Cs promoted Ag/Al2O3 catalysts for selective catalytic reduction of NOx by methane: effect of SO2 and H2O
Komateedi N.
Rao
and
Heon Phil
Ha
*
Interfacial Engineering Research Center, Korea Institute of Science and Technology (KIST), Seoul 130-650, Republic of Korea. E-mail: heonphil@kist.re.kr; drnraok@gmail.com; Fax: +82 2 9585379; Tel: +82 2 9585461
First published on 19th December 2011
Abstract
Highly active alumina supported silver nano-particles were evaluated for SCR of NOx using methane in an excess oxygen atmosphere. Cs promoted Ag/Al2O3 catalysts showed high NO reduction activity. In addition, the prepared materials exhibited promotional effects in the presence of SO2 and H2O. The X-ray diffraction profiles of fresh and used catalysts indicated that the prepared materials are thermally stable up to 70 h of time-on-stream reaction. Activity results well correlated with the characterization data, which revealed that the high deNOx ability was due to superior NO adsorption properties as well as the presence of an appropriate amount of Ag+ and metallic Ag particles.
1. Introduction
The selective catalytic reduction (SCR) of NOx using methane is an attractive approach to pollution control due to the hydro-carbon (HC) availability through natural gas infrastructures. This after-treatment technology would be particularly applicable to stationary sources such as natural gas engines and small-scale gas boilers, in which unburned methane remains as a main component. A number of catalysts have been reported for HC-SCR in the presence of excess oxygen, in which silver based catalysts are highly promising.1–8 Unfortunately, many of the catalysts have limitations in the practical application such as lower activity, insufficient thermal stability and tend to be poisoned with impurities.8 Researchers have achieved higher NOx conversions using several reductants like methanol, octane, propane and ammonia.9 However, it is still a challenging task to obtain higher methane SCR activity, particularly, in the presence of O2. Therefore, in the present study we described the design of a novel alkali promoted catalytic system, which provides high activity as well as thermal stability in the presence of SO2 and H2O.Alkali metals are effective catalysts for the gasification of coal and carbon by H2O and CO2.10,11 Because of the ability to transfer oxygen between gaseous molecules or between a gaseous reactant and the support, these systems could also be effective for the reduction of other oxygen-containing compounds such as NOx. Thus in this work, Cs doped Ag/Al2O3 catalysts were synthesized to evaluate the SCR of NOx using methane as a reductant. The main objectives were to find the highly active, SO2 resistant and thermally stable caesium promoted catalysts for the title reaction. The resulting fresh and used samples were characterized with BET surface area, XRD, TEM, UV-Vis DRS, NO TPD and quadruple mass spectrometry (Q-MS) techniques.
2. Experimental
The Ag/Al2O3 catalysts were prepared by wet-impregnation of commercial γ-Al2O3 (Alfa Aesar, surface area 255 m2 g−1) with an aqueous solution of AgNO3 (Junsei Chem.). To synthesize 2 wt% Cs doped samples, required amounts of Cs2CO3 (Aldrich) were used. The Ag content of 3 wt% has been found as an optimal Ag loading for the high deNOx performance of the present catalytic system. After impregnation, the samples were dried at 120 °C overnight and calcined at 550 °C for 5 h under air atmosphere, and labelled Ag/Al2O3 and Cs–Ag/Al2O3.The catalytic activity was evaluated in a fixed-bed flow-type quartz reactor. Prior to the reaction, about 0.4 g of the catalyst was pretreated with 10% O2/He gas flow at 550 °C for 2 h. A mixture of 500 ppm NO, 4000 ppm methane, 6% O2 and 10% H2O in He was fed into the reactor system through mass flow controllers and a bubbler (H2O) at 9000 h−1 of GHSV (total flow 150 mL min−1). The NOx concentrations were analyzed with an online NDIR Fuji NO analyzer. The other reactants and products were analyzed online by using a VARIAN Micro GC equipped with TCD.
Powder X-ray diffraction patterns were recorded on a Bruker-8 using Ni filtered Cu Kα radiation. The BET surface area measurements were carried out by N2 adsorption/desorption at liquid-nitrogen temperature using a Micromeritics ASAP 2000 instrument. TEM images and the corresponding EDAX were obtained using an FEI TECNAI G2 F20 instrument. The UV-Vis DRS measurements were performed over a wavelength range between 180 and 700 nm using an Agilent Cary 5000 UV-Vis-NIR spectrophotometer. NO TPD experiments were performed using a Micromeritics AutoChem II-2720 chemisorptions instrument from 50 to 900 °C. Prior to TPD studies, samples were pre-treated at 300 °C and saturated with 1000 ppm NO in He at 50 °C and subsequently flushed with He. For the analysis of the gases evolving in the NO TPD, quadruple mass spectroscopy (Q-MS) was used. The signals for NO (m/e = 30), N2 (m/e = 28), N2O (m/e = 44), O2 (m/e = 32) and H2O (m/e = 18) were monitored by a Hiden HPR-20 QIC bench top gas analysis system connected to an AutoChem II outlet.
3. Results and discussion
3.1. Activity studies
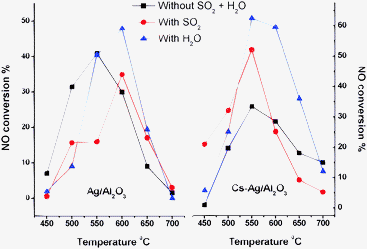
The deNOx performance of Ag/Al2O3 and Cs doped Ag/Al2O3 materials was evaluated at a temperature range of 450 to 700 °C in the presence of SO2 and H2O stream. The obtained results are plotted in Fig. 1. In addition, Tables 1 and 2 show a comparison of NO conversion and the amount of CO evolved from CH4. From Table 1, unpromoted Ag/Al2O3 showed high activity at 550 °C, but at the same temperature the catalytic activity was dropped in the presence of SO2. However, the activity at 600 °C was not suppressed even in the presence of SO2. This indicates that the activation energy of NO to N2 was increased and thereby the shift in the reaction temperature was observed.8 In addition, the conversion of methane was significantly enhanced by SO2. As noted in the table, the presence of SO2 from 500 to 600 °C leads to a substantial promotion in the CO concentration. Usually, CO ppm values are directly related to the methane oxidation. This indicates that the activation of methane was enhanced by the presence of SO2. In general, activation of methane is an important step in the CH4-SCR. The SCR process commonly involves the H2O stream, however, most of the researchers did not consider the H2O effect in their systems.4–9 In the present investigation, a stable catalytic activity was observed in the presence of a 10% water stream.Table 2 presents the activity data of Cs promoted samples. As could be seen in Table 2, Cs–Ag/Al2O3 samples were highly resistant to SO2 and H2O, even at low temperatures. Interestingly, increases in the NO conversion as well as CO concentrations were noticed in the presence of SO2 and water streams. It is also observed that the activity obtained with a SO2 stream was preserved in further reactions with H2O. The higher deNOx activity in the presence of SO2 is mainly due to the SO2 involvement in the reaction mechanism as well as surface modification.1,12 Our recent studies also revealed the sulfation and formation of alkyl-sulfur-oxide species in the reaction stream.2 Of late, Angelidis and Kruse1 and She et al.7 have reported the enhancement of NOx conversion with SO2. Moreover, the higher NO conversion in the temperature range between 500 and 600 °C well matched with the peak obtained in NO TPD. To understand the thermal and mechanical stability of the systems time-on-stream reaction was carried out in each case. The obtained results clearly showed the superior properties of the synthesized materials.
3.2. Catalyst characterization
Fig. 2 presents the standard X-ray diffraction patterns of the Ag/Al2O3 and Cs–Ag/Al2O3 fresh and used samples. The shown patterns are mainly due to the gamma (γ) phase of alumina (JCPDS-50 0741). The absence of XRD lines corresponding to Ag or Cs illustrates the nominal metal loading and the resulting nano-sized particle distribution. Interestingly, the XRD patterns of used samples reveal that about 70 h of time on stream reaction didn't affect the compositional changes of the materials. In general, coagulation or sintering of silver particles under the oxygen atmosphere has been observed by several authors.7,10,13 However, in the present study the silver particles are mostly unchanged under the reaction conditions. This is further supported by the TEM analysis. BET SA measurements showed a moderate surface area for the Cs doped sample (222 g2 m−1), which is a little higher than that of the unpromoted Ag/Al2O3 (210 g2 m−1) sample. | ||
| Fig. 2 XRD profiles of fresh and used Ag/Al2O3 and Cs–Ag/Al2O3 samples. | ||
The high dispersion and the available surface area usually facilitate the number of active sites for a particular reaction. Hence the nano-sized particles with good dispersion would be a contributor for the higher activity obtained in the Cs doped sample.
Fig. 3A and 3B and B′ exhibit the TEM images of used Ag/Al2O3 and Cs–Ag/Al2O3 samples, respectively. The silver particles in both the samples are well dispersed and range from 5 to 25 nm in size. The morphology of the fresh and used samples revealed that no drastic sintering of active silver particles occurred. Particularly, it is found that the silver grains are grown by the addition of Cs. As shown in Fig. 3A, along with nano-sized silver particles a few silver clusters are also observed. Interestingly, as shown in Fig. 3B the silver particles are unchanged under severe reaction conditions. In caesium prompted samples the silver particles are found at the outside of the alumina matrix with nearly spherical texture. These findings are well supported by EDAX results. The spherical particles at the outside of the matrix were found to be single crystals with (2 0 0) surface plane. In the case of Ag/Al2O3 samples, the silver particles are mainly located at the inside of the matrix. The high NO conversions of the Cs promoted sample might also be due to the crystallization and growth of stable silver particles. Several authors have reported that stable oxide Ag catalysts had high NO conversion by generating intermediates like isocyanate (–NCO).4,6,8,10,13 These findings are well supported by UV-Vis DRS studies.
 | ||
| Fig. 3 TEM images of used Ag/Al2O3 (A) and Cs–Ag/Al2O3 (B & B′) samples. Inset shows selected area electron diffraction patterns of used Cs–Ag/Al2O3. | ||
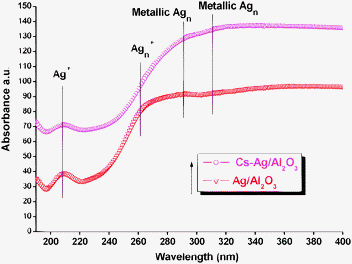
To determine the active reaction site of prepared catalysts in the present reaction system, UV-Vis DRS techniques have been employed to characterize Ag on the catalyst surface. The UV-Vis DRS patterns of Ag/Al2O3 and Cs–Ag/Al2O3 samples calcined at 550 °C are depicted in Fig. 4. There are four absorption bands representing the state of Ag. The bands obtained at 210 and 260 nm were attributed to Ag+, clustered Ag+ species and peaks at 295 and 318 nm are assigned to metallic Ag clusters, respectively.6,14 As noticed from Fig. 4, the unpromoted Ag/Al2O3 exhibited higher Ag+ species than that of the Cs promoted sample. The band related to cluster Ag+ is also low intense in the case of Cs–Ag/Al2O3. Moreover, addition of Cs to silver–alumina promoted the formation of the metallic Ag. It is described in the literature that an appropriate combination of the ionic and metallic Ag is critical for the high deNOx performance.15 Hence, the higher activity for the Cs promoted sample is due to the balanced combination of Ag+ and Ag0 situated over the catalysts' surface.
To understand the adsorption properties of prepared samples NO TPD was conducted. Usually, adsorption behaviours of materials substantially affect the SCR of NO. Fig. 5A displays the NO TPD profiles of Ag/Al2O3 and Cs–Ag/Al2O3 samples calcined at 550 °C. As noticed from Fig. 5A the initial peak was due to physically adsorbed NO and the other signal is due to strong NO adsorptions sites. The activity data describe that the adsorption properties of the sample from 500 to 650 °C are significant in the present systems. However, in the case of Ag/Al2O3 catalysts the strong NO adsorption band ranged from 300 to 580 °C, whereas for the Cs–Ag/Al2O3 sample the signal was located between 300 and 700 °C. The intense and wider NO desorption band for Cs doped sample reveals that the addition of a nominal amount of Cs improves the NO adsorption sites of Ag/Al2O3. Recently, Ito et al.10 reported that the strong basicity of Cs and the oxidative adsorption of NO also increase to form nitrate on the solid surface. Thus, the existence of a broad area peak from 350 to 700 °C is the reason for the wide temperature activity obtained for Cs–Ag/Al2O3. To evaluate gases from NO TPD were analyzed by Q-MS. The temperature dependent mass profiles of Cs–Ag/Al2O3 are plotted in Fig. 5B. As is shown the patterns of N2, NO2 and NO were the major products. Moreover, small amounts of water were also detected from the hydroxyl groups over the material. Along with the desorption of NO there is a formation of a small amount of N2 at around 420 °C without the reducing gas such as methane or H2.9 This is mainly caused by the involvement of silver particles in the NO reduction reaction. From TPD studies it is clearly seen that the doping of Cs promoted the performance of the parent Ag/Al2O3 sample.
 | ||
| Fig. 5 NO TPR patterns of Ag/Al2O3 and Cs–Ag/Al2O3 samples. (B) Q-MS of evaluated gases from NO TPR of Cs–Ag/Al2O3. | ||
4. Conclusions
The addition of Cs to Ag/Al2O3 has been found to greatly promote the deNOx at a wide temperature window. In the present investigation, the high NO conversion in the temperature range between 500 and 650 °C was explained by the desorption bands in NO TPD. Furthermore, synthesized samples exhibited high deNOx activity in the presence of SO2 and H2O. XRD profiles revealed the strong thermal and mechanical stabilities of Ag/Al2O3 and Cs–Ag/Al2O3 samples. TEM images of the used Cs–Ag/Al2O3 sample disclosed the highly dispersed spherical silver particles located out of alumina particles. UV-Vis DRS results reveal the presence of appropriate combination of the ionic and metallic Ag over the catalyst surface. In conclusion, the strong NO adsorption properties and stable Ag crystallization are suggested to be the reason for higher deNOx activity of Cs promoted samples.Acknowledgements
This work was funded by ‘Future Core Technology’ program from KIST. A grant also from Fundamental R&D Program for the core technology of materials supported the research, funded by the Ministry of Knowledge Economy of the Republic of Korea.References
- T. N. Angelidis and N. Kruse, Appl. Catal., B, 2001, 34, 201–212 CrossRef CAS
.
- P. A. Kumar, M. P. Reddy, B. Hyun-Sook and H. H. Phil, Catal. Lett., 2009, 131, 85–97 CrossRef CAS
.
- A. Keshavaraja, X. She and M. Flytzani-Stephanopoulos, Appl. Catal., B, 2000, 27, L1–L9 CrossRef CAS
.
- R. Brosius, K. Arve, M. H. Groothaert and J. A. Martens, J. Catal., 2005, 231, 344–353 CrossRef CAS
.
- J. R. H. Carucci, K. Arve, S. Bartova, K. Eranen, T. Salmi and D. Y. Murzin, Catal. Sci. Technol., 2011, 1, 1456–1465 Search PubMed
.
- Z. Li and M. Flytzani-Stephanopoulos, J. Catal., 1999, 182, 313–327 CrossRef CAS
.
- X. She, M. Flytzani-Stephanopoulos, C. Wang, Y. Wang and C. H. F. Peden, Appl. Catal., B, 2009, 88, 98–105 CrossRef CAS
.
- R. Burch, J. P. Breen and F. C. Meunier, Appl. Catal., B, 2002, 39, 283–303 CrossRef CAS
.
- K.-I. Shimizu and A. Satsuma, Phys. Chem. Chem. Phys., 2006, 8, 2677–2695 RSC
.
- K. Ito, K. Kishikawa, A. Watajima, K. Ikeue and M. Machida, Catal. Commun., 2007, 8, 2176–2180 CrossRef CAS
.
- Z. H. Zhu, G. Q. Lu and R. T. Yang, J. Catal., 2000, 192, 77–87 CrossRef CAS
.
- R. Ke, J. Li, X. Liang and J. Hao, Catal. Commun., 2007, 8, 2096–2099 CrossRef CAS
.
- R. Ke, Q. Chen, J. Li, Y. Zhu and J. Hao, Catal. Commun., 2007, 8, 589–592 CrossRef CAS
.
- K. Shimizu, K. Sawabe and A. Satsuma, Catal. Sci. Technol., 2011, 1, 331–341 CAS
.
- X. She and M. Flytzani-Stephanopoulos, J. Catal., 2006, 237, 79–93 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2012 |