Transfer hydrogenation of carbonyl compounds and carbon–carbon multiple bonds by zeolite supported Cu nanoparticles†
Thirumeni
Subramanian
and
Kasi
Pitchumani
*
School of Chemistry, Madurai Kamaraj University, Madurai 625021, India. E-mail: pit12399@yahoo.com; Fax: +91-0452-2459181
First published on 1st December 2011
Abstract
Efficient reduction of carbonyl compounds and C–C multiple bonds to the corresponding reduced products with 2-propanol as a green reducing agent is achieved by highly active copper nanoparticles supported on a zeolite framework. These nanoparticles are stable in a zeolite framework and are recycled with good catalytic activity in the absence of base. The synthesized zeolite supported Cu nanoparticles were characterized using UV-DRS, XRD, XPS and HR-TEM.
Introduction
In the current trend towards more economically and environmentally sustainable processes, porous materials like zeolites are emerging as promising catalysts in chemical processes relevant to the fine chemicals and pharmaceutical industry.1–3 Crystalline zeolites with pore sizes typically at 0.4–1.2 nm are among the most useful catalysts in industrial processes such as oil refining and organic synthesis, due to their large surface area, high adsorption capacity, hydrothermal stabilities and well defined micro pores with excellent shape selectivity in catalysis.4–7Zeolite-Y and FAU-type zeolites are considered to be suitable hosts providing highly ordered larger cavities and can be used for the assembly of metal and metal oxide nanoclusters as the pore size could limit the growth of nanoparticles and prevents further aggregation of nanoparticles.8 This class of heterogeneous catalysts are proved easy to prepare, easy to handle and recyclable. In recent years, work in the field of metal nanoparticles as catalysts in synthetic organic chemistry has gained much attention9–11 and metal catalysts on heterogeneous supports emerged as active and selective catalysts for a broad array of organic transformations including chemoselective reduction of unsaturated carbonyl compounds.12 Among the different metal nanoparticles, Cu nanoparticles have received considerable attention because of their unusual properties, potential applications in diverse fields and have been utilized for carbon–carbon bond formation,13 click chemistry,14–17Mannich reaction,18etc.The reduction of carbonyl compounds to the corresponding alcohols is an important transformation in the synthesis of biologically active compounds.19,20 Recently Alonso and Yus et al. had reported nickel nanoparticles catalyzed hydrogen transfer reduction of carbonyl compounds under base free conditions.21,22 A variety of reducing systems are available to carry out reductions, which include Au/TiO2,23RhCl(PPh3)3,24RuCl(CNN)(dppb),25[Ir(cod)Cl]2,26Ni-MCM-4127 and Pt/C.28,29 However, these methods suffer from drawbacks such as highly sensitive or pyrophoric nature of the reagents, toxicity, cost of metal catalyst and these aspects create the demand for more sustainable methods.30 For example RANEY®-Ni catalyst is flammable and presents hazards during handling. It is therefore worthwhile to develop new green catalysts that would enhance the reaction rate. Copper metal and Cu nanoparticles are more acceptable to the environment and copper appears as a potential alternative to the above expensive transition metal catalyst and very less studied in hydrogen transfer reactions with 2-propanol. In this study, we have presented a novel protocol for the chemoselective reduction of aromatic and aliphatic carbonyl compounds by transfer hydrogenation with a sacrificial donor 2-propanol in the presence of zeolite supported copper nanoparticles in high yields.
Reduction of C–C multiple bonds is important for the manufacture of fragrances and other fine chemicals. Recently Naota et al. had reported aerobic transfer hydrogenation of olefins by neutral flavins31 and Garcia et al. had reported selective reduction of C–C multiple bonds by Metal–Organic Frameworks (MOFs) as heterogeneous catalysts.32 These systems involve hydrazine hydrate as a reducing agent since the hydrazine hydrate is a more powerful and hazardous source. A variety of other reagents such as sodium borohydride,40thiourea dioxide33 and ammonium formate34 have also been employed to achieve carbonyl compounds transformation. Despite the fact that a plethora of reducing agents is available for this operation, green reagents especially the catalytic versions are still highly desirable. 2-Propanol is a very popular hydrogen donor since it is less expensive, non-toxic, volatile, possesses good solvent properties and it is readily transformed into acetone, which is environmentally friendly and easy to remove from the reaction system.39
Our interest in development of heterogeneous catalyst supports35,36 enjoys advantages such as fast and simple isolation of the reaction products by filtration, recyclability and minimization of metallic waste. Our studies focusing on use of inexpensive and green reagents for organic transformations37 prompted us to report, for the first time, the use of Cu nanoparticles which can effectively catalyse the heterogeneous transfer hydrogenation of C–C multiple bonds using 2-propanol as the hydrogen donor. 2-Propanol was found to provide the best results in terms of yields and reaction time compared to other hydrogen sources.
In this paper, we have presented a highly efficient and expeditious protocol for the chemoselective reduction of aromatic and aliphatic carbonyl compounds and C–C multiple bonds by the remarkable efficiency of zeolite supported Cu nanoparticles along with 2-propanol. Our selective and active hydrogenation catalyst can replace some common heterogeneous and homogenous olefin hydrogenation catalysts such as Fe,38Ni,39,40Pt41 and Pd42,43 (Table 5) also reduce costs since these catalysts are normally used in excess and generate poisonous waste end products after the reaction is complete. Compared with other conventional catalytic hydrogenation processes, the present system has the following significant advantages: (i) high selectivity, high yields in the reduction of aromatic and aliphatic aldehydes, (ii) milder conditions and (iii) without the direct use of molecular hydrogen.
Results and discussions
Cu nanoparticles were prepared from Cu exchanged Y-zeolite (CuII-Y)44 by the reduction with hydrazine hydrate at 60 °C for 3 h. They were characterized by UV-DRS, powder XRD, XPS and HRTEM (Fig. S2–S5, ESI†). The size of the nanoparticles was estimated to an average diameter of 8 nm from HRTEM (Fig. 1).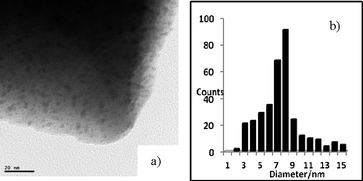 | ||
| Fig. 1 (a) HRTEM image and (b) size distribution of zeolite supported Cu nanoparticles. | ||
Catalytic activities of various copper sources for selective reduction of carbonyl compounds are studied and the results are summarized in Table 1. This reaction did not take place in the absence of any catalyst with 2-propanol acting as a reducing agent and solvent at room temperature (Table 1, entry 1). The reaction was also not very effective with CuNPs/Y zeolite without 2-propanol (entry 2). When CuCl, Cu(NO3)2·3H2O and CuI were used as catalysts, under identical reaction conditions the product formation was only 13, 24 and 54% respectively (Table 1, entries 3–5). Only lower conversion was observed when the reaction was carried out with CuI–Y zeolite catalyst (Table 1, entry 6). When the reaction was carried at room temperature, the yield was very low. An attempt to increase the reaction temperature to 80 °C led to an increase in the yield from 34 to 54% in 10 hours. When the experiment was carried out in an autoclave, the yield was better and reaction was complete within one hour compared to other copper sources. The reason for the high catalytic activity of CuNPs/Y zeolite is probably the high dispersion of Cu nanoparticles with the zeolite surface, temperature and pressure of hydrothermal conditions.
| Entry | Catalyst | Amount of Cu/mg | Temp/°C | Time/h | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: acetophenone 1 mmol, 2-propanol 2 mL, CuNPs/Y zeolite 30 mg at 70 °C in an autoclave. b Yield was determined by GC. c Without 2-propanol. d Amount of copper is calculated as 3 mg based on weight percentage data (10.56 weight%) from EDX spectra. e Reaction carried out in an oil bath at 80 °C. | |||||
| 1 | None | — | 70 | 12 | — |
| 2 | CuNPs/Y zeolitec | 3/30d | 70 | 12 | — |
| 3 | CuCl | 50 | 70 | 10 | 13 |
| 4 | Cu(NO3)2·3H2O | 50 | 70 | 10 | 24 |
| 5 | CuI | 50 | 70 | 10 | 54 |
| 6 | CuI-Y zeolite | 30 | 70 | 10 | 63 |
| 7 | CuNPs/Y zeolite | 3/30d | rt | 12 | 34 |
| 8 | CuNPs/Y zeolitee | 3/30d | 80 | 10 | 54 |
| 9 | CuNPs/Y zeolite | 3/30d | 70 | 1 | 98 |
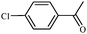
After optimization of the reaction conditions, the scope of zeolite supported copper nanoparticles catalysed reduction of aromatic carbonyl compounds in the presence of 2-propanol was extended to other substituents. A smooth conversion was observed in each case, affording the desired products in good to excellent yields (Table 2). Both electron deficient and rich aromatic ketones were converted readily to the corresponding alcohol under the reaction conditions (Table 2, entries 3–6). High yields were attained for 4-chloroacetophenone reduction, without hydrodechlorination or ring reduction (Table 2, entry 4). Other functional groups, viz., –Br and –OCH3 were tolerated. This reduction was also successfully carried out for heterocyclic and unsaturated carbonyl compounds which were reduced selectively with the heterocyclic ring remaining intact.
| Entry | Substrate | Product | Yieldb (%) | |
|---|---|---|---|---|
| a Reaction conditions. substrate 1 mmol, 2-propanol 2 mL, CuNPs/Y zeolite 30 mg (amount of copper is calculated as 3 mg based on weight percentage data (10.56 weight%) in EDX spectra) at 70 °C in an autoclave for 1 h. b Yield was determined by GC. | ||||
| 1 |
|
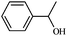
|
1a | 98 |
| 2 |
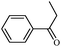
|
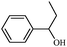
|
1b | 96 |
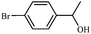
| 3 |
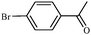
|
|
1c | 97 |
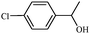
| 4 |
|
|
1d | 96 |
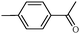
| 5 |
|
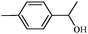
|
1e | 97 |
| 6 |
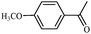
|
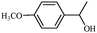
|
1f | 86 |
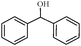
| 7 |
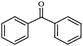
|
|
1g | 96 |
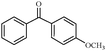
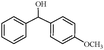
| 8 |
|
|
1h | 89 |
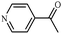
| 9 |
|
|
1i | 95 |
| 10 |
|
|
1j | 88 |
| 11 |
|
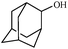
|
1k | 80 |
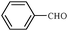
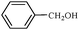
| 12 |
|
|
1l | 78 |
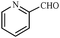
| 13 |
|
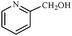
|
1m | 95 |
| 14 |
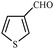
|
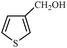
|
1n | 93 |
| 15 |
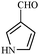
|
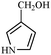
|
1o | 96 |
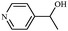
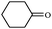
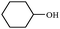
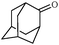
Synthetically useful examples such as pyridine, pyrrole, cyclohexanone and thiophene carboxaldehyde were converted selectively to the corresponding primary alcohols (Table 2, entries 9–15). It is also important to note that the hydrogenation of ketones proceeds efficiently under base free conditions. Further, the catalytic activity of CuNPs/Y zeolites was also extended to reduction of aldehydes (Table 2, entries 12–15).
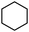
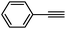
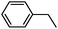
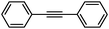
To broaden the scope of the above catalyst, we then studied with reduction of carbon–carbon multiple bonds. Our studies include the reduction of a variety of alkenes and alkynes, the results are shown in Table 3. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds in all the substrates were smoothly hydrogenated at 70 °C (Table 3, entries 1–8). Terminal alkenes were easily reduced to the corresponding alkanes in quantitative yield (Table 3, entries 1 and 2). The same behaviour was also observed for acenaphthylene (Table 3, entry 3). The electron rich aromatics, 4-methoxystyrene, were also nicely reduced in high yields (Table 3, entry 2). trans-Stilbene was readily transformed to 1,2-diphenylethane. Longer reaction time (2 h) was needed for the geminal alkene 1,1-diphenylethylene in comparison with trans-stilbene (Table 3, entry 5). In our system, high yields of only C
C double bonds in all the substrates were smoothly hydrogenated at 70 °C (Table 3, entries 1–8). Terminal alkenes were easily reduced to the corresponding alkanes in quantitative yield (Table 3, entries 1 and 2). The same behaviour was also observed for acenaphthylene (Table 3, entry 3). The electron rich aromatics, 4-methoxystyrene, were also nicely reduced in high yields (Table 3, entry 2). trans-Stilbene was readily transformed to 1,2-diphenylethane. Longer reaction time (2 h) was needed for the geminal alkene 1,1-diphenylethylene in comparison with trans-stilbene (Table 3, entry 5). In our system, high yields of only C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond reduced products are obtained and substituted alkenes do not undergo double bond isomerisation. 1,1-Diphenylethylene was hydrogenated successfully without dimerization. Cyclohexene was successfully converted to cyclohexane (Table 3, entry 6). Good results were obtained for aliphatic olefin (Table 3, entry 7).
C double bond reduced products are obtained and substituted alkenes do not undergo double bond isomerisation. 1,1-Diphenylethylene was hydrogenated successfully without dimerization. Cyclohexene was successfully converted to cyclohexane (Table 3, entry 6). Good results were obtained for aliphatic olefin (Table 3, entry 7).
| Entry | Substrate | Product | Yieldb (%) | |
|---|---|---|---|---|
| a Reaction conditions: substrate 1 mmol, 2-propanol 2 mL, CuNPs/Y zeolite 30 mg (amount of copper is calculated as 3 mg based on weight percentage data (10.56 weight%) from EDX spectra) at 70 °C in an autoclave for 1 h. b Yield was determined by GC. | ||||
| 1 |
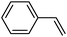
|
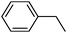
|
2a | 97 |
| 2 |
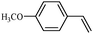
|
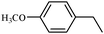
|
2b | 80 |
| 3 |
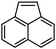
|
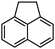
|
2c | 92 |
| 4 |
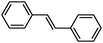
|
|
2d | 72 |
| 5 |
|
|
2e | 90 |
| 6 |
|
|
2f | 95 |
| 7 |

|

|
2g | 97 |
| 8 |
|
|
2h | 92 |
| 9 |
|
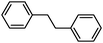
|
2i | 77 |
| 10 |
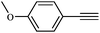
|
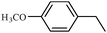
|
2j | 82 |
| 11 |
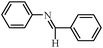
|
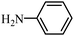
|
2k | 54 |
| 12 |
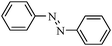
|
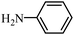
|
2l | 45 |
Data on reaction conditions, activity and efficiency of the different metal and supported metal nanoparticles for reduction of carbonyl compounds and C–C multiple bonds are given in Table 4. Comparison of the results indicates that our catalytic system (entries 8 and 18) exhibits better catalytic activity compared to conventional catalysts such as Fe, Ni, Pd, Pt and Au. These systems require additional base (entries 4–7), external solvent (entries 1, 3, 9, 10, 11, 13–15 and 17), higher reaction time (entries 1–7, 9–11,13–15 and 17), higher quantity of catalyst (entries 4, 5, 9, 11, 13 and 17), need excess amount of reducing agents (entries 1–7 and 10) and use hazardous reducing reagents (entries 9, 11 and 15).
| Entry | Catalyst | Size/nm | Reducing agent/mL | Solvent | Base | Temp/°C | Time/h | Yield (%) | Reusability of catalyst |
|---|---|---|---|---|---|---|---|---|---|
| a After 4th run, the percentage of product was reduced from 95 to 65. b Reaction conditions: benzophenone 1 mmol, 2-propanol 2 mL, CuNPs/Y zeolite 30 mg (amount of copper is calculated as 3 mg based on weight percentage data (10.56 weight%) from EDX spectra) at 70 °C in an autoclave for 1 h. | |||||||||
| (a) Carbonyl compounds | |||||||||
| 1 | NiNPs (1 mmol)22 | <2 | i-PrOH (4) | THF | — | 76 | 1–24 | 42–82 | 4a |
| 2 | NiNPs (1 mmol)21 | 0.7–2.8 | i-PrOH (4) | — | — | 1–5 | 30–80 | 4 | |
| 3 | NiNPs (10 mol%)19 | 45 | HCOONH4 | THF | — | 25 | 1–4 | 60–97 | — |
| 4 | NiHMA (100 mg)27b | — | i-PrOH (10) | — | KOH | 83 | 1.5–3 | 90 | 6 |
| 5 | Ni-MCM-41 (100 mg)27a | — | i-PrOH (10) | — | KOH | 83 | 3.5–5 | 94 | 6 |
| 6 | Pt/C (0.77 mol%)29 | 5–100 | i-PrOH (4) | — | KOH | 76 | 4–48 | >90 | 8 |
| 7 | Au/TiO2 (0.78 mol%)23 | 3–3.5 | i-PrOH (10) | — | KOH | 82 | 2–10 | >90 | 5 |
| 8 | CuNPs/Y zeolite (30 mg)b | 7–8 | i-PrOH (2 ml) | — | — | 70 | 1 | 98 | 6 |
| (b) Carbon–Carbon multiple bonds | |||||||||
| 9 | Ni(0)-K10 clay (100 mg)35 | 15–20 | N2H4·H2O | EtOH | — | 70 | 8 h | 78–88 | 3 |
| 10 | NiNPs (20 mol%)39 | 0.7–2.8 | i-PrOH (5) | — | — | 1–5 | >90 | 4 | |
| 11 | Al2(BDC)3 (150 mg) (MOF)32 | — | N2H4·H2O | ACN | 25 | 24 | >90 | 4 | |
| 12 | PdNPs (2.78 × 10−4 mmol)42b | 7–9 | H2 (10 atm) | — | — | 70 | 1.3–2.75 | >99.5 | 10 |
| 13 | Pd/C (10 wt%)41 | — | Hantzsch ester | EtOH | — | Reflux | 4–8 | 99 | — |
| 14 | FeNPs (5 mol%)38 | 2.6–0.6 | H2 (1 bar) | THF | — | rt | 0.5–15 | 11–100 | — |
| 15 | Neutral flavins (2 mol%)31 | — | N2H4·H2O | CH3CN | — | 30 | 24 | 85–99 | 3 |
| 16 | PdNPs (1.67 × 10−5 mol)42a | 3.9–4.1 | H2 (1 atm) | — | — | 35 | 1–4 | 100 | 8 |
| 17 | Pd-MCM-41 (50 mg)42c | — | HCOONH4 | MeOH | — | 70 | 1.5–5 | 62–87 | 3 |
| 18 | CuNPs/Y zeolite (30 mg)b | 7–8 | i-PrOH (2 ml) | — | — | 70 | 1 | 97 | 6 |
The advantage of using CuNPs/Y zeolite in the above reduction of acetophenone and styrene was examined by its recovery and reuse after the first run (Table 5). After each cycle, CuNPs/Y zeolite were recovered through simple filtration, followed by the addition of more isopropanol and the substrate. Slight decrease of activity was observed for styrene, after the third and fourth runs albeit the yields were still reasonably good.
| Reuseb | 1st | 2nd | 3rd | 4th | 5th | 6th |
|---|---|---|---|---|---|---|
| a Reaction conditions: CuNPs/Y zeolite 30 mg, substrate 1 mmol, 2-propanol 2 mL at 70 °C in an autoclave for 1 h. b Yield was determined by GC. | ||||||
| Acetophenone | 98 | 96 | 95 | 93 | 92 | 90 |
| Styrene | 97 | 94 | 92 | 89 | 87 | 86 |
Conclusions
In summary, we have developed a novel method for the reduction of carbonyl, C–C, C–N and N–N multiple bonds. In addition, we have demonstrated for the first time that copper nanoparticles can effectively catalyse the heterogeneous transfer hydrogenation of olefins using 2-propanol as the hydrogen donor. This heterogeneous method offers several advantages such as atom efficiently, greener, economic, ecofriendly protocols as the reaction requires neither high temperature nor harsh acids or bases and produces high yields with good chemoselectivity. The work-up and product isolation from the catalyst is easy, moreover this heterogeneous zeolite supported copper nanoparticles catalyst can be reused six times with slight change of activity. Further mechanism and application of this catalytic system to many other key transformations is currently being explored.Experimental section
The CuNPs/Y zeolite catalyst (30 mg) was suspended in 2 mL of 2-propanol followed by the addition of substrate (1 mmol). This heterogeneous solution was maintained in a closed autoclave bomb at 70 °C for 1 h, then cooled down to room temperature and products were quantitatively recovered by simple extraction with polar solvent. The extracted solution was filtered, the solvent dried and the mixture was analyzed by gas chromatography with authentic samples and products confirmed by NMR techniques.Acknowledgements
We are grateful to the University of Grants Commission (UGC), New Delhi, for UGC-BSR grant and UGC-BSR-JRF.Notes and references
- (a) D. E. De Vos and P. A. Jacobs, in Catalytic Science Series Vol. 3, Zeolites for Cleaner Technologies, ed. Michel Guisnet and Jean-Pierre Gilson, Imperial College Press, London, 2002, pp. 261–280 Search PubMed; (b) D. E. De Vos, M. Dams, B. F. Sels and P. A. Jacobs, Chem. Rev., 2002, 102, 3615 CrossRef CAS.
- (a) P. Levecque, D. W. Gammon, P. Jacobs, D. E. De Vos and B. Sels, Green Chem., 2010, 12, 828–835 RSC; (b) A. Corma and H. Garcia, Catal. Today, 1997, 38, 257–308 CrossRef CAS.
- S. Chassaing, M. Kumarraja, A. Sani Souna Sido, P. Pale and J. Sommer, Org. Lett., 2007, 9, 883 CrossRef CAS.
- A. Corma, Chem. Rev., 1997, 97, 2373–2419 CrossRef CAS.
- A. Corma, Chem. Rev., 1995, 95, 559–614 CrossRef CAS.
- M. E. Davis, Nature, 2002, 417, 813–821 CrossRef CAS.
- X. Meng, F. Nawaz and F.-S. Xiao, Nano Today, 2009, 4, 292–301 CrossRef CAS.
- M. Zahmakıran, S. Akbayrak, T. Kodairab and S. Ozkar, Dalton Trans., 2010, 39, 7521–7527 RSC.
- N. Yan, C. Xiao and Y. Kou, Coord. Chem. Rev., 2010, 254, 1179–1218 CrossRef CAS.
- F. Alonso, P. Riente and M. Yus, Acc. Chem. Res., 2011, 44, 379–391 CrossRef CAS.
- B. Baruwati, V. Polshettiwar and R. S Varma, Tetrahedron Lett., 2009, 50, 1215–1218 CrossRef CAS.
- J. M. Campelo, D. Luna, R. Luque, J. M. Marinas and A. A. Romero, ChemSusChem, 2009, 2, 18–45 CrossRef CAS.
- H. Sharghi, R. Khalifeh and M. M. Doroodmand, Adv. Synth. Catal., 2009, 351, 207–218 CrossRef CAS.
- F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Adv. Synth. Catal., 2010, 352, 3208 CrossRef CAS.
- F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Eur. J. Org. Chem., 2010, 1875 CrossRef CAS.
- N. Kidwai, K. Mishra, V. Bansala, A. Kumar and S. Mozumdar, Tetrahedron Lett., 2009, 50, 1355–1358 CrossRef.
- F. Alonso, Y. Moglie, G. Radivoy and M. Yus, Tetrahedron Lett., 2009, 50, 2358 CrossRef CAS.
- D. Raut, K. Wankhede, V. Vaidya, S. Bhilare, N. Darwatkar, A. Deorukhkar, G. Trivedi and M. Salunkhe, Catal. Commun., 2009, 10, 1240–1243 CrossRef CAS.
- M. Kidwai, V. Bansal, A. Saxena, R. Shankar and S. Mozumdar, Tetrahedron Lett., 2006, 47, 4161–4165 CrossRef CAS.
- J. W. Yang, M. T. H. Fonseca and B. List, Angew. Chem., Int. Ed., 2004, 43, 6660–6662 CrossRef.
- F. Alonso, P. Riente and M. Yus, Tetrahedron Lett., 2008, 49, 1939–1942 CrossRef CAS.
- F. Alonso, P. Riente and M. Yus, Tetrahedron, 2008, 64, 1847–1852 CrossRef CAS.
- F.-Z. Su, L. He, J. Ni, Y. Cao, H.-Y. He and K.-N. Fan, Chem. Commun., 2008, 3531–3533 RSC.
- Z. Baan, Z. Finta, G. Keglevichb and I. Hermecz, Green Chem., 2009, 11, 1937–1940 RSC.
- W. Baratta, K. Siega and P. Rigo, Adv. Synth. Catal., 2007, 349, 1633–1636 CrossRef CAS.
- S. Sakaguchi, T. Yamaga and Y. Ishii, J. Org. Chem., 2001, 66, 4710–4712 CrossRef CAS.
- (a) S. K. Mohapatra, S. U. Sonavane, R. V. Jayaram and P. Selvam, Org. Lett., 2002, 4, 4297–4300 CrossRef CAS; (b) P. Selvam, S. K. Mohapatra, S. U. Sonavane and R. V. Jayaram, Tetrahedron Lett., 2004, 45, 2003–2007 CrossRef CAS.
- F. Alonso, P. Riente, F. Rodriguez-Reinoso, J. Ruiz-Martinez, A. Sepulveda-Escribano and M. Yus, J. Catal., 2008, 260, 113–118 CrossRef CAS.
- F. Alonso, P. Riente, F. Rodriguez-Reinoso, J. Ruiz-Martinez, A. Sepulveda-Escribano and M. Yus, ChemCatChem, 2009, 1, 75–77 CrossRef CAS.
- N. S. Shaikh, K. Junge and M. Beller, Org. Lett., 2007, 9, 5429–5432 CrossRef CAS.
- Y. Imada, T. Kitagawa, T. Ohno, H. Iida and T. Naota, Org. Lett., 2010, 12, 32–35 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Adv. Synth. Catal., 2009, 351, 2271–2276 CrossRef CAS.
- S. Sambher, C. Baskar and R. S. Dhillon, Synth. Commun., 2008, 38, 2150–2157 CrossRef CAS.
- A. Saha and B. C. Ranu, J. Org. Chem., 2008, 73, 6867–6870 CrossRef CAS.
- A. Dhakshinamoorthy and K. Pitchumani, Tetrahedron Lett., 2008, 49, 1818–1823 CrossRef CAS.
- S. Vijaikumar, T. Subramanian and K. Pitchumani, J. Nanomater., 2008 DOI:10.1155/2008/257691 , article ID257691.
- A. Dhakshinamoorthy, A. Sharmila and K. Pitchumani, Chem.–Eur. J., 2010, 16, 1128–1132 CrossRef CAS.
- P.-H. Phua, L. Lefort, A. Jeroen, F. Boogers, M. Tristanyb and J. G. de Vries, Chem. Commun., 2009, 3747–3749 RSC.
- F. Alonso, P. Riente and M. Yus, Tetrahedron, 2009, 65, 10637–10643 CrossRef CAS.
- J. M. Khurana and P. Sharma, Bull. Chem. Soc. Jpn., 2004, 77, 549–552 CrossRef CAS.
- Q. Liu, J. Li, X. Shen, R. Xing, J. Yang, Z. Liu and B. Zhou, Tetrahedron Lett., 2009, 50, 1026–1028 CrossRef CAS.
- (a) Y. Lan, M. Zhang, W. Zhang and L. Yang, Chem.–Eur. J., 2009, 15, 3670–3673 CrossRef CAS; (b) X. Ma, T. Jiang, B. Han, J. Zhang, S. Miao, K. Ding, G. An, Y. Xie, Y. Zhou and A. Zhu, Catal. Commun., 2008, 9, 70–74 CrossRef CAS; (c) P. Selvam, S. U. Sonavane, S. K. Mohapatra and R. V. Jayaram, Tetrahedron Lett., 2004, 45, 3071–3075 CrossRef CAS.
- T. Ueno, M. Suzuki, T. Goto, T. Matsumoto, K. Nagayama and Y. Watanabe, Angew. Chem., Int. Ed., 2004, 43, 2527–2530 CrossRef CAS.
- A. Seidel, J. Loos and B. Boddenberg, J. Mater. Chem., 1999, 9, 2495–2498 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, EDX, UV-DRS, XPS, powder XRD and HRTEM micrographs and particle size distributions for CuNPs/Y zeolite and 1H-NMR for reduced products. See DOI: 10.1039/c1cy00383f |
| This journal is © The Royal Society of Chemistry 2012 |