Synthesis of high-quality I–III–VI semiconductor supported Au particles and their catalytic performance†
Xiao
Wang
,
Dapeng
Liu
,
Shuyan
Song
* and
Hongjie
Zhang
*
State Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 5625 Renmin Street, Changchun, 130022, P. R. China. E-mail: songsy@ciac.jl.cn; hongjie@ciac.jl.cn; Fax: +86-431-85698041; Fax: +86-431-85685653
First published on 12th January 2012
Abstract
High-quality I–III–VI semiconductor supported Au particles have first been successfully synthesized via a simple seeded growth approach. Compared with the catalytic performance of the two obtained hybrids of Au–AgInS2 and –CuInS2, it is found that sample Au–AgInS2 shows higher photocatalytic activity towards the reduction of 4-nitrophenol by NaBH4. However, sample Au–CuInS2 exhibited a much better performance on oxidation of 3,3,5,5-tetramethylbenzidine di-hydrochloride in the presence of H2O2, indicating their strong composition-related catalytic properties.
Owing to excellent catalytic performance, noble metal Au catalysts have been paid much more attention in past decades.1 It has been identified that decreasing particle size is an efficient way to improve the catalytic activity of Au catalysts, and the ultrasmall sized Au particles could be supported on the binary semiconductors to achieve hetero-structures. Thus obtained hybrid nanocrystals (HNCs), such as Au-CdSe, Au-PbS, and Au-CdS etc.2–14 are usually prepared via the “seeded growth” strategy, and they have shown better electro-optical properties and greatly enhanced photocatalytic activities due to energy transfer that has been clearly observed between the two components of Au and semiconductors.15,16 The band gap of the binary semiconductors can be tuned by their nanocrystal size due to quantum confinement, and consequently the HNCs will show tunable optical, electrical and catalytic properties.
In comparison with those binary ones, the I–III–VI ternary semiconductors have more abundant varieties of compositions, thus their physico-chemical properties can also be tuned by changing one or two of the compositions besides the nanocrystal size and shape.17 They absorb lights more efficiently in the much broader wavelength range, which make them exhibit amazing photocatalytic performance under visible and even near IR light irradiation. Moreover the lattice of the I–III–VI semiconductors in cubic phase matches well with that of Au, which benefits them forming hybrid structures with Au. So if the I–III–VI semiconductor supported Au particles were achieved, the enhanced photocatalytic capability under sunlight and their composition-related catalytic properties should be the most desired.
In this study we have successfully synthesized two kinds of high-quality I–III–VI semiconductor supported Au particles (Au–CuInS2, and Au–AgInS2, dubbed as Au-CIS, and Au-AIS, respectively) via a rationally designed seeded growth approach. To achieve high loading of Au particles, the polyhedric CIS nanodisks are first chosen as seeds because their abundant high-energy edges and corners in polyhedrons facilitate further growth of Au components more effectively. Experimentally, the CIS nanodisks were prepared according to the Korgel’s report with only minor revision18 (for details see ESI†). Then the obtained nanodisks were mixed with 5 mL of the HAuCl4/oleylamine solution (0.01 mmol mL−1) with vigorous stirring for at least 12 h at room temperature. The final products were washed several times, and further isolated by centrifugation. For preparing AIS polyhedrons the process was kept in the same case with that of CIS except for the usage of AgNO3 instead of CuCl.
The transmission electron microscope (TEM) images in Fig. 1A indicate that the as-prepared CIS nanodisks are monodisperse with an average diameter of about 20 nm, and show self-assembly behavior. It can be seen in Fig. 1B that after the seeded growth process for 12 h the Au particles have grown on the surface of the CIS nanodisks to form Au-CIS HNCs, in which the CIS nanodisks remained their original size and shape. The Au particles of about 2 nm with a narrow size distribution are evenly dispersed on the HNCs. No isolated Au particles could be observed from TEM images, indicating the absence of independent nucleation of Au. The HRTEM images (Fig. 1C and D) show that both the CIS and AIS nanodisks are of good crystallinity in hexagonal crystal phases, which could be also identified by the X-ray diffraction (XRD) patters (Fig. S1, ESI†).‡ In the case of AIS seen in Fig. 1D, the only difference from CIS is that they have a smaller average size of 16 nm. The size of Au particles are also around 2 nm. ICP analysis has been taken to further identify the compositions of the two as-prepared hybrids to show that the final loading amount of Au is consistent with the initial dosage.
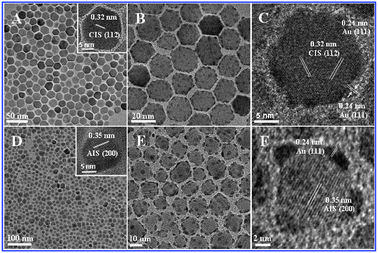 | ||
| Fig. 1 TEM images of CIS (A) and AIS (D) nanodisks, Au-CIS (B and C) and Au-AIS (E and F) HNCs. | ||
To get detailed growth profiles, here the CIS was taken as an example. First we picked samples acquired from the reaction system at different reaction intervals of 4 h, 8 h, and 12 h. The three samples were all harvested by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, and then redispersed in toluene for further characterization. It has been found in their TEM images (Fig. S5, ESI†) that Au particles prefer growing on the corners and edges of the CIS nanodisks at the initial 4 h. Generally it is difficult for oleylamine to reduce Au3+ ions at room temperature, so the growth of Au particles becomes quite slow that the whole growth process even needs a few days. However, the presence of semiconductors can accelerate the reduction of Au3+ ions, because free electrons existing in semiconductors are beneficial for the nucleation of Au.19 As a result, Au particles will prefer growing on the more active surface sites of semiconductors with higher free electron densities. After reaction for 8 h, Au particles began to occur on the facets of CIS nanodisks. It is important to point that from 4 h to 8 h the size and shape of Au particles stayed the same except for their growth positions and total numbers. In other words, if the edges and corners were fully occupied, Au particles should then grow on the facets of the nanodisks, so growth might occur in the following order: corners > edges > facets, staying consistent with their surface energies from high to low. When the amount of HAuCl4 was changed from 0.03 to 0.10 mmol in the experiments, the size of Au particles would increase from 1.7 nm to 2.4 nm (Fig. S6, ESI†). However the numbers of Au particles either on the corners, the edges, or the facets have almost no obvious changes by counting over 200 particles. That means the amount of active growth sites on the CIS nanodisks decides the numbers of Au particles in the HNCs. More importantly, the nucleation of Au needs to overcome higher energy barriers, so Au will prefer to grow on the CIS nanodisks with lower energy cost. That is why isolated Au particles could not be found in the samples but appeared when the amount of HAuCl4 added was more than 0.05 mmol.
000 rpm for 10 min, and then redispersed in toluene for further characterization. It has been found in their TEM images (Fig. S5, ESI†) that Au particles prefer growing on the corners and edges of the CIS nanodisks at the initial 4 h. Generally it is difficult for oleylamine to reduce Au3+ ions at room temperature, so the growth of Au particles becomes quite slow that the whole growth process even needs a few days. However, the presence of semiconductors can accelerate the reduction of Au3+ ions, because free electrons existing in semiconductors are beneficial for the nucleation of Au.19 As a result, Au particles will prefer growing on the more active surface sites of semiconductors with higher free electron densities. After reaction for 8 h, Au particles began to occur on the facets of CIS nanodisks. It is important to point that from 4 h to 8 h the size and shape of Au particles stayed the same except for their growth positions and total numbers. In other words, if the edges and corners were fully occupied, Au particles should then grow on the facets of the nanodisks, so growth might occur in the following order: corners > edges > facets, staying consistent with their surface energies from high to low. When the amount of HAuCl4 was changed from 0.03 to 0.10 mmol in the experiments, the size of Au particles would increase from 1.7 nm to 2.4 nm (Fig. S6, ESI†). However the numbers of Au particles either on the corners, the edges, or the facets have almost no obvious changes by counting over 200 particles. That means the amount of active growth sites on the CIS nanodisks decides the numbers of Au particles in the HNCs. More importantly, the nucleation of Au needs to overcome higher energy barriers, so Au will prefer to grow on the CIS nanodisks with lower energy cost. That is why isolated Au particles could not be found in the samples but appeared when the amount of HAuCl4 added was more than 0.05 mmol.
The electrons will be drawn from the semiconductors to the polar interface of Au and the semiconductor after forming hybrids, and thus the obtained HNCs could show enhanced photocatalytic performance. The model reaction of the reduction of 4-nitrophenol (4-NP) by NaBH4 has been chosen to evaluate the as-prepared Au-CIS and Au-AIS catalysts first. In this reaction NaBH4 was used in large excess to make sure the process followed a pseudo-first-order reaction. The results show both pure CIS and AIS nanodisks have very weak catalytic activities. After 30 min, less than 5% of 4-NP had been reduced by NaBH4. In addition, another control experiment has been performed to test the catalytic capability of pure Au NPs with similar particle sizes of 1–2 nm. The previous studies have confirmed that such a small Au particle was hard to obtain by simple protection of oleylamine.20,21 A key factor of our system is the hard template effect of CIS and AIS NPs which could stabilize the ultrasmall Au NPs efficiently. Therefore, the pure Au NPs were obtained from CIS-Au after treatment with hydrochloric acid (Fig. S7, ESI†). The pure Au catalysts only exhibited much lower catalytic performance with a TOF (turnover frequency) value of 680 h−1. The lack of supporting material might be the main reason for the poor catalytic activity. However in the presence of even a trace amount of Au-AIS catalyst, the reduction suddenly accelerates. For instance, if 40 μL of Au-AIS catalysts were added, the reaction could be finished in 40 s under sunlight (inset of Fig. 2A). Meanwhile the bright yellow solution completely faded to colorless, and there was no characteristic absorption of 4-NP that could be detected at 400 nm. In Fig. 2A for both Au-CIS and Au-AIS it shows a strong liner relationships between ln(Ct/C0) and time, where C is the concentration of 4-NP and t is the reaction time. The kinetic constant (k) was calculated by the equation of ln(Ct/C0) = kt.22 The turnover frequency (TOF), which is defined as the number of moles of reduced 4-NP per mole surface Au atoms per hour when the conversion has reached 90%. Compared with the highest TOF (1363 h−1) of Au catalysts reported by Yin's group,22 the Au-CIS catalyst shows a lower value of about 1080 h−1, however the TOF reaches to 1740 h−1 for Au-AIS. When the reactions were conducted in the dark, the TOFs suddenly decreased to be about 910 h−1 for Au-CIS and 1450 h−1 for Au-AIS. This proves that under light irradiation photo-generated electrons of semiconductors might be captured by Au, and the enrichment of electrons might be more helpful for the above reduction, and hence the catalytic activities of Au particles are greatly enhanced.15,16
 | ||
| Fig. 2 Catalytic performance of the two Au catalysts on (A) reduction of nitrophenol into aminophenol by NaBH4; (B) oxidation of TMB in the presence of H2O2 molecules. | ||
Besides the catalytic reduction of 4-NP, the two Au catalysts also employed to catalyze the peroxide-like oxidation of 3,3,5,5-tetramethylbenzidine di-hydrochloride (TMB) in the presence of H2O2 (for details see ESI†). Previously, peroxide-like catalysis has been observed in the case of Fe3O4,23 FeS,24 CeO225 and some kinds of noble metals and their alloys.26,27 In this reaction we chose the buffer solution of pH = 4.5, and the reaction temperature of 40 °C. The initial oxidation rate is evaluated by monitoring the absorbance increase of the oxidized products of TMB at 652 nm referring to the reports before.27 It demonstrates in Fig. 2B that neither the pure AIS nanodisks nor the Au-AIS HNCs have activity for the reaction, however all of the pure CIS nanodisks, Au NPs and Au-CIS HNCs exhibit quite high catalytic oxidation activity. In addition, unlike the reduction of 4-NP, sunlight irradiation has no influence on the catalytic performance. Obviously the catalytic activity must depend on the semiconductor compositions containing univalent copper rather than the Au particles, because after the reaction the black precipitation forms immediately while even in the presence of only a drop of sodium diethyldithiocarbamate aqueous solution, a very good indicator of Cu2+ ions. That means that Cu+ plays a key role in the TMB oxidation.
In summary, we have demonstrated a facile way to synthesize high-quality I–III–VI semiconductor supported Au HNCs. It is found that Au particles prefer growing on CIS and AIS nanodisks with lower energy costs rather than independently nucleating until the amount of HAuCl4 is more than 0.05 mmol. Growth occurs in the following order: corners > edges > facets, staying consistent with their surface energies from high to low. In the reduction of 4-NP both the two Au catalysts of Au-CIS and Au-AIS have shown enhanced photocatalytic performance. However for the oxidation of TMB, only the two Cu+ containing CIS and Au-CIS samples have good activities, indicating the composition-related catalytic properties of I–III–VI semiconductors. It will guide us to rationally design the new type of HNCs with enhanced photocatalytic performance and also composition-dependent properties for future applications.
The authors are grateful for the financial aid from the National Natural Science Foundation of China (Grant No. 21071140 and 21001101) and the National Natural Science Foundation for Creative Research Group (Grant No. 20921002).
Notes and references
- M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS.
- G. Menagen, A. Salant, I. Popov, D. Dorfs and U. Banin, Chem. Mater., 2008, 20, 6900 CrossRef CAS.
- R. Paiva and R. D. Felice, ACS Nano, 2008, 2, 2225 CrossRef.
- S. E. Habas, P. D. Yang and T. Mokar, J. Am. Chem. Soc., 2008, 130, 3294 CrossRef CAS.
- R. Costi, G. Cohen, A. Salant, E. Rabani and U. Banin, Nano Lett., 2009, 9, 2031 CrossRef CAS.
- E. Shaviv and U. Banin, ACS Nano, 2010, 4, 1529 CrossRef CAS.
- S. Chakrabortty, J. A. Yang, Y. M. Tan, N. Mishra and Y. Chan, Angew. Chem. Int. Ed., 2010, 49, 2888 CAS.
- X. Wang, X. G. Kong, Y. Yu and H. Zhang, J. Phys. Chem. C, 2007, 111, 3836 CAS.
- Y. C. Liu, M. Y. Zhong, G. Y. Shan, Y. J. Li, B. Q. Huang and G. L. Yang, J. Phys. Chem. B, 2008, 112, 6484 CrossRef CAS.
- J. S. Lee, E. V. Shevchenko and D. V. Talapin, J. Am. Chem. Soc., 2008, 130, 9673 CrossRef CAS.
- J. Yang, H. I. Elim, Q. B. Zhang, J. Y. Lee and W. Ji, J. Am. Chem. Soc., 2006, 128, 11921 CrossRef CAS.
- W. L. Shi, H. Zeng, Y. Sahoo, T. Y. Ohulchanskyy, Y. Ding, Z. L. Wang, M. Swihart and P. N. Prasad, Nano Lett., 2006, 6, 875 CrossRef CAS.
- Z. K. Zheng, B. B. Huang, X. Y. Qin, X. Y. Zhang, Y. Dai and M. Whangbo, J. Mater. Chem., 2011, 21, 9079 RSC.
- H. F. Chen, B. B. Huang, P. Wang, Z. Y. Wang, Z. Z. Lou, J. P. Wang, X. Y. Qin, X. Y. Zhang and Y. Dai, Chem. Commun., 2011, 47, 7054 RSC.
- E. Elmalem, A. E. Saunders, R. Costi, A. Salant and U. Banin, Adv. Mater., 2008, 20, 4312 CrossRef CAS.
- R. Costi, A. E. Saunders, E. Elmalem, A. Salant and U. Banin, Nano Lett., 2008, 8, 637 CrossRef CAS.
- I. Tsuji, H. Kato, H. Kobayashi and A. Kudo, J. Am. Chem. Soc., 2004, 126, 13406 CrossRef CAS.
- B. Koo, P. N. Patel and B. A. Korgel, Chem. Mater., 2009, 21, 1962 CrossRef CAS.
- J. Yang, L. Levina, E. H. Sargent and S. O. Kalley, J. Mater. Chem., 2006, 16, 4025 RSC.
- S. Y. Song, R. X. Liu, Y. Zhang, J. Feng, D. P. Liu, X. Yan, F. Y. Zhao and H. J. Zhang, Chem.–Eur. J., 2010, 16, 6251 CrossRef CAS.
- P. Li, Z. Wei, T. Wu, Q. Peng and Y. D. Li, J. Am. Chem. Soc., 2011, 133, 5660 CrossRef CAS.
- J. P. Ge, Q. Zhang, T. R. Zhang and Y. D. Yin, Angew. Chem., Int. Ed., 2008, 47, 8924 CrossRef CAS.
- S. H. Liu, F. Lu, R. M. Xing and J. J. Zhu, Chem.–Eur. J., 2011, 2, 620 CrossRef.
- A. Asati, S. Santra, C. Kaittanis, S. Nath and J. M. Perez, Angew. Chem. Int. Ed., 2009, 48, 2308 CAS.
- Z. Dai, S. Liu, J. Bao and H. Ju, Chem.–Eur. J., 2009, 15, 4321 CrossRef CAS.
- W. W. He, X. C. Wu, J. B. Liu, K. Zhang, W. G. Chu, L. L. Feng, X. N. Hu, W. Y. Zhou and S. S. Xie, Langmuir, 2010, 26, 4443 CrossRef CAS.
- W. W. He, X. C. Wu, J. B. Liu, X. N. Hu, K. Zhang, S. Hou, W. Y. Zhou and S. S. Xie, Chem. Mater., 2010, 22, 2988 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedure, XRD, ICP analysis of as-prepared products, further TEM images of products at lower magnification, TEM images of growth mechanism of Au-CIS . See DOI: 10.1039/c2cy00372d |
| ‡ The XRD patterns were recorded on a Rigaku X-ray diffractometer (D/max-2550 with Cu-Kα radiation, λ = 1.5418 Å. UV-vis absorption measurements were carried out using a Shimadzu UV-3600 Spectrophotometer. The TEM images were recorded with a Philips TF-F20 transmission electron microscope operating at 200 kV. ICP-MS analyses were determined using an inductively coupled plasma-atomic emission spectrometer (ICP-MS, XSeriesII, ThermoScientific, USA). |
| This journal is © The Royal Society of Chemistry 2012 |
