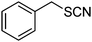
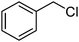
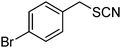
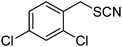
β-Cyclodextrin based polyurethane as eco-friendly polymeric phase transfer catalyst in nucleophilic substitution reactions of benzyl halides in water: An efficient route to synthesis of benzyl thiocyanates and acetates†
Ali Reza
Kiasat
* and
Simin
Nazari
Chemistry Department, Faculty of Sciences, Shahid Chamran University, Ahvaz, 61357-4-3169, Iran. E-mail: akiasat@scu.ac.i; Fax: +98 611 3738044; Tel: +98 611 3331042
First published on 17th February 2012
Abstract
β-Cyclodextrin, a cyclic sugar with a hydrophobic central cavity that has extensive applications in the pharmaceutical, food and biomedical industries, was polymerized with hexamethylene diisocyanate in dry dimethylformamide. Water-insoluble β-cyclodextrin-polyurethane (β-CDPU) polymer has demonstrated the ability to catalyst the nucleophilic substitution reaction of benzyl halides with thiocyanate and acetate anions in water. No evidence for the formation of by-product, for example isothiocyanate, was observed and the products were obtained in pure form without further purification. The polymeric phase transfer catalyst, easily recovered by simple filtration, shows no appreciable loss of activity when recycled several times.
Introduction
β-Cyclodextrin (βCD) is torus-shaped cyclic oligosaccharide composed of seven D-glucopyranose units connected by an α-(1,4)-linkage. This macromolecule possesses a characteristic toroidal shape with a well-defined lipophilic cavity and hydrophilic exterior that is suitable for the inclusion binding of appropriately sized guest compounds.1,2 This outstanding property has long been utilized in pharmaceutical, food, cosmetic and textile industries and has found applications in the field of catalysis, environmental remediation, chemical sensing, and enantiomeric separations.3–8The practical utility of βCD as a catalyst in aqueous media could be extended further if it can be rendered water insoluble. Immobilization of βCD on solid supports or its conversion to insoluble polymeric derivatives have been suggested as alternative ways of getting around the problems. Using these strategies, the recycling of βCD will be feasible and its discharge into the environment would be significantly minimized to non-harmful levels. Various methods for insoluble CD production and/or immobilization on solid supports have been successfully developed and used.8–10 Cyclodextrins when crosslinked with diisocyanates form an insoluble resin (cyclodextrin-polyurethane, β-CDPU) which exhibits specific adsorption based on inclusion complex formation and was successfully used for removal of organic pollutants and heavy metals in water and separation of enantiomers.11,12
Even though some anticancer natural products, drug candidates, synthetic intermediates, antiasthmatic drugs, biocidal compounds and insecticides possess thiocyanate or acetate functional groups, there are few reported practical synthetic routes in the literature for this class of compounds.13,14 By having this goal in minds and in continuation of our research on using water as a reaction medium,14–17 herein, we report the synthetic applicability of β-CDPU resin as polymeric phase transfer catalyst for the facile preparation of benzyl thiocyanates and acetates in water by nucleophilic substitution reaction.
Experimental
General
Benzyl halides and other chemical materials were purchased from Fluka and Merck and used without further purification. β-Cyclodextrin was heated at 80 °C under vacuum for 30 min before use to remove traces of moisture. Products were characterized by comparison of their physical data, IR and 1H & 13C NMR spectra with known samples. NMR spectra were recorded in CDCl3 on a Bruker Avance DPX 400 MHz instrument spectrometer using TMS as internal standard. IR spectra were recorded on a BOMEM MB-Series 1998 FT-IR spectrometer. The purity determination of the products and reaction monitoring were accomplished by TLC on silica gel polygram SILG/UV 254 plates.Preparation of β-cyclodextrin-polyurethane polymer
β-Cyclodextrin-polyurethane polymer (β-CDPU) was synthesized by reacting β-CD with hexamethylene diisocyanate (HMDI) in dry DMF according to Yilmaz et al.18 Briefly, two grams of β-CD (1.76 mmol) was dissolved in 15 mL of dry DMF in a 100 mL round bottom flask at room temperature. To the solution, 17.6 mmol of HMDI in 5 mL of dry DMF was added dropwise. Then the mixture was stirred at 70 °C for 3 h. The resin was filtered and washed with acetone several times. The polymer was dried under vacuum at 80 °C overnight.Typical procedure for the conversion of benzyl halides to the corresponding benzyl thiocyanates and acetates
To a mixture of the benzyl halide (1.0 mmol) and nucleophilic reagents (KSCN or NaOAc) (2 mmol) in water (5 ml), β-CDPU resin (0.2 g) was added. The suspension was magnetically stirred at 90 °C for the time shown in Table 1. After complete consumption of starting material as judged by TLC (using n-hexane/ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent), the insoluble polymeric catalyst was filtered off and the filtrate extracted with diethyl ether (2 × 10 mL). The organic phase was dried over calcium chloride, and evaporated in vacuo to give the corresponding product in 78–89% isolated yields.
1) as eluent), the insoluble polymeric catalyst was filtered off and the filtrate extracted with diethyl ether (2 × 10 mL). The organic phase was dried over calcium chloride, and evaporated in vacuo to give the corresponding product in 78–89% isolated yields.
Results and discussion
β-CD as a microvessel can be easily incorporated into cross linked polymeric forms using a variety of linker molecules. Hexamethylene diisocyanate, a widely used linker for fabrication of drug delivery vehicles with reportedly a very low degree of toxicity, was used in the present work as a linker and crosslinking agent. Water-insoluble β-cyclodextrin-polyurethane (β-CDPU) polymer was synthesized in the reaction depicted in Scheme 1.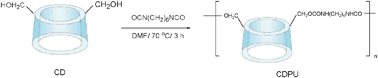 | ||
| Scheme 1 Synthesis of β-CD based polymer. | ||
The polymerization reaction was confirmed by IR analysis. The FTIR spectrum of the polymer show characteristic adsorption bands at 3360 and 1718 cm−1 correspond to NH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. The NHCO stretching was also observed at 1570 cm−1. In addition, the absence of a peak at 2280 cm−1 (corresponding to isocyanate group) indicate that polymerization did take place.
O groups. The NHCO stretching was also observed at 1570 cm−1. In addition, the absence of a peak at 2280 cm−1 (corresponding to isocyanate group) indicate that polymerization did take place.
In order to investigate the possible catalytic properties of β-CDPU resin in the nucleophilic substitution reaction, the reaction of benzyl halides with thiocyanate anion in water were chosen. Initially, the mixture of benzyl chloride and KSCN in water was chosen as the model reaction to determine whether the use of the polymeric phase transfer catalyst was efficient and to investigate the optimal conditions. After some experiments, it was found that the use of 2 equiv. of KSCN per benzyl chloride in the presence of β-CDPU (0.2 g) in water were the best conditions and after stirring for 10 min at 90 °C, the clean formation of a product with lower Rf value was observed. It should be pointed out that in the absence of β-CDPU, the reaction was sluggish and even after prolonged reaction time a considerable amount of starting material was remained. Moreover, the reaction mixture was contaminated with benzyl alcohol.
The success of the conversion of benzyl chloride to benzyl thiocyanate using β-CDPU as a polymeric phase transfer catalyst encouraged us to expand the scope of the reaction to other benzyl halides (Table 1). In all cases, a very clean reaction was observed. It is noteworthy that no evidence for the formation of by-products such as alcohols or isothiocyanates were observed and the products obtained in pure form without further purification. 13C resonance of the SCN and NCS groups at ∼111 and ∼145 ppm, respectively, are very characteristic for thiocyanate and isothiocyanate functionalities.14
It is worthy to note that β-CDPU resin does not suffer from extensive mechanical degradation after operating and could be quantitatively recovered by simple filtration and washing with water and methanol. The recovered resin has been reused three times for the nucleophilic substitution reaction of benzyl chloride with thiocyanate anion. The results clearly showed that the catalyst does not show any loss in its activity and produced benzyl thiocyanate in 87%, 84% and 85% yield, respectively.
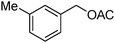
With this promising result in hand and establishing the advantages of β-CDPU polymer as polymeric phase transfer catalyst, we focused our attention in the another nucleophilic substitution reaction, conversion of benzyl halides to the corresponding acetate (Scheme 2).
 | ||
| Scheme 2 Preparation of benzyl acetates catalyzed by β-CDPU. | ||
Table 2 summarizes the data and clearly shown that the desired products, benzyl acetates, were formed in good isolated yields and no side products were observed. It is worthy to note that this method is not suitable for aliphatic alkyl halides. In this case, the reaction was sluggish, and even after prolonged reaction time a considerable amount of starting material was remained. Moreover, the reaction mixture was contaminated with alcohol. This observation was confirmed with the presence of alcohol on the TLC plate.
The structures of all of the benzyl thiocyanate and acetate products were determined from their analytical and spectral (IR, 1H & 13C NMR) data and by direct comparison with authentic samples.
The high reaction rate observed in nucleophilic substitution reaction could be attributed to the fact that, in water, hydrophobic central cavities of β-CD units in the β-CDPU polymer act as microvessels and accommodate nonpolar benzyl halides. In addition the hydrophilic exterior due to the outer OH of the β-CD cavity formed complexes with cations and these complexes cause the anion to be activated (Scheme 3).
 | ||
| Scheme 3 Postulated roles of β-CDPU in the nucleophilic substitution reaction of benzyl halides. | ||
Conclusions
The data as a whole show that β-CDPU, which combines easy availability and good stability with excellent complexing properties and high catalytic activity, represents a valid alternative to the more sophisticated crown ethers as catalysts in solid–liquid phase-transfer reactions. In conclusion, we have developed an easy to operate, safe and cost-effective method for the preparation of benzyl thiocyanates and acetates in water by nucleophilic substitution.Acknowledgements
We are grateful to the Research Council of Shahid Chamran University for financial support.Notes and references
- A. R. Kiasat and S. Sayyahi, Catal. Commun., 2010, 11, 484–486 CrossRef CAS.
- L. A. Wessjohann, Mol. Divers., 2005, 9, 171–186 CrossRef CAS.
- C. A. Kozlowski and W. Sliwa, Carbohyd. Polym., 2008, 74, 1–9 CrossRef CAS.
- D. V. Emm, Process Biochem., 2004, 39, 1033–1046 CrossRef.
- J. Szejtli, Chem. Rev., 1998, 98, 1743–1754 CrossRef CAS.
- J. Szejtli, J. Mater. Chem., 1997, 7, 575–587 RSC.
- K. Uekama, F. Hirayama and T. Irie, Chem. Rev., 1998, 98, 2045–2076 CrossRef CAS.
- D. Zhao and L. Zhao, Carbohyd. Polym., 2009, 78, 125–130 CrossRef CAS.
- I. Velaz, J. R. Isasi, M. Sanchez, M. Uzqueda and G. Ponchel, J. Incl. Phenom. Macro., 2007, 57, 65–68 CrossRef CAS.
- G. Mocanu, D. Vizitiu and A. Carpov, J. Bioact. Compat. Polym., 2001, 16, 315–342 CrossRef CAS.
- M. Bhaskar, Anal. Chim. Acta, 2004, 509, 39–45 CrossRef CAS.
- M. A. Esmaeili and R. Yazdanparast, Protein J., 2008, 27, 334–342 CrossRef CAS.
- A. R. Kiasat and M. Fallah-Mehrjardi, Bull. Korean Chem. Soc., 2008, 29, 2346–2348 CrossRef CAS.
- A. R. Kiasat, R. Badri and S. Sayyahi, Chin. Chem. Lett., 2008, 19, 1301–1304 CrossRef CAS.
- A. R. Kiasat and M. Fallah-Mehrjardi, Catal. Commun., 2008, 9, 1497–1500 CrossRef CAS.
- A. R. Kiasat, R. Badri, B. Zargar and S. Sayyahi, J. Org. Chem., 2008, 73, 8382–8385 CrossRef CAS.
- R. Kiasat and S. Sayyahi, Mol. Divers., 2010, 14, 155–158 CrossRef.
- E. Y. Ozmen, M. Sezgin and M. Yilmaz, J. Mol. Catal. B: Enzym., 2009, 57, 109–11 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c2cy00375a |
| This journal is © The Royal Society of Chemistry 2012 |