index {522} facets (Fig. 3e), and about 90.8% of the MO was degraded after 5 h (see Fig. 4e, panel D). It can be obviously demonstrated that the high-index {522} facet plays a vital role in accelerating the degradation of MO dyes, and the photocatalytic advantage of the 50-facet Cu2O can be also attributed to their partial exposure with high-index {522} facets building blocks. As compared with the abovementioned 50-facet Cu2O microcrystals with high-index {211} facets, the photocatalytic performance of 50-facet Cu2O microcrystals with high-index {522} facets is much better. A plot of the extent of photodegradation of MO by different Cu2O catalysts at different intervals is shown in Fig. S3 (see ESI†). Fig. 4e shows the decomposition of the MO aqueous solution at 5 h of UV irritation in the presence of the abovementioned four types of as-obtained samples as follows: 50-facet polyhedral Cu2O with high-index {522} facets (90.8%) > 50-facet polyhedral Cu2O with high-index {211} facets (82.3%) > 26-facet polyhedral Cu2O cubes (78.9%) >26-facet polyhedral Cu2O octahedra (72.1%).
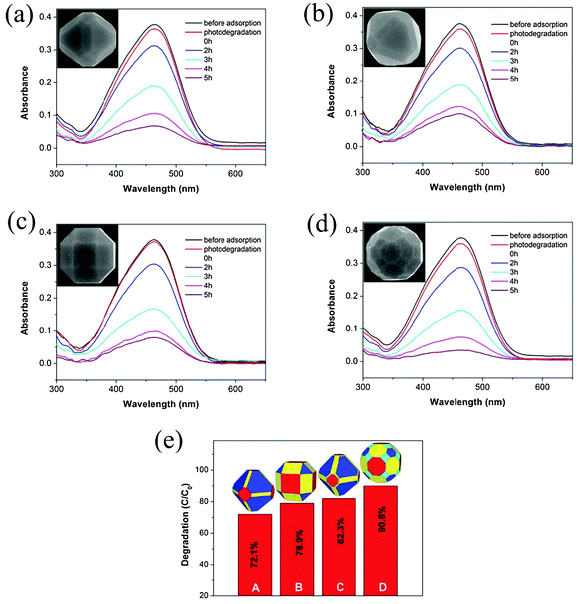 | ||
| Fig. 4 (a) Absorption spectrum of a MO solution in the presence of the 26-facet truncated edge octahedral Cu2O microcrystals (inset: the corresponding morphology of the particle); (b) absorption spectrum of a MO solution in the presence of the 50-facet polyhedral Cu2O microcrystals partially exposed with {211} facets (inset: the corresponding morphology of the particle); (c) absorption spectrum of a MO solution in the presence of the 26-facet truncated edge cubic Cu2O microcrystals (inset: the corresponding morphology of the particle); (d) absorption spectrum of a MO solution in the presence of the 50-facet polyhedral Cu2O microcrystals partially exposed with {522} facets (inset: the corresponding morphology of the particle); (e) a plot of the extent of photodegradation of MO by different catalysts, panel A: 26-facet truncated edge octahedral Cu2O microcrystals, panel B: 26-facet truncated edge cubic Cu2O microcrystals, panel C: 50-facet polyhedral Cu2O microcrystals partially exposed with {211} facets, panel D: 50-facet polyhedral Cu2O microcrystals partially exposed with {522} facets. | ||
The results clearly imply that exposure of a greater proportion of highly active facets leads to higher photocatalytic activity, which provides more active sites for Cu2O crystals to excite the electrons and holes during the photocatalytic process. When the Cu2O crystals were irradiated with UV light, oxygen vacancies in Cu2O can act as potential wells to trap electrons, being in favor of the separation of electron–hole pairs. The holes can be captured by OH−, leading to the formation of hydroxyl radical species (˙OH), and the electrons can be trapped by the adsorbed O2 and form the superoxide anion radicals (˙O2−), which can finally be reduced to ˙OH radicals.32,33 It has been demonstrated that the ˙OH radicals are in favor of oxidizing organic contaminates because of their high oxidative capacity.34 The OH− ions could preferentially adsorb onto the highly active surfaces because of their coordination unsaturated “Cu” dangling bonds (positive charge), resulting in a greater rate of production of ˙OH radicals. In order to shed light on the high-index facet-dependent surface energies of Cu2O crystals, the density functional theory (DFT) calculations (see ESI†) were performed to investigate the structures of Cu2O(522) and Cu2O(211). Fig. 5 shows the optimized (522) and (211) surface structures, and it can be seen that there are greater amounts of unsaturated “Cu” dangling bonds and surface oxygen vacancies in (522) facets, and the surface energy of (522) facets is 6.61 eV nm−2, and it is larger than that of (211) facets (6.02 eV nm−2). Hence, it is proposed that the higher activity of the (522) surfaces may be attributed to their intrinsically higher surface energies. However, we are not clear how the proportion of different-index facets of polyhedral Cu2O microcrystals effects the photocatalytic activity, so further study is still needed to uncover the origin of the high photocatalytic activity of the as-obtained multi-index facets of polyhedral Cu2O microcrystals.
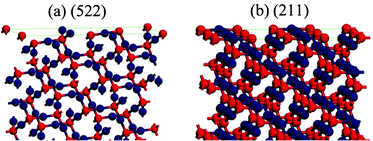 | ||
| Fig. 5 (a) Optimized structures of Cu2O(522) and (211) surfaces: (a) side view of the Cu2O(522) surface; (b) side view of the Cu2O(211) surface. The red and blue balls represent oxygen and copper atoms, respectively. | ||
In summary, we have successfully synthesized different types of perfect polyhedral Cu2O microcrystals via a template-free complex-precursor solution route. The crystal-facet-dependent effect of polyhedral Cu2O microcrystals on photodegradation of MO has been systematically investigated. The sequence of photodegradation of the MO aqueous solution under UV irritation in the presence of different polyhedral Cu2O microcrystals is as follows: 50-facet polyhedral Cu2O with high-index {522} facets > 50-facet polyhedral Cu2O with high-index {211} facets > 26-facet Cu2O cubes > 26-facet polyhedral Cu2O octahedra. The photocatalytic superiority of the novel 50-facet polyhedral architectures can be attributed to the introduction of highly active components of high-index surfaces, which can offer more amount of unsaturated “Cu” dangling bonds and surface oxygen vacancies, and accelerate the formation of highly oxidative ˙OH radicals, leading to the enhancement of the decomposition of MO dyes. This study not only provides the convincing evidence that the performance of catalysts can be enhanced by crystal-facet tailoring, but also promotes the synthesis and application of other polyhedral crystals with high-index surfaces in the catalytic field.
Acknowledgements
We thank the support from the National Basic Research Program of China (No. 2010CB635101), National Science Foundation of China (NSFC No. 51071116).References
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638 CrossRef CAS.
- H. B. Lu, S. M. Wang, L. Zhao, J. C. Li, B. H. Dong and Z. X. Xu, J. Mater. Chem., 2011, 21, 4228 RSC.
- Y. P. Bi, S. X. Ouyang, N. T. Umezawa, J. Y. Cao and J. H. Ye, J. Am. Chem. Soc., 2011, 133, 6490 CrossRef CAS.
- N. Tian, Z. Y. Zhou, S. G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732 CrossRef CAS.
- T. Ming, W. Feng, Q. Tang, F. Wang, L. D. Sun, J. F. Wang and C. H. Yan, J. Am. Chem. Soc., 2009, 131, 16350 CrossRef CAS.
- R. N. Briskman, Sol. Energy Mater. Sol. Cells, 1992, 27, 361 CrossRef CAS.
- C. H. Kuo and M. H. Huang, Nano Today, 2010, 5, 106 CrossRef CAS.
- H. G. Zhang, Q. S. Zhu, Y. Zhang, Y. Wang, L. Zhao and B. Yu, Adv. Funct. Mater., 2007, 17, 2766 CrossRef CAS.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Taracon, Nature, 2000, 407, 496 CrossRef CAS.
- Z. Y. Wang, D. Y. Luan, C. M. Li, F. B. Su, S. Madhavi, F. Y. C. Boey and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 16271 CrossRef CAS.
- H. Pang, F. Gao and Q. Y. Lu, Chem. Commun., 2009, 1076 RSC.
- A. Paracchino, V. Laporte, K. Sivula, M. Grätzel and E. Thimsen, Nat. Mater., 2011, 10, 456 CrossRef CAS.
- V. M. Leng, M. Z. Liu, Y. B. Zhang, Z. Q. Wang, C. Yu, X. G. Yang, H. J. Zhang and C. Wang, J. Am. Chem. Soc., 2010, 132, 17084 CrossRef.
- A. Chen, S. Haddad, Y. C. Wu, T. N. Fang, S. Kaza and Z. Lan, Appl. Phys. Lett., 2008, 92, 13503 CrossRef.
- Y. W. Tan, X. Y. Xue, Q. Peng, H. Zhao, T. H. Wang and Y. D. Li, Nano Lett., 2007, 7, 3723 CrossRef CAS.
- M. L. Pang and H. C. Zeng, Langmuir, 2010, 26, 5963 CrossRef CAS.
- Y. Chang and H. C. Zeng, Cryst. Growth Des., 2004, 4, 273 CAS.
- H. W. Zhang, X. Zhang, H. Y. Li, Z. K. Qu, S. Fan and M. Y. Ji, Cryst. Growth Des., 2007, 7, 820 CAS.
- C. H. Lu, L. M. Qi, J. H. Yang, X. Y. Wang, D. Y. Zhang, J. L. Xie and J. M. Ma, Adv. Mater., 2005, 17, 2562 CrossRef CAS.
- Y. M. Sui, W. Y. Fu, H. B. Yang, Y. Zeng, Y. Y. Zhang, Q. Zhao, Y. G. Li, X. M. Zhou, Y. Leng, M. H. Li and G. T. Zou, Cryst. Growth Des., 2010, 10, 99 CAS.
- X. Zhao, Z. Y. Bao, C. T. Sun and D. F. Xue, J. Cryst. Growth, 2009, 311, 711 CrossRef CAS.
- X. D. Liang, L. Gao, S. W. Yang and J. Sun, Adv. Mater., 2009, 21, 2068 CrossRef CAS.
- S. D. Sun, F. Y. Zhou, L. Q. Wang, X. P. Song and Z. M. Yang, Cryst. Growth Des., 2010, 10, 541 CAS.
- S. D. Sun, C. C. Kong, S. C. Yang, L. Q. Wang, X. P. Song, B. J. Ding and Z. M. Yang, CrystEngComm, 2011, 13, 2217 RSC.
- S. D. Sun, D. C. Deng, C. C. Kong, Y. Gao, S. C. Yang, X. P. Song, B. J. Ding and Z. M. Yang, CrystEngComm, 2011, 13, 5593 Search PubMed.
- S. D. Sun, H. Zhang, X. P. Song, S. H. Liang, C. C. Kong and Z. M. Yang, CrystEngComm, 2011, 13, 6040 RSC.
- S. D. Sun, X. P. Song, C. C. Kong and Z. M. Yang, CrystEngComm, 2011, 13, 6616 RSC.
- J. Zhang, M. R. Langille, M. L. Personick, K. Zhang, S. Y. Li and C. A. Mirkin, J. Am. Chem. Soc., 2010, 132, 14012 CrossRef CAS.
- J. S. Xu and D. F. Xue, Acta Mater., 2007, 55, 2397 CrossRef CAS.
- Z. L. Wang, J. Phys. Chem. B, 2000, 104, 1153 CrossRef CAS.
- Y. Zhang, B. Deng, T. R. Zhang, D. M. Gao and A. W. Xu, J. Phys. Chem. C, 2010, 114, 5073 CAS.
- Z. H. Wang, S. P. Zhao, S. Y. Zhu, Y. L. Sun and M. Fang, CrystEngComm, 2011, 13, 2262 RSC.
- Y. H. Zheng, L. R. Zheng, Y. Y. Zhan, X. Y. Lin, Q. Zheng and K. M. Wei, Inorg. Chem., 2007, 46, 6980 CrossRef CAS.
- W. W. Lu, S. Y. Gao and J. J. Wang, J. Phys. Chem. C, 2008, 112, 16792 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, theoretical calculation, XRD pattern, FESEM images, and a plot of the extent of photodegradation of MO by various Cu2O catalysts at different intervals. See DOI: 10.1039/c2cy00530a |
| This journal is © The Royal Society of Chemistry 2012 |
