A nanocluster design for the construction of artificial cellulosomes†
Do-Myoung
Kim
a,
Hikaru
Nakazawa
a,
Mitsuo
Umetsu
*a,
Takashi
Matsuyama
b,
Nobuhiro
Ishida
b,
Akinori
Ikeuchi
b,
Haruo
Takahashi
b,
Ryutaro
Asano
a and
Izumi
Kumagai
a
aDepartment of Biomolecular Engineering, Graduate School of Engineering, Tohoku University, Aoba 6-6-11, Aramaki, Aoba-ku, Sendai, 980-8579, Japan. E-mail: mitsuo@kuma.che.tohoku.ac.jp; Fax: +81-22-795-7276; Tel: +81-22-795-7276
bToyota Central R&D Lab, Yokomichi 41-1, Oaza Nagakute, Nagakute-cho, Aichi-gun, 480-1192, Japan
First published on 13th January 2012
Abstract
We describe here the construction of artificial cellulosomes by nanoclustering recombinant cellulolytic modules on non-cellulosome-derived scaffolds. Catalytic and cellulose-binding domain modules derived from cellulosomes were assembled on streptavidin and on inorganic nanoparticles. Heteroclustering of the modules significantly promoted the activity of the assembled catalytic modules for degradation of water-insoluble substrates.
Cellulose is a linear polysaccharide of glucosyl units connected by β-1,4-linkages. This biomass material, which is an abundant carbon resource on earth, is water-insoluble and hard, but cellulolytic bacteria and fungi degrade it to low-molecular-weight sugars by means of cellulolytic enzymes called cellulases. Cellulases are generally modular proteins with an independent catalytic domain (CD) and a carbohydrate-binding module (CBM), and in some bacteria, CDs with different functions are clustered on a giant scaffold protein containing CBM, via cohesin–dockerin interaction, to improve the degradation efficiency of the CD module.1,2 The dockerin domain fused to the CD module non-covalently interacts with one of the cohesin domains tandemly arranged in a giant scaffold protein to form a complicated protein complex called a cellulosome. The clustered catalytic modules are adsorbed on cellulose via CBMs on the scaffold protein and synergistically degrade biomass materials by means of coupled hydrolysis reactions.3,4
The high performance of cellulosomes with regard to their efficiency of degradation is attractive for the production of alternative fuels from renewable biomass resources with low energetic and environmental loads. However, extracting substantial amounts of cellulosomes from native bacteria is too difficult to utilize native cellulosomes for the production of alternative fuels. Several studies have reported the preparation of small cellulosomes from recombinant proteins expressed in Escherichia (E.) coli,3,5–7 but the recombinant preparation of intact cellulosomes with the same length and activity as those of the native form remains challenging. Therefore, a new means of fabricating highly clustered enzyme complexes is needed.
Cellulases mainly comprise endoglucanases (E.C. 3.2.1.4) and exoglucanases (E.C.3.2.1.91): endoglucanases prefer to randomly hydrolyze celluloses, and exoglucanases can degrade the chain ends of cellulose. Recently, we used GH family 12 endoglucanase A (EglA) from Aspergillus niger, which has only a CD module and is easily expressed by E. coli, as a model enzyme to cluster the enzymes together with CBDs on non-cellulosome-derived scaffold units, thus demonstrating the multivalent design of CBDs.8 In this study, we propose a new design for artificial cellulosomes by reassembling independently prepared building blocks of cellulosomes in vitro on the surface of non-cellulosome-derived scaffold structures to efficiently improve the enzymes' degradation activity.
Endoglucanase D (CelD) is an endoglucanase belonging to GH family 9 from Clostridium thermocellum.9 CelD has higher activity than EglA and it is a component of cellulosomes. The structure of CelD is composed of a typical CD (CDCelD) and a dockerin domain, which binds to any of the nine cohesin domains in a cellulosome integrating protein (CipA) containing a family 3a CBM (CBM3a), thus forming cellulosomes.10–13 To cluster CDCelD and CBM3a, CDCelD and CBM3a with an IgA hinge linker (SPSTPPTPSPSTPP) and then a biotin acceptor peptide (AviTag: GGLNDIFEAQKIEWH)14 at the C-terminus (Scheme 1) were independently prepared in E. coli within biotin ligase by recombinant means (see ESI†, experimental), so that the recombinant CDCelD and CBM3a each had a biotin molecule only on the lysine residue at the C-terminus of the AviTag-peptide. The biotinylated CDCelD and CBM3a were mixed with streptavidin or streptavidin-conjugated cadmium selenide (CdSe) nanoparticles (size: 20 nm) to cluster the modules on streptavidin or the nanoparticles (Scheme 1). In this study, the molecular ratios were varied (CDCelD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CBM3a
CBM3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) streptavidin = 1
streptavidin = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 1
0, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 4
0, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; CDCelD
1; CDCelD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CBM3a
CBM3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CdSe = 30
CdSe = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 23
1, 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 15
1, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 7
1, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to yield several complexes in which CDCelD and CBM3a were clustered on a streptavidin molecule or nanoparticle at different ratios (see ESI†, experimental).
1) to yield several complexes in which CDCelD and CBM3a were clustered on a streptavidin molecule or nanoparticle at different ratios (see ESI†, experimental).
 | ||
| Scheme 1 Schematic illustration of the clustering of CDCelD and CBM on streptavidin and on streptavidin-conjugated CdSe nanoparticles. | ||
To analyse the degradation activity of CelD for cellulose substrates, degradation activity for phosphoric-acid-swollen cellulose (PSC) was measured by means of a tetrazolium blue chloride assay (see ESI†, experimental).15 The concentration of CelD or biotinylated CDCelD in the reaction solution was adjusted to 40 nM to analyse the activity change of the catalytic domain in the clusters, and the reaction was carried out at 45 °C to compare the activity of clustered CDCelD with non-thermophilic CBM later. CelD alone degraded PSC to reducing sugars at a concentration of 0.07 mg mL−1 over a reaction time of 1 h, but this degradation increased only slightly to 0.10 mg mL−1 after 96 h (open black circles in Fig. 1a). Biotinylated CDCelD also showed the same performance as CelD (open black squares in Fig. 1a), indicating that the removal of the dockerin domain and the fusion of the biotin acceptor peptide did not influence the degradation activity of CDCelD. Previously, recombinant CBM3a with a cohesin domain at the C-terminus was designed to be bound to CelD via cohesin–dockerin interaction, and the formation of CelD–CBM3a complexes promoted the enzyme's activation.16 We also prepared the CelD–CBM3a complexes by mixing CelD with the cohesin-fused CBM3a in equal amounts. The resulting CelD–CBM3a complexes produced reducing sugars at concentrations that were 1.9 and 2.6 times those produced by CelD alone (0.13 and 0.26 mg mL−1, respectively) after 1 and 96 h, respectively (closed black circles in Fig. 1a). This enhancement of degradation activity was not observed for a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mixture of CDCelD (without dockerin) and CBM3a (without cohesin) (closed black squares in Fig. 1b). These results indicate that the conjugation of CBM3a to CDCelD could effectively enhance degradation activity for water-insoluble substrates.
50 mixture of CDCelD (without dockerin) and CBM3a (without cohesin) (closed black squares in Fig. 1b). These results indicate that the conjugation of CBM3a to CDCelD could effectively enhance degradation activity for water-insoluble substrates.
 | ||
| Fig. 1 Amounts of reducing sugars produced from 1 mg ml−1 PSC in a 50 mM MES buffer solution (pH 6.0, 10 mM CaCl2) at 45 °C for 96 h in the presence of unclustered CelD and CDCelD (a), and CDCelD–CBM3a clusters on streptavidin (b) and on CdSe nanoparticles (c). All the experiments were carried out at the CelD or CDCelD concentration of 40 nM. Each experiment was conducted three times, and the average values are plotted with error bars representing standard variation. | ||
The effectiveness of CBM3a toward cellulose degradation was noticeable for the clusters on streptavidin. Even without CBM3a, the clustering of CDCelD on streptavidin (open red circles in Fig. 1b) produced an amount of reducing sugars that was comparable to that produced by CelD–CBM3a complexes formed from CelD and cohesin-fused CBM3a, but the heteroclustering of CDCelD with CBM3a drastically enhanced the degradation activity of CDCelD with increasing the concentrations of CBM3a. Finally, the CDCelD–CBM3a clusters with 1 CDCelD and 3 CBM3a modules per streptavidin molecule produced reducing sugars at concentrations that were 2.7 and 5.5 times those produced by CelD alone (0.20 and 0.57 mg mL−1, respectively) after 1 and 96 h, respectively (closed red squares in Fig. 1b). The addition of CBM3a without streptavidin did not enhance the degradation activity of CDCelD, indicating that the clustering was critical for the enhancement of degradation activity.
In native cellulosomes, CDs with a dockerin domain are clustered on a giant scaffold protein with tandemly arranged cohesins and a CBM to efficiently concentrate the CDs on water-insoluble substrates. Previous studies on the assembly of cellulases on a scaffold protein have focused on the clustering of CDs to enhance the degradation activity of the enzymes. For example, 10 CD modules with a dockerin domain have been clustered on a hexameric stable protein 1 with cohesins fused to each domain,17 and several different cellulases with dockerin domains have been assembled on a rosettasome with cohesins fused to each of the 18 subunits.18 Both types of clusters exhibited enhanced degradation activity that was 2–3.5 times that of the free enzymes.
In our study, CD and CBM were clustered on non-cellulosome-derived scaffold units of streptavidin via biotin–avidin interactions,19 thus demonstrating the multivalent effect of CBMs on the degradation activity of clustered cellulase complexes. The biotin acceptor peptide at the C-terminus of each module was fused at the C-terminus of each module to homogeneously cluster selectively biotinylated modules on streptavidin, and the insertion of the rigid IgA hinge linker between the biotin acceptor peptide and module enables each module to independently fulfill its function.14 Consequently, the design of multivalent CBMs drastically enhanced the activity of the CDs. Fierobe et al. reported that more than one CBM per designer cellulosome decreases activity,3 and indeed the cluster formats with several CBMs have not been found in any of the natural cellulosomes. Although we cannot make a simple comparison between our results and the previously reported results, our results show the potential of multivalent design of CBMs for the construction of artificial cellulosomes.
As an alternative scaffold unit, inorganic nanoparticles were used in place of streptavidin to increase the valence of the CBMs in the CDCelD–CBM3a clusters. Cadmium selenide (CdSe) nanoparticles used in this study have 20 nm particle size and streptavidins were conjugated on the surface to provide ∼30 biotin-binding sites per particle.8Fig. 1c shows the degradation activity of CdSe nanoparticles with CDCelD and CBM3a for PSC substrates. CDCelD clustered without CBM3a on the nanoparticle (open blue circles in Fig. 1c) produced a comparable amount of reducing sugars as did CelD–CBM3a complexes formed from CelD and cohesin-fused CBM3a. However, heteroclustering of CDCelD with CBM3a at a ratio of 3 CDCelD to 1 CBM3a (closed blue circles in Fig. 1c) produced fewer reducing sugars than did clusters with only CDCelD. The degradation activity was enhanced by increasing the valence of CBM3a on the nanoparticle, but the enhancement was lower than that observed when the valence of CBM3a was increased on streptavidin. Therefore, the inclusion of highly multivalent CBM3a on the nanoparticle did not efficiently increase the degradation activity of CDCelD.
To analyse the influence of CBM properties on the enhancement of degradation activity, we clustered CDCelD with another CBM, which is the second N-terminal domain in endoglucanase C from Cellulomonas fimi belonging to family 4 (CBM4).20,21 Although the addition of biotinylated CBM4 without clustering slightly enhanced the degradation activity (closed black squares in Fig. 2a), heteroclustering of biotinylated CDCelD and CBM4 on streptavidin and on CdSe nanoparticles resulted in a gradual enhancement of degradation activity as the valence of CBM4 increased (Fig. 2). This enhancement was more pronounced for the clusters on nanoparticles than for the clusters on streptavidin. For a reaction time of 8 h, the degradation rate of CDCelD–CBM4 clusters at a ratio of 1 CDCelD to 3 CBM4 per streptavidin molecule (closed red squares in Fig. 2a) was comparable to that of clusters with the same ratio per nanoparticle (closed blue squares in Fig. 2b), but after 96 h the clusters on nanoparticles had produced 1.9 times the amount of reducing sugars formed by the clusters on streptavidin. Consequently, the CDCelD–CBM4 clusters on nanoparticles produced 1.3 times the sugars formed by the most active clusters among the complexes with CBM3a.
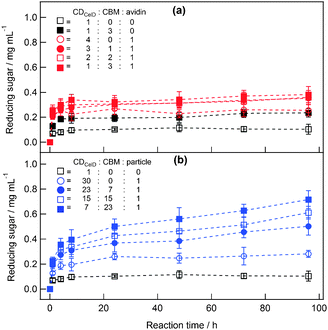 | ||
| Fig. 2 Amounts of reducing sugars produced from 1 mg ml−1 PSC in a 50 mM MES buffer solution (pH 6.0, 10 mM CaCl2) at 45 °C for 96 h in the presence of CDCelD–CBM4 clusters on streptavidin (a) and on nanoparticles (b). All the experiments were carried out at the CDCelD concentration of 40 nM. Each experiment was conducted three times, and the average values are plotted with error bars representing standard variation. | ||
The clustering of CBM4 on streptavidin-conjugated CdSe nanoparticles mostly enhanced the CDCelD activity of the CD–CBM clusters constructed in this study. To carefully analyze the degradation rate of the CDCelD–CBM4 clusters, degradation was measured on the timescales of minutes (Fig. 3a) and hours (Fig. 3b). The degradation rate after 10 min for the clusters on streptavidin was faster than that observed for the clusters on nanoparticles; however, for reaction times longer than 10 min, the degradation rate of the clusters on streptavidin was slightly decreased, and no additional reducing sugars were produced for times longer than 8 h (Fig. 3b). In contrast, the clusters on nanoparticles continued to degrade PSC; as a result, after 4 h, the amount of reducing sugars produced by the clusters on nanoparticles surpassed that produced by the clusters on streptavidin (Fig. 3b). Therefore, the increased degradation of PSC by the clusters on nanoparticles was due to sustained degradation of PSC over long reaction times.
 | ||
| Fig. 3 Amounts of reducing sugars produced from 1 mg ml−1 PSC in a 50 mM MES buffer solution (pH 6.0, 10 mM CaCl2) at 45 °C for 20 min (a) and for 24 h (b). Each reaction was performed in the presence of CDCelD (open black squares), a mixture of 1 CDCelD and 3 CBM4 (closed black squares), CDCelD–CBM4 clusters at a ratio of 1 CDCelD to 3 CBM4 per streptavidin molecule (closed red squares), and CDCelD–CBM4 clusters at a ratio of 7 CDCelD to 23 CBM4 per nanoparticle (closed blue squares). All the experiments were carried out at the CDCelD concentration of 40 nM. Each experiment was conducted three times, and the average values are plotted with error bars representing standard variation. | ||
PSC is a non-crystalline substrate. It is easily degraded by simple endoglucanases in comparison to crystalline substrates such as avicel, which present greater challenge to cellulases. Fig. 4 shows the degradation of avicel substrates by using the CD–CBM clusters. Consequently, although the enhancement of CDCelD activity for avicel is less drastic than that for PSC, the clustering of CDCelD with CBM was also effective for the degradation of avicel (Fig. 4), and the correlation between clustering format and activity enhancement denoted the same tendency of the degradation of PSC: the clustering with CBM3a on streptavidin more enhanced CDCelD activity than on nanoparticles, while CBM4 enhanced CDCelD activity by clustering on nanoparticles. The use of one type of endoglucanase usually enables degradation of simple substrates, but our clustering design using streptavidin and nanoparticles was effective for the degradation of crystalline cellulose by using only one type of endoglucanase. Addition of a second type of enzyme to the complex might be helpful in further promoting the degradation of crystalline substrates.
 | ||
| Fig. 4 Amounts of reducing sugars produced from 10 mg ml−1 avicel in a 50 mM MES buffer solution (pH 6.0, 10 mM CaCl2) at 45 °C for 96 h in the presence of CDCelD–CBM3a clusters (a) and CDCelD–CBM4 clusters (b). Each reaction was performed in the presence of CDCelD (open black squares), a mixture of 1 CDCelD and 3 CBM (closed black squares), CDCelD–CBM clusters at a ratio of 1 CDCelD to 3 CBM per streptavidin molecule (closed red squares), and CDCelD–CBM clusters at a ratio of 7 CDCelD to 23 CBM per nanoparticle (closed blue squares). All the experiments were carried out at the CDCelD concentration of 2.5 μM. Each experiment was conducted three times, and the average values are plotted with error bars representing standard variation. | ||
CBMs play a role in localizing CDs on cellulose surfaces so that the substrate concentration surrounding the CDs is substantially increased. CBMs are classified into CBM families with different substrate specificity: CBM3a binds to the surface of the microcrystalline substrate,16 and CBM4 appears to bind to amorphous substrates.20,21 The affinity and specificity of CBMs for cellulose surfaces influence the catalytic properties of CD–CBM complexes.22 In this study, appropriate clustering format for enhancing CDCelD degradation activity depended on the type of CBM used in the clustered complexes: CBM3a drastically promoted CDCelD activity when clustered on streptavidin, whereas CBM4 increased the activity of CDCelD clustered on nanoparticles. We measured the binding affinity of each CBM for PSC and avicel: CBM3a and CBM4 were adsorbed on PSC with similar Kd values (CBM3a: 1.4 μM, CBM4: 1.8 μM; Fig. 5a), while CBM3a had higher affinity for avicel than CBM4 (CBM3a: 1.5 μM, CBM4: 4.5 μM; Fig. 5b). We could not find straightforward correlation between the binding affinity of CBM and the activity of the clustered complex, suggesting that other crucial factors determine the appropriate multivalent number of CBMs in the CD–CBM clusters.
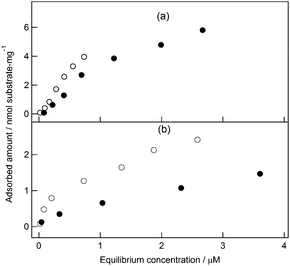 | ||
| Fig. 5 Adsorption of CBM3a (open circles) and CBM4 (closed circles) on 1 mg ml−1 PSC (a) and 1 mg ml−1 avicel (b) in 50 mM MES buffer solution (pH 6.0, 10 mM CaCl2). | ||
In conclusion, we constructed artificial cellulosomes from independently prepared CD and CBM of cellulosomes on non-cellulosome-derived scaffolds, and we demonstrated the effectiveness of a multivalent CBM design for drastic enhancement of cellulose degradation. Selection of an appropriate combination of CBM module and scaffold was critical for the enhancement of degradation activity. The combination of a cellulose-disruptive CBM and a nanoparticle scaffold provided the greatest enhancement of degradation activity, suggesting the availability of nanoscale scaffold units for clustering the cellulose-disruptive CBM. The cluster design proposed in this study can easily be used to generate various cellulolytic enzymes with multiple types of CBMs. This design also may be utilized more generally to create artificial cellulosomes.
This work was partly supported by the New Energy and Industrial Technology Development Organization (NEDO) of Japan (M.U.), by a Scientific Research Grant from the Ministry of Education, Science, Sports, and Culture of Japan (M.U., I.K.), and by Advanced Low Carbon Technology Research and Development Program Grant from Japan Science and Technology (JST) Agency (M.U.).
Notes and references
- E. A. Bayer, J. P. Belaich, Y. Shoham and R. Lamed, Annu. Rev. Microbiol., 2004, 58, 521 CrossRef CAS.
- H. J. Gilbert, Mol. Microbiol., 2007, 63, 1568 CrossRef CAS.
- H.-P. Fierobe, E. A. Bayer, C. Tardif, M. Czjzek, A. Mechaly, A. Bélaïch, R. Lamed, Y. Shoham and J.-P. Bélaïch, J. Biol. Chem., 2002, 277, 49621 CrossRef CAS.
- I. Fendri, C. Tardif, H.-P. Fierobe, S. Lignon, O. Valette, S. Pagès and S. Perret, FEBS J., 2009, 276, 3076 CrossRef CAS.
- H.-P. Fierobe, A. Mechaly, C. Tardif, A. Bélaïch, R. Lamed, Y. Shoham, J.-P. Bélaïch and E. A. Bayer, J. Biol. Chem., 2001, 276, 21257 CrossRef CAS.
- H.-P. Fierobe, F. Mingardon, A. Mechaly, A. Bélaïch, M. T. Rincon, S. Pagès, R. Lamed, C. Tardif, J.-P. Bélaïch and E. A. Bayer, J. Biol. Chem., 2005, 280, 16325 CrossRef CAS.
- F. Mingardon, A. Chanal, A. M. López-Contreras, C. Dray, E. A. Bayer and H.-P. Fierobe, Appl. Environ. Microbiol., 2007, 73, 3822 CrossRef CAS.
- D.-M. Kim, M. Umetsu, K. Takai, T. Matsuyama, N. Ishida, H. Takahashi, R. Asano and I. Kumagai, Small, 2011, 7, 656 CrossRef CAS.
- G. Joliff, P. Béguin and J. P. Aubert, Nucleic Acids Res., 1986, 14, 8605 CrossRef CAS.
- K. Tokatlidis, S. Salamitou, P. Béguin, P. Dhurjati and J. P. Aubert, FEBS Lett., 1991, 291, 185 CrossRef CAS.
- T. Fujino, P. Béguin and J. P. Aubert, FEMS Microbiol. Lett., 1992, 94, 165 CrossRef CAS.
- D. M. Poole, E. Morag, R. Lamed, E. A. Bayer, G. P. Hazlewood and H. J. Gilbert, FEMS Microbiol. Lett., 1992, 99, 181 CrossRef CAS.
- U. T. Gerngross, M. P. Romaniec, T. Kobayashi, N. S. Huskisson and A. L. Demain, Mol. Microbiol., 1993, 8, 325 CrossRef CAS.
- S. M. Cloutier, S. Couty, A. Terskikh, L. Marguerat, V. Crivelli, M. Pugnières, J. Mani, H. Leisinger, J. Mach and D. Deperthes, Mol. Immunol., 2000, 37, 1067 CrossRef CAS.
- C. K. Jue and P. N. Lipke, J. Biochem. Biophys. Methods, 1985, 11, 109 CrossRef CAS.
- G. Carrard, A. Koivula, H. Söderlund and P. Béguin, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 10342 CrossRef CAS.
- A. Heyman, Y. Barak, J. Caspi, D. B. Wilson, A. Altman, E. A. Bayer and O. Shoseyov, J. Biotechnol., 2007, 131, 433 CrossRef CAS.
- S. Mitsuzawa, H. Kagawa, Y. Li, S. L. Chan, C. D. Paavola and J. D. Trent, J. Biotechnol., 2009, 143, 139 CrossRef CAS.
- E. A. Bayer, T. Viswanatha and M. Wilchek, FEBS Lett., 1975, 60, 309 CrossRef CAS.
- J. B. Coutinho, N. R. Gilkes, R. A. J. Warren, D. G. Kilburn and C. A. Haynes, Mol. Microbiol., 1992, 6, 1243 CrossRef CAS.
- E. Brun, P. E. Johnson, A. L. Creagh, P. Tomme, P. Webster, C. A. Haynes and L. P. McIntosh, Biochemistry, 2000, 39, 2445 CrossRef CAS.
- M. Linder and T. T. Teeri, J. Biotechnol., 1997, 57, 15 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, kinetic analysis, binding analysis of cellulose-binding domain. See DOI: 10.1039/c2cy00371f |
| This journal is © The Royal Society of Chemistry 2012 |
