High H2O2 yield in the direct oxidation of H2 with O2 on mono dispersed Pd–Au nano colloid under pressurized conditions
Tatsumi
Ishihara
*,
Ryota
Nakashima
and
Yohei
Nomura
Department of Applied Chemistry, Faculty of Engineering, Kyushu University Motooka 744, Nishi-ku, Fukuoka 819-0395, Japan. E-mail: ishihara@cstf.kyushu-u.ac.jp; Fax: +81-92-802-2871
First published on 16th January 2012
Abstract
Pd–Au (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) nano colloid was prepared by using various reductants and it was found that the mono dispersed Pd–Au nano colloid with 4.7 nm in diameter was prepared by reduction with oxalic acid and this Pd–Au nano colloid is highly active for the synthesis of H2O2 by direct oxidation of H2 with O2. The yield of H2O2 increased with increasing reaction pressure and at 1 MPa, H2O2 yield achieved a value of 30%. Under these conditions, H2 conversion is as high as 80%. H2O2 yield was further improved by increasing H2 partial pressure, and the optimum H2 partial pressure was 10% at 1 MPa because of safety (out of the explosion limit concentration in reactor) and the formation rate of H2O2. Because H2O2 decomposition is prevented, H2O2 yield also increased with decreasing reaction temperature and at 268 K, H2O2 yield and production rate are achieved as high as 46% and 25 mmol h−1, respectively, in aqueous solution. H2O2 concentration reaches a value of 5.8% in aqueous solution after 20 h at 268 K.
25) nano colloid was prepared by using various reductants and it was found that the mono dispersed Pd–Au nano colloid with 4.7 nm in diameter was prepared by reduction with oxalic acid and this Pd–Au nano colloid is highly active for the synthesis of H2O2 by direct oxidation of H2 with O2. The yield of H2O2 increased with increasing reaction pressure and at 1 MPa, H2O2 yield achieved a value of 30%. Under these conditions, H2 conversion is as high as 80%. H2O2 yield was further improved by increasing H2 partial pressure, and the optimum H2 partial pressure was 10% at 1 MPa because of safety (out of the explosion limit concentration in reactor) and the formation rate of H2O2. Because H2O2 decomposition is prevented, H2O2 yield also increased with decreasing reaction temperature and at 268 K, H2O2 yield and production rate are achieved as high as 46% and 25 mmol h−1, respectively, in aqueous solution. H2O2 concentration reaches a value of 5.8% in aqueous solution after 20 h at 268 K.
Introduction
Demands for hydrogen peroxide (H2O2) are currently increasing, with the increasing importance of green chemistry. Therefore, the market for H2O2 is expanding in various fields, like breaching. In particular, H2O2 is expected to become an important oxidant for use in catalytic reactions,1–5 such as the epoxidation of olefins. At present, H2O2 is synthesized by the so-called anthraquinone method, which consists of anthraquinone hydrogenation followed by the auto-thermal oxidation of hydroanthraquinone.6,7 This process is useful and efficient for production of H2O2 only at a large scale. On the other hand, the direct synthesis of H2O2 from gaseous hydrogen and oxygen is attracting much interest as a simple H2O2 production method, in particular, for small scale production, because of the high production rate and simple process. Studies of various catalysts, particularly Pd- and Pt-based catalysts, have been conducted.8–24 Lunsford et al. reported the high activity of colloidal Pd,8–11 and Edwards et al. reported that the addition of Au to Pd improves the yield and that high productivity is achieved using TiO2 (P-25), which is a mixed phase of rutile and anatase, as a support.12–14 We have also reported that a rutile-type TiO2 support leads to a high H2O2 yield in the direct oxidation of H2 on a Pd–Au bimetallic catalyst under atmospheric pressure. In addition, we found that Pd–Au nano colloid at Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 85
Au = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 exhibits high activity for H2O2 formation rate15 since a sharp particle size distribution and a large metal surface area can be achieved.25 In this study, the effects of the type of reductant for Pd–Au nano colloid preparation on H2O2 synthesis were investigated. Optimization of reaction conditions is also performed. Although high H2O2 yield is reported by using methanol for catalyst suspension solvent,26,27 separation of H2O2 from solvent containing methanol is highly difficult and so high yield of H2O2 formation in aqueous solution is highly important and so we used water for solvent in spite of low H2 and O2 dissolved amount.
15 exhibits high activity for H2O2 formation rate15 since a sharp particle size distribution and a large metal surface area can be achieved.25 In this study, the effects of the type of reductant for Pd–Au nano colloid preparation on H2O2 synthesis were investigated. Optimization of reaction conditions is also performed. Although high H2O2 yield is reported by using methanol for catalyst suspension solvent,26,27 separation of H2O2 from solvent containing methanol is highly difficult and so high yield of H2O2 formation in aqueous solution is highly important and so we used water for solvent in spite of low H2 and O2 dissolved amount.
Experiment
Pd–Au bimetallic colloid was prepared by the chemical reduction of HAuCl4 and PdCl2 in a mixture of 1 ml of 5.6 M HCl solution, 25 ml of H2O, 25 ml of C2H5OH and 1 g of oxalic acid or ca. 0.25 mmol of hydrazine (namely 1 ml of 0.8 vol% hydrazine). The total amount of Pd and Au was maintained at 50 μmol and the Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au ratio was maintained at 75
Au ratio was maintained at 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 mol% for the catalyst. In order to mono dispersion of the colloid, 0.43 g of polyvinylpyrrolidone (PVP, Kishida Pure Chem. Co. Ltd.) was added for preventing aggregation of nano colloid. Since the amount of PVP as dispersion reagents is insufficient, aggregation of Pd–Au nano colloid occurs easily and the catalytic activity is significantly decreased. However, it is confirmed that the H2O2 formation rate is hardly dependent on the amount of PVP when the amount of PVP is larger than 0.4 g. The aqueous solution was refluxed at 353 K for 60 min under atmospheric conditions with a magnetic stirring mixer and the solution gradually changed to a brownish red, which indicated the formation of nano colloid particles. The nano colloidal catalyst was kept in liquid phase and when reaction activity was tested, the liquid phase catalyst was put into the reaction solution to be 17.8 mg in total amount of Pd–Au nano colloid with colloidal solution, unless otherwise noted.
25 mol% for the catalyst. In order to mono dispersion of the colloid, 0.43 g of polyvinylpyrrolidone (PVP, Kishida Pure Chem. Co. Ltd.) was added for preventing aggregation of nano colloid. Since the amount of PVP as dispersion reagents is insufficient, aggregation of Pd–Au nano colloid occurs easily and the catalytic activity is significantly decreased. However, it is confirmed that the H2O2 formation rate is hardly dependent on the amount of PVP when the amount of PVP is larger than 0.4 g. The aqueous solution was refluxed at 353 K for 60 min under atmospheric conditions with a magnetic stirring mixer and the solution gradually changed to a brownish red, which indicated the formation of nano colloid particles. The nano colloidal catalyst was kept in liquid phase and when reaction activity was tested, the liquid phase catalyst was put into the reaction solution to be 17.8 mg in total amount of Pd–Au nano colloid with colloidal solution, unless otherwise noted.
The synthesis of H2O2 from a gaseous mixture of H2 and O2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ratio in general) was tested in a stainless steel autoclave reactor with magnetic stirring at 283 K, 1 MPa. This reactor was charged with an aqueous solution containing a catalyst comprising 17.8 mg in total concentration of Pd and Au, 84 mM of NaCl and 0.368 M of H2SO4 for experiments performed under all conditions used. In addition, a gaseous mixture of H2–O2–N2 (2.5
9 ratio in general) was tested in a stainless steel autoclave reactor with magnetic stirring at 283 K, 1 MPa. This reactor was charged with an aqueous solution containing a catalyst comprising 17.8 mg in total concentration of Pd and Au, 84 mM of NaCl and 0.368 M of H2SO4 for experiments performed under all conditions used. In addition, a gaseous mixture of H2–O2–N2 (2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19.5
19.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 volume ratio, assuming the H2 diluted with air) was mainly fed through a porous glass filter (pore size, 10 μm in diameter) and the catalyst suspension was stirred mechanically at 800 rpm using a motor. It is also noted that the hydrogen partial pressure used (2.5%) is smaller than that of the explosion limit (4.5%). A back pressure valve (TESCOM type 2500) was used for pressurizing the reactor and a thermal flow controller were used for gas flow rate control. The reactor (Tiatsu Glass Co. Ltd.) was immersed in temperature-controlled water (283 K) using a cryostat (Taitec CR-80R). Since H2O2 yield decreased with reaction time, we used the reaction data after 2 h reaction for comparison unless otherwise mentioned.
78 volume ratio, assuming the H2 diluted with air) was mainly fed through a porous glass filter (pore size, 10 μm in diameter) and the catalyst suspension was stirred mechanically at 800 rpm using a motor. It is also noted that the hydrogen partial pressure used (2.5%) is smaller than that of the explosion limit (4.5%). A back pressure valve (TESCOM type 2500) was used for pressurizing the reactor and a thermal flow controller were used for gas flow rate control. The reactor (Tiatsu Glass Co. Ltd.) was immersed in temperature-controlled water (283 K) using a cryostat (Taitec CR-80R). Since H2O2 yield decreased with reaction time, we used the reaction data after 2 h reaction for comparison unless otherwise mentioned.
The amount of H2O2 formed was analyzed by the UV absorption method, in which TiO(SO4)2 was used as the pigment. The amounts of gaseous H2 and O2 were measured with a TCD gas chromatograph (Shimadzu GC 8A). The selectivity of H2 to H2O2 was defined as the H2O2 formation rate divided by the H2 consumption rate. Samples for XPS measurement were prepared by drying the colloidal solution on a clean glass plate at 333 K and the commercial equipment (Shimadzu type 165). It was confirmed no change in surface composition with XPS and no elution of Pd and Au during the reaction over 5 h with ICP measurements. TEM observation was performed by using FEI Tecnai type G2 with 200 kV accelerated voltage and around 200 particles of Pd–Au colloid were used for estimating the particle size distribution.
Results and discussion
H2O2 synthesis at ambient pressure
Fig. 1 shows TEM images of the Pd–Au nano colloid obtained by using oxalic acid (a), hydrazine (b), and NaBH4 (c) as reductant. As shown in Fig. 1(a), Pd–Au colloid with a particle size of 1–7 nm was obtained when oxalic acid was used for the reductant. However, finer (1–4 nm) metal particles were obtained using hydrazine as the reductant (Fig. 1(b)). In contrast, slightly larger but similar size particles to those of oxalic acid preparation are obtained by using NaBH4 as reductant (Fig. 1(c)). The particles obtained by hydrazine reductant were uniform in the fine size area (1–4 nm), but some of particles are aggregated and so a bimodal distribution is observed. Fig. 1(d) shows the lattice image of the nano colloid obtained with oxalic acid reductant. Considering the lattice constant of Pd–Au particles (0.228 nm), which is intermediate between Pd and Au, Pd–Au alloy seems to form in the Pd–Au nano colloids prepared using both of these reductants. The surface composition of the obtained Pd–Au nano colloids was also analyzed quantitatively by XPS using a mixture of Pd and Au powders as a standard and the results are shown in Table 1. Note that the surface of the Pd–Au nano colloids is slightly rich in Pd compared with that of the bulk. Considering the clear lattice image is observed, it seems that Pd–Au nano colloid is a functionally graded structure. Such enrichment of Pd has also been reported for a Pd–Au bimetallic supported on TiO2 or Al2O3.28 Compared with the surface composition of the colloid obtained using the oxalic acid as reductant, that of the Pd–Au colloid obtained by the hydrazine reductant is slightly rich in Au. In contrast, in the case of NaBH4 reductant, the surface is enriched with Au. According to the quantum calculations,29,30 a surface composition of Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 3
Au = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is ideal for H2O2 synthesis by direct oxidation of H2. This is because dissociation of the O–O bond is prevented and desorption of H2O2 formed is accelerated with Au. Therefore, an excessively enriched composition with Au or Pd is not suitable for H2O2 synthesis.
1 is ideal for H2O2 synthesis by direct oxidation of H2. This is because dissociation of the O–O bond is prevented and desorption of H2O2 formed is accelerated with Au. Therefore, an excessively enriched composition with Au or Pd is not suitable for H2O2 synthesis.
 | ||
| Fig. 1 TEM images and particle distribution of Pd–Au nano colloids obtained using oxalic acid (a), hydrazine (b) and NaBH4 (c) as reductants. (d) A high resolution image of nano colloid obtained by oxalic acid. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) nano colloids on results of H2O2 synthesis by H2 oxidation and surface composition
25) nano colloids on results of H2O2 synthesis by H2 oxidation and surface composition
| Reductant | H2 Conversion/% | H2O2 Formation | Surface Pd amount/mol (%) | |||
|---|---|---|---|---|---|---|
| Rate/mmol h−1 | Productivity mmol/(h g-cat) | Selectivityb/% | Yield/% | |||
Pressure, atmospheric pressure. Glass reactor, flow rates: H2, 50 ml min−1; O2, 50 ml min−1; H2SO4, 0.368 M; NaCl, 84 mM; amount of Pd–Au catalyst, 23.9 mg; volume of water, 100 ml. Temperature: 283 K.a Pd–Au/rutile TiO2: Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 82 Au = 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 molar ratio (Pd–Au 25.5mg).b Hydrogen base value. 18 molar ratio (Pd–Au 25.5mg).b Hydrogen base value. |
||||||
| C2H2O4 | 6.1 | 5.96 | 249 | 99.6 | 6.1 | 0.84 |
| N2H4 | 7.7 | 7.58 | 317 | 78.5 | 6.1 | 0.79 |
| NaBH4 | 8.6 | 4.04 | 169 | 37.9 | 3.3 | 0.68 |
| Rutile-TiO2 supporteda | 2.9 | 3.60 | 141 | 99.7 | 2.9 | — |
The H2O2 formation rates, H2O2 concentrations for 2 h accumulation, H2O2 selectivity, and H2 conversion rates are summarized in Table 1 for the Pd–Au nano colloids prepared using three different reductants. For this experiment, we used a glass reactor and slightly larger amount of the calayst (23.9 mg) was used. In Table 1, the H2O2 synthesis results on rutile-TiO2-supported Pd–Au are also listed for comparison. Although the optimized composition of Pd–Au for H2O2 synthesis differed between the nano colloid and the TiO2-supported one, all Pd–Au nano colloid catalysts showed much higher H2O2 formation rates and also higher H2 conversion rates than the TiO2-supported one. Therefore, it is clear that a high H2O2 formation rate can be obtained using Pd–Au nano colloids. Moreover, H2O2 formation activity clearly varies markedly depending on the type of reductant used for the colloid preparation, despite the use of the same type and ratio of starting materials. H2O2 formation rate increases in the following order of reductants used: hydrazine > oxalic acid > NaBH4. Although the high selectivity is exhibited on Pd–Au nano colloid obtained by oxalic acid, H2O2 formation rate, namely, H2O2 yield, is important considering the difficulty in recycling the reactant when air is used for H2O2 synthesis. There are two possible reasons for the difference by reductant for the preparation of the colloid: one is a difference in particle size resulting in a difference in surface area and the other is a difference in surface composition. As shown in Fig. 1, smaller particles tend to be obtained by using hydrazine than oxalic acid. In addition, a slightly higher concentration of Au on the surface is also effective for suppressing H2O2 decomposition. However, in the case of NaBH4, Au concentration becomes excessively high, thereby decreasing the H2O2 formation rate. This is because Au has a low activity for H2O2 formation. Therefore, among those reductants examined, Pd–Au nano colloid prepared by using hydrazine has a high activity for H2O2 formation by H2 direct oxidation under the high H2 concentration condition, which is a problem for safety.
Although PVP was used as a dispersion agent for the Pd–Au nano colloid, it is expected that PVP is also effective for preventing H2O2 decomposition. Effects of PVP on H2O2 formation rate were further studied (Fig. 2) by using the nano colloid prepared by oxalic acid. Evidently, H2O2 formation rate as well as selectivity increased with increasing PVP concentration and saturated at a PVP concentration higher than 40 μM. Under these conditions, H2O2 selectivity is close to 100%. This suggests that surface PVP may also have a function of preventing the decomposition of formed H2O2. This is because the H2O2 synthesis catalyst is also an active H2O2 decomposition catalyst. In addition, a slight excess amount of PVP is also effective for preventing the aggregation of the nano colloid during the reaction. Since the saturated value was achieved and the mono dispersion of Pd–Au nano colloid is stably sustained under reaction conditions, a PVP concentration of 40 μM is used in the following part of this study.
 | ||
| Fig. 2 H2O2 formation rate as a function of polyvinylpyrrolidone (PVP) dispersion concentration. Reaction conditions: 283 K, 0.1 MPa, H2: 50 vol%, O2:5 0 vol%, total flow rate: 100 ml min−1. | ||
Fig. 3 shows H2O2 concentration as a function of reaction time for the Pd–Au nano colloid prepared by using the hydrazine reductant under various H2 and O2 concentrations at atmospheric pressure. A low concentration of H2O2 is the most significant drawbacks of the direct synthesis of H2O2 from H2 oxidation. In the most reports, H2O2 concentration is smaller than 0.5% when water is used as the solvent for catalyst suspension, while much higher H2O2 concentration is required for application of breaching etc., at least 5 wt%. The H2O2 formation rate increases with increasing H2 concentration, and the H2O2 accumulated amount is also strongly dependent on the H2/O2 ratio. H2O2 concentration tends to increase with decreasing H2/O2 ratio. At H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 = 1
O2 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, H2O2 concentration reaches a value as high as 2 wt.% in spite of atmospheric pressure and water for solvent. Since the H2O2 decomposition rate increases with increasing H2O2 concentration, accumulation of a high concentration of H2O2 means a low H2O2 decomposition rate. In reply to the improved decomposition rate of H2O2, the selectivity of H2O2 is high (ca. 80%) in the initial 2 h but decreased drastically with reaction period.
5, H2O2 concentration reaches a value as high as 2 wt.% in spite of atmospheric pressure and water for solvent. Since the H2O2 decomposition rate increases with increasing H2O2 concentration, accumulation of a high concentration of H2O2 means a low H2O2 decomposition rate. In reply to the improved decomposition rate of H2O2, the selectivity of H2O2 is high (ca. 80%) in the initial 2 h but decreased drastically with reaction period.
 | ||
| Fig. 3 H2O2 concentration as a function of reaction time for the Pd–Au nano colloid prepared by using the hydrazine reductant under various H2 and O2 concentrations. Reaction condition: 283 K, 0.1 MPa, total gas flow: 100 ml min−1 and no dilute gas. | ||
Fig. 4 shows the H2O2 formation rate, H2O2 yield and H2 conversion for the Pd–Au colloid prepared using the hydrazine reductant as a function of H2 concentration. Since the H2 concentration in Table 1 is high and an explosion problem is anticipated, a high H2O2 formation rate at diluted H2 concentration is strongly requested from safety issues. H2O2 formation rate monotonously decreased with decreasing H2 concentration (CH2) from 50 to 0.5 vol%, suggesting that reaction is mass transfer limited one. In contrast, H2 conversion increased with decreasing H2 concentration. Hence, the H2O2 formation rate and the H2 conversion rate showed the opposite dependency on hydrogen concentration (CH2). Since H2 conversion increased, H2O2 yield also increased with decreasing CH2. At a CH2 of 0.5 vol%, H2 conversion and H2O2 yield as high as 32% and 30% were achieved, even at atmospheric pressure. It is also noted that selectivity of H2O2 is as high as 93% at this CH2 and is always higher than 90%. Since the amount of hydrogen dissolved in water decreases with decreasing CH2, an increase in H2 conversion rate with decreasing CH2 is opposite to the expected result. In fact, in the case of Pd–Au/rutile TiO2, H2 conversion the H2O2 formation rate decreased with decreasing CH2. At present, the reason Pd–Au nano colloids exhibit a small dependency on hydrogen partial pressure (rH2O2 ∝ PH20.85) remains unclear comparing with that of TiO2 one (rH2O2 ∝ PH21.35); however, it seems that the high surface activity of Pd–Au nano colloids is related to such observations and that a high activity for hydrogen activation is sustained under a low hydrogen concentration.
 | ||
| Fig. 4 H2O2 formation rate, H2O2 yield and H2 conversion for the Pd–Au colloid prepared using the hydrazine reductant as a function of H2 concentration. Reaction condition: 283 K, 0.1 MPa, H2: X vol%, O2: 50 vol%, N2: 50 − X vol%. Total flow rate 100 ml min−1. | ||
H2O2 synthesis under pressurized conditions
Table 2 summarizes the results of H2O2 synthesis on a Pd–Au nano colloid catalyst prepared using the hydrazine or oxalic acid as reductant under 1 MPa pressurized conditions. For pressurization, the reactor was changed from a glass reactor to a stainless steel autoclave. In Table 2, comparison of H2O2 formation rate in a glass reactor and stainless reactor was also shown under atmospheric pressure. H2O2 formation rate and H2O2 selectivity decreased markedly at 0.1 MPa with the change of the reactor because of H2O2 decomposition on the stainless steel parts in the reactor, such as the paddle, and the formation of a much larger bubble of the reactant gas mixture. In fact, we confirmed that H2O2 decomposition occurred rapidly in an autoclave reactor under H2 flow. However, as shown in Table 2, an increase in reaction pressure is highly effective for improving the H2O2 formation rate because of the increased amount of hydrogen dissolved in water, and H2 conversion becomes higher than 95% at 283 K. In contrast, H2O2 selectivity decreased markedly with the change of the reactor (from 95 to 38%) and slightly with pressurization (from 38 to 33%). Since the oxidation of hydrogen by H2O2 occurs with increasing hydrogen concentration, decrease in H2O2 selectivity at 1 MPa could be explained by the improved H2O2 decomposition rate as a result of the increased amount of hydrogen in water. Because the H2 conversion is as high as 95% at 1 MPa on Pd–Au nano colloid, it is expected that a high H2O2 yield will be achieved by the suppression of H2O2 decomposition under pressurized conditions. On the other hand, a slightly higher selectivity is achieved on Pd–Au nano colloid obtained using the oxalic acid reductant at 1 MPa because of the low surface activity for H2O2 decomposition; thus a H2O2 yield higher than 40% is obtained at 1 MPa. At present, the reason why Pd–Au prepared with oxalic acid reductant shows slightly higher selectivity to H2O2 at 1 MPa is not clear, however, mono dispersion of metal particles seems to effectively work for synthesis of H2O2 selectively at high pressure. H2O2 synthesis on Pd–Au nano colloid obtained by oxalic acid under pressurized conditions was studied in detail in the following part of this study.| Catalyst | H2 Conversion/% | H2 Conversion/% | H2O2 Formation | |||
|---|---|---|---|---|---|---|
| Rate/mmol h−1 | Productivity mmol/(h g-cat) | Selectivitya/% | Yield/% | |||
Stainless steel autoclave reactor with PTFE inner vessel; Total flow rate: 100 ml min−1, flow rates of each gas: H2, 1.25 ml min−1; O2, 9.75 ml min−1; N2, 15 ml min−1; Ar, 24 ml min−1; amount of catalyst: 17.9 mg; volume of water, 75 ml. H2SO4: 0.368 M, NaCl: 84 mM Pd–Au(O): Pd–Au obtained using oxalic acid reductant Pd–Au(H): Pd–Au obtained using hydrazine reductant. Rutile TiO2 supported: Pd–Au (Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 82 Au = 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 molar ratio) supported on rutile TiO2.a H2O2 amount after 2-h reactionb Glass reactor; flow rates: H2, 2.5 ml min−1; O2, 19.5 ml min−1; Ar, 78 ml min−1; volume of water, 100 ml. 18 molar ratio) supported on rutile TiO2.a H2O2 amount after 2-h reactionb Glass reactor; flow rates: H2, 2.5 ml min−1; O2, 19.5 ml min−1; Ar, 78 ml min−1; volume of water, 100 ml. |
||||||
| Pd–Au(H)b | 0.1 | 30.7 | 1.53 | 92 | 95.4 | 29.0 |
| Pd–Au(H) | 0.1 | 60.2 | 0.67 | 54 | 37.5 | 22.6 |
| Pd–Au(H) | 1.0 | 96.6 | 0.98 | 78 | 33.1 | 32.0 |
| Pd–Au(O) | 1.0 | 84.4 | 1.24 | 92 | 48.0 | 32.9 |
| Rutile TiO2 supported | 1.0 | 54.2 | 0.92 | 64 | 56.0 | 30.3 |
Fig. 5 shows the effects of total flow rate on the formation rate, selectivity, and yield of H2O2 and also H2 conversion on Pd–Au nano colloid prepared by oxalic acid. Evidently, high H2 conversion which was close to 90% was exhibited on Pd–Au nano colloid and sustained up to the 233 ml min−1 examined. This suggests that the surface of Pd–Au nano colloid is highly active for H2 activation. With increasing flow rate, yield as well as selectivity was slightly decreased, however, the decrease is quite small. Therefore, H2O2 formation rate is monotonously increased with increasing total flow rate. At 233 ml min−1, the H2O2 formation rate achieved a value of 4.5 mmol h−1. Therefore, in the following part, we fixed 233 ml min−1 for total gas flow rate, which is the maximum flow rate for the flow controlling system used in this study.
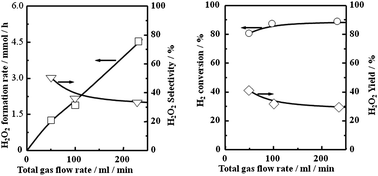 | ||
| Fig. 5 Effects of total gas flow rate on H2O2 formation rate, selectivity, yield and H2 conversion. Reaction condition; 283 K, H2 2.5%, O2 19.5%, N2 78%, 1.0 MPa, 800 rpm. | ||
The effects of reaction pressure on H2O2 synthesis were further studied for increasing H2O2 formation rate by using Pd–Au nano colloids prepared with oxalic acid as the reductant. Here it is also noted that in Fig. 6, we used air composition for oxygen, which is also industrially important. Since the concentration of H2 dissolved in water is small, mass transport of H2 from the gas to water phase tends to be a rate determining step and an increase in the dissolved amount of H2 into water seems to be effective for increasing the H2O2 formation rate. Fig. 6 shows the H2 conversion, H2O2 formation rate, and H2O2 yield as a function of reaction pressure. In this experiment, H2 partial pressure was fixed at 2.5%, which is lower than that of the explosion limit. Evidently, as expected, the H2O2 formation rate as well as H2 conversion monotonously increased with increasing total pressure and at 1 MPa, the H2O2 formation rate achieved a value of ca. 4.5 mmol h−1, which corresponds to 251 mmol h−1 g-cat−1. Comparing with the formation rate on Pd–Au/TiO2 (160 mmol h−1 g-cat−1 in 66% CH3OH solution) reported by Edwards et al.,31,32 H2O2 formation rate of 4.5 mmol g−1 achieved is higher even using H2O solution, however, for industrial application, H2O2 yield is still not high enough.
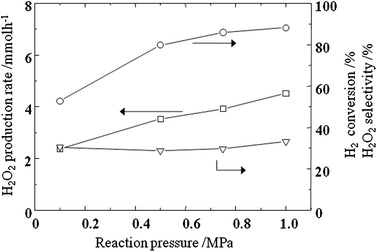 | ||
| Fig. 6 H2 conversion, H2O2 formation rate, and H2O2 yield as a function of reaction pressure. 283 K, H2 2.5 vol%, O2 19.5 vol%, N2 78 vol%, total flow rate: 233 ml min−1. | ||
Fig. 7 shows the H2O2 formation rate, H2O2 selectivity, and H2 conversion as a function of H2 partial pressure in feed gas. As discussed, the dissolved amount of H2 in water is an important factor for achieving high H2O2 yield, and so increase in H2 partial pressure seems to be positive effects on H2O2 formation rate. As expected, H2 formation rate monotonously increased with increasing H2 partial pressure and at 10% PH2, it achieved a value as high as ca. 17 mmol h−1. In contrast, selectivity to H2O2 slightly decreased with increasing H2 partial pressure because of the improved activity to H2O2 decomposition with increasing PH2. However, even at 10% PH2, selectivity to H2O2 is close to 40%, which is reasonably high comparing with that reported for a H2O2 direct synthesis catalyst. On the other hand, H2 conversion is also increased by increasing H2 partial pressure and at 10% H2, H2 conversion was close to 90%. Although the explosion limit of H2 is around 4.5%, 90% of H2 fed was converted and so on this Pd–Au nano colloid catalyst, it seems that H2 partial pressure of 10% can be used within an explosion limit. Under this condition, H2O2 production rate and H2O2 yield are as high as 531 mmoh h−1 g-cat−1 and 33%, respectively.
 | ||
| Fig. 7 H2O2 formation rate, H2O2 selectivity, and H2 conversion as a function of H2 partial pressure in feed gas. 283 K, H2: X vol%, O2: 0.2 × (100 − X) vol%, N2: 0.8 × (100 − X) vol%, total flow rate: 233 ml min−1. | ||
Fig. 8 shows the H2O2 concentration on Pd–Au colloid prepared with oxalic acid as a function of the reaction period. Another important issue for the direct synthesis method for H2O2 is the concentration of H2O2 accumulated as discussed because the reaction rate for H2O2 decomposition was much improved with increasing H2O2 concentration. As shown in Fig. 8, H2O2 concentration monotonously increased with reaction period at initial 20 h and achieved a value of ca. 2.6 wt%. However, after 20 h, concentration of H2O2 hardly increased with time and this suggests that the formation rate and decomposition rate of H2O2 was balanced because the decomposition reaction rate increased as the amount of H2O2 increases. Here it is noted that H2O2 concentration of 2.6 wt% is one of the highest values in water solvent reported in the open literature. Since the H2O2 accumulated concentration is determined by formation rate and decomposition rate of the formed H2O2. Therefore, by preventing H2O2 decomposition, much higher H2O2 concentration could be achieved.
 | ||
| Fig. 8 H2O2 concentration on Pd–Au colloid prepared with oxalic acid as a function of the reaction period. 283 K, H2: 2.5 vol%, O2: 19.5 vol%, N2: 78 vol%, total flow rate: 233 ml min−1. | ||
Since H2O2 decomposition could be suppressed by decreasing reaction temperature, temperature dependence of H2O2 formation rate was studied and Fig. 9 shows H2O2 formation rate, H2O2 selectivity and H2 conversion as a function of reaction temperature. Because of freezing point depression and exothermic reaction, direct synthesis of H2O2 was performed at 268 K without freezing of catalyst suspension. As expected, H2O2 formation rate and selectivity to H2O2 are monotonously increased with decreasing reaction temperature. At 268 K, H2O2 formation rate is achieved at 25 mmol h−1 and the selectivity is also higher than 54%. In addition, H2 conversion is almost independent of reaction temperature and so H2O2 yield achieved a value as high as 46%, which is the highest class value for yield reported considering water used for solvent and the average value over 2 h after reaction started. Under these conditions, H2O2 production rate is achieved to a value of 782.5 mmol h−1 g-cat−1, which is 5 times higher than that reported by Edwards et al.31,32 This high yield of H2O2 suggests that the primary product in H2 oxidation on Pd–Au nano colloid is H2O2 and preventing the formed H2O2 decomposition is highly important for achieving the high yield of H2O2. Because the surface activity of Pd–Au nano colloid for H2 activation is high, high H2 conversion e.g. 85% was still exhibited even at 268 K. Therefore, decrease in reaction temperature is highly effective for obtaining the high yield of H2O2. Here, it is also noted that one targeted yield for commercialization of the direct H2O2 synthesis method is 50% yield with one-pass reaction and so 46% H2O2 yield achieved in this study is highly interesting from the viewpoint of an industrial process.
 | ||
| Fig. 9 H2O2 formation rate, H2O2 selectivity and H2 conversion as a function of reaction temperature. Reaction condition: H2: 10 vol%, O2: 18 vol%, N2: 72 vol%, total flow rate: 233 ml min−1. | ||
Since a high yield of H2O2 is achieved, accumulation of H2O2 was further studied at 268 K. H2O2 concentration at 268 K was shown in Fig. 10 as a function of reaction period. The H2O2 formation rate is much improved and the H2O2 concentration also increased greatly, as shown in Fig. 10. After 10 h, the H2O2 concentration achieved a value of 5.8% and then it is saturated because the H2O2 decomposition rate is balanced with formation rate. It is said that one of the application areas of H2O2 is a bleaching process in paper and the required concentration of H2O2 is around 5%. Therefore, it is expected that 5.8% H2O2 concentration achieved in this study is reasonably high. In addition, we used water as a solvent for catalyst suspension, and so it is expected that aqueous H2O2 water can be obtained easily. Comparing the ethanol or methanol solvent, which usually leads to a high yield or concentration of H2O2, no separation process of formed H2O2 is required for the present reaction system and this is the great advantage for the use of aqueous solution.26,27 As discussed before, high H2O2 concentration is another important issue for direct synthesis of H2O2 from H2 and so Pd–Au nano colloid prepared by oxalic acid is highly useful for the synthesis of H2O2 with reasonably high concentration. In any way, Pd–Au nano colloid is highly active to H2O2 synthesis and a relatively high concentration of H2O2 can be obtained by direct oxidation of H2 with O2.
 | ||
| Fig. 10 H2O2 concentration at 268 K as a function of reaction period. Reaction condition: 268 K, H2: 10 vol%, O2: 18 vol%, N2: 72 vol%, total flow rate: 233 ml min−1. | ||
For H2O2 selective synthesis, suppression of H2O2 decomposition is important. A H2O2 synthesis catalyst is also active to H2O2 decomposition, in particular, under a H2 coexisting atmosphere. Decomposition activity of H2O2 on Pd–Au nano colloid was studied as a function of reaction temperature. Under the conditions used, simple decomposition of H2O2 into H2O is hardly observed and so the Pd–Au under addition of HCl and H2SO4 is almost inactive for the simple decomposition of H2O2. On the other hand, the H2O2 decomposition proceeds quickly when H2 is fed. Therefore, H2O2 decomposition mainly occurs by reaction with H2 and H2O2 (H2 + H2O2 = 2H2O), which is also reported by several groups.32,33Fig. 11 shows the H2O2 decomposition rate on Pd–Au prepared with oxalic acid as a function of reaction temperature. As expected, the H2O2 decomposition rate was monotonously decreased with decreasing reaction temperature and at 276 K, the decomposition rate is almost half of that at 283 K. Therefore, a much improved H2O2 yield or formation rate at 268 K can be explained by suppression of H2O2 decomposition with H2 remaining in reactor. Since H2O2 selectivity is slightly higher than 50%, direct oxidation of H2 into H2O (H2 + ½O2 = H2O) seems also to proceed simultaneously and more significantly with reaction period. Therefore, preventing the simple oxidation of H2 with O2 should be requested for achieving further higher H2O2 yield.
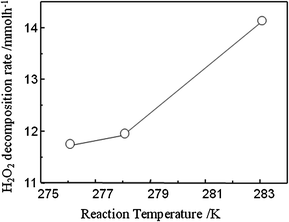 | ||
| Fig. 11 H2O2 decomposition rate on Pd–Au prepared with oxalic acid as a function of reaction temperature. Reaction condition: H2O2: 2 wt% (566.8 mM), H2: 2 vol%, N2: 98 vol%, total flow rate 233 ml min−1. | ||
In summary, this study revealed that Pd–Au nano colloid is highly active and selective for H2O2 synthesis by direct oxidation of H2 and that a high H2O2 yield of 46% and H2O2 concentration of 5.8% was achieved at 10 vol% H2, 1 MPa, 268 K.
Conclusions
Development of an active catalytic process for H2O2 synthesis from H2 is a highly important subject from green chemistry. Mono dispersed Pd–Au nano colloid was successfully prepared by using oxalic acid as reductant. Under atmospheric pressure, a slightly higher yield of H2O2 is obtained on Pd–Au nano colloid prepared by hydrazine as reductant because of finer particles. However, under pressurized conditions, Pd–Au nano colloid with mono dispersion seems to be important and so Pd–Au nano colloid prepared with oxalic acid shows higher H2O2 yield. The reaction mechanism for H2O2 formation on Pd–Au metal surface has been estimated with quantum chemistry calculations.29,30 From the theoretical approaches, H2O2 formation proceeds through hydrogenation of oxygen molecule and water forms dominantly when dissociation of oxygen molecules occurs. Addition of Au into Pd is effective for preventing O–O bond dissociation and also makes the desorption of H2O2 easier from the surface. Quantum calculations suggest that clusters consisting of Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 3
Au = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are highly important for H2O2 selective synthesis, and larger particles may not be active for this reaction because of the larger portion of Pd domain. Therefore, we think that Pd–Au nano colloid with a small size and mono dispersion is highly important for the synthesis of H2O2 selectively. The high activity of Pd–Au nano colloid prepared with oxalic acid may suggest that the highly disperse state of Pd–Au particle is important for a high yield of H2O2. Because H2O2 decomposition with H2 was suppressed by decreasing temperature and high H2 conversion is sustained, a much higher H2O2 yield of 46% and formation rate of 25 mmol h−1 were achieved at 268 K. Under these conditions, H2O2 concentration can be improved to 5.8 wt%. Cooling the reaction media lower than ambient temperature requires energy resulting in increased cost. Therefore, a reaction temperature slightly higher than room temperature is ideal for an industrial process for direct synthesis of H2O2 from H2. Since H2O2 formation rate drastically decreased with increasing reaction temperature, a reaction temperature slightly lower than ambient temperature is requested at present. We think that optimum reaction temperature may relate with added halogen for preventing H2O2 decomposition and so, further detailed study on optimizing the halogen compound added may lead to increased reaction temperature and this is now under investigation. The results will be reported in the future. Consequently, this study reveals that Pd–Au nano colloid with Pd
1 are highly important for H2O2 selective synthesis, and larger particles may not be active for this reaction because of the larger portion of Pd domain. Therefore, we think that Pd–Au nano colloid with a small size and mono dispersion is highly important for the synthesis of H2O2 selectively. The high activity of Pd–Au nano colloid prepared with oxalic acid may suggest that the highly disperse state of Pd–Au particle is important for a high yield of H2O2. Because H2O2 decomposition with H2 was suppressed by decreasing temperature and high H2 conversion is sustained, a much higher H2O2 yield of 46% and formation rate of 25 mmol h−1 were achieved at 268 K. Under these conditions, H2O2 concentration can be improved to 5.8 wt%. Cooling the reaction media lower than ambient temperature requires energy resulting in increased cost. Therefore, a reaction temperature slightly higher than room temperature is ideal for an industrial process for direct synthesis of H2O2 from H2. Since H2O2 formation rate drastically decreased with increasing reaction temperature, a reaction temperature slightly lower than ambient temperature is requested at present. We think that optimum reaction temperature may relate with added halogen for preventing H2O2 decomposition and so, further detailed study on optimizing the halogen compound added may lead to increased reaction temperature and this is now under investigation. The results will be reported in the future. Consequently, this study reveals that Pd–Au nano colloid with Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 3
Au = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is highly active for H2O2 synthesis by H2 oxidation.
1 is highly active for H2O2 synthesis by H2 oxidation.
Acknowledgements
This work was supported in part by an R&D grant for novel interdisciplinary fields based on nanotechnology and materials from MEXT.Notes and references
- K. Kamata, K. Yonehara, Y. Sumida and K. Yamaguchi, Science, 2003, 300, 964–966 CrossRef CAS.
- T. M. Kin, K. Markus, B. Santosh, D. Christian, A. Gopinathan, H. Herbert, M. Wolfgang and B. Matthias, Org. Lett., 2005, 7, 987–990 CrossRef.
- D. Bianchi, R. D’Aloisio, R. Bortolo and M. Ricci, Appl. Catal., A, 2007, 327, 295–299 CrossRef CAS.
- Y. Sawaki and Y. Ogata, Bull. Chem. Soc. Jpn., 1965, 38, 2103–2106 CrossRef CAS.
- N. Ullrich, B. Kolbe and N. Bredemeyer, ThyssenKrupp Techforum, 2007, 1, 38–43 Search PubMed.
- G. Goor, W. Kunkel and O. Weiberg, in Ulman’s Encyclopedia of Industrial Chemistry, WILEY-VCH, Weinheim, 1989, vol. A13, pp. 443–466 Search PubMed.
- J. M. Campos-Martin, G. Blanco-Brieva and J. L. G. Fierro, Angew. Chem., Int. Ed., 2006, 45, 6962–6984 CrossRef CAS.
- D. P. Dissanayake and J. H. Lunsford, J. Catal., 2002, 206, 173–176 CrossRef CAS.
- Y. F. Han and J. H. Lunsford, J. Catal., 2005, 230, 313–316 CrossRef CAS.
- Q. Liu and J. H. Lunsford, J. Catal., 2006, 239, 237–243 CrossRef CAS.
- Q. Liu and J. H. Lunsford, Appl. Catal., A, 2006, 314, 94–100 CrossRef CAS.
- J. K. Edwards, A. Thomas, B. E. Solsona, P. Landon, A. F. Carley and G. J. Hutchings, Catal. Today, 2007, 122, 397–402 CrossRef CAS.
- J. K. Edwards, B. E. Solsona, P. Landon, A. F. Carley, A. Herzing, C. J. Kiely and G. J. Hutchings, J. Catal., 2005, 236, 69–79 CrossRef CAS.
- P. Landon, P. J. Collier, A. F. Carley, D. Chadwick, A. J. Papworth, A. Burrows, C. J. Kiely and G. J. Hutchings, Phys. Chem. Chem. Phys., 2003, 5, 1917–1923 RSC.
- T. Ishihara, Y. Hata, Y. Nomura, K. Kaneko and H. Matsumoto, Chem. Lett., 2007, 36, 878–879 CrossRef CAS.
- G. B. Brieva, E. C. Serrano, J. M. C. Martin and J. L. G. Fierro, Chem. Commun., 2004, 1184–1185 RSC.
- C. Burato, P. Centomo, M. Rizzoli, A. Biffis, S. Campestrini and B. Corain, Adv. Synth. Catal., 2006, 348, 255–259 CrossRef CAS.
- K. P. Reis, V. K. Joshi and M. E. Thompson, J. Catal., 1996, 161, 62–67 CrossRef CAS.
- K. Kamata, K. Yonehara, Y. Sumida and K. Yamaguchi, Science, 2003, 300, 964–966 CrossRef CAS.
- V. V. Krishnan, A. G. Dokoutchaev and M. E. Thompson, J. Catal., 2000, 196, 366–374 CrossRef CAS.
- S. E. Park, L. Huang. C. W. Lee and J.-S. Chang, Catal. Today, 2000, 61, 117–122 CrossRef CAS.
- Q. Chen and E. J. Beckman, Green Chem., 2007, 9, 802–808 RSC.
- A. G. Gaikwad, S. D. Sansare and V. R. Choudhary, J. Mol. Catal. A: Chem., 2002, 181, 143–149 CrossRef CAS.
- S. Melada, R. Rioda, F. Menegazzo, F. Pinna and G. Strukul, J. Catal., 2006, 239, 422–430 CrossRef CAS.
- Y. Nomura, H. Matsumoto and T. Ishihara, ChemSusChem, 2008, 1(7), 619–621 CrossRef CAS.
- S. Park, D. R. Park, J. H. Choi, T. J. Kim, Y. M. Chung, S. H. Oh and I. K. Song, J. Mol. Catal. A: Chem., 2011, 336, 78–86 CrossRef CAS.
- R. Burch and P. R. Ellis, Appl. Catal., B, 2003, 42, 203–211 CrossRef CAS.
- A. A. Herzing, A. F. Carley, J. K. Edwards, G. J. Hutchings and C. J. Kiely, Chem. Mater., 2008, 20, 1492–1501 CrossRef CAS.
- A. Staykov, T. Kamachi, T. Ishihara and K. Yoshizawa, J. Phys. Chem. C, 2008, 112(49), 19501–19505 CAS.
- J. Li, A. Staykov, T. Ishihara and K. Yoshizawa, J. Phys. Chem. C, 2011, 115, 7392–7398 CAS.
- J. K. Edwards, B. Solsona, E. Ntainjua N, A. F. Carley, A. Herzing, C. J. Kiety and G. J. Hutchings, Science, 2009, 323, 1037–1041 CrossRef CAS.
- M. Piccinini, E. Ntainjuas N, J. F. Edwards, A. F. Carley, J. A. Moulijn and G. J. Hutching, Phys. Chem. Chem. Phys., 2010, 12, 2488–2492 RSC.
- T. Deguchi and M. Iwamoto, Ind. Eng. Chem. Res., 2010, 50, 4351–4358 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
