Highly efficient and selective reduction of nitroarenes with hydrazine over supported rhodium nanoparticles†
Pingfei
Luo
,
Kunling
Xu
,
Rui
Zhang
,
Lei
Huang
,
Jun
Wang
,
Weihong
Xing
and
Jun
Huang
*
State Key Laboratory of Materials-Oriented Chemical Engineering, College of Chemistry and Chemical Engineering, Nanjing University of Technology, Nanjing 210009, China. E-mail: junhuang@njut.edu.cn; Fax: +86 25-83172261
First published on 1st December 2011
Abstract
A highly efficient and selective Rh nanocatalyst was demonstrated for the reduction of nitroarenes with hydrazine monohydrate under mild conditions. Functional groups such as halides (F, Cl, Br and I), CN, NH2, OH, alkene, ester and amide groups were untouched during the hydrogenation of the nitroarenes, and the corresponding anilines were obtained quantitatively.
Nitro aromatics are applied widely in both industrial and academic laboratories. The reduction of nitro aromatics is an important method for the preparation of functional anilines, which are intermediates for agrochemicals, pharmaceuticals, dyes, and pigments.1 Traditionally, the reduction of nitro aromatics with Fe/HCl produces a large amount of waste, which is not environmentally acceptable nowadays. Recyclable catalysts are highly favorable for the hydrogenation of nitro aromatics to anilines, and a number of transition metal catalysts (based on Pt, Pd, Ruetc.) were reported to be efficient for the transformation.2–4 However, the selective reduction of a nitro group is not easy when other reducible groups are present in the same molecule. Other metal catalysts (such as Au, Ag and Cu) were discovered to be selective for the hydrogenation of nitro groups, but the catalysts were always not active enough even under harsh reaction conditions (for example, T > 100 °C and P > 1.0 atm H2).5 In addition, other hydrogen sources (such as CO/H2O and H2NNH2) were applied for the catalytic hydrogenation of nitro compounds, and high selectivity was obtained.6 Due to the importance of selective reduction of aromatic nitro compounds, the development of improved chemoselective methods remains highly desirable.
Recently, highly porous ionic copolymer (PICP) materials based on ionic liquids were reported, and the related Pd and CuO nanocatalysts were prepared and applied as efficient catalysts for aryl C–C and C–O coupling reactions.7 Furthermore, a Pt nanocatalyst was developed as a highly selective catalyst for the hydrogenation of nitroarenes under mild conditions, and the PICP materials were proved to be excellent stable agents for metal nanoparticles.7,8 Using hydrazine as a hydrogen source, a rhodium catalyst was reported for the hydrogenation of nitro aromatics and alkenes, but the selectivity was studied scarcely and the efficiency was not high enough for wide applications.9 Furthermore, a Rh nanocatalyst was reported to be active for the decomposition of hydrazine to H2 and N2.10 Inspired by these results, herein we have reported a Rh nanocatalyst, which was highly efficient and selective for the hydrogenation of nitro aromatics to the corresponding anilines under mild conditions (Scheme 1).
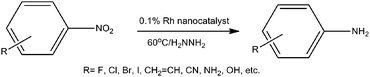 | ||
| Scheme 1 Rh catalyzed selective hydrogenation of nitroarenes. | ||
The PICP material and the Rh/PICP (2 wt% Rh contained) catalyst were prepared using the reported method,8 and the detailed procedure is provided in ESI.† The SEM image showed that the PICP material had a sponge cake structured surface (Fig. S1 in ESI†). As shown from the thermogravimetric analysis, some water was released before 250 °C, and the PICP material was stable until 330 °C (see Fig. S2 in ESI†). The PICP material and the Rh/PICP catalyst were highly porous, and the specific surface areas of the PICP material and the Rh/PICP were 528 m2 g−1 and 358 m2 g−1, respectively, based on the nitrogen adsorption–desorption analysis (Fig. S3 and S4 in ESI†). The Rh/PICP catalyst was characterized by TEM and the Rh particles were dispersed well on the PICP material (Fig. 1). The mean diameter of the Rh particles was around 2–3 nm (Fig. 1, left), which suggested high activity of the Rh/PICP catalyst.
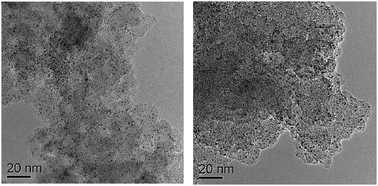 | ||
| Fig. 1 TEM images of the Rh/PICP nanocatalyst. The fresh Rh/PICP nanocatalyst (left); the 4th time recovered Rh/PICP nanocatalyst (right), scale bar for both images is 20 nm. | ||
The hydrogenation of nitrobenzene was performed as the model reaction with the Rh/PICP nanocatalyst, and the results are listed in Table 1. The Rh/PICP catalyst was highly active for the hydrogenation of nitrobenzene to aniline with low Rh loading (0.1 mol% Rh loading) at 60 °C in ethanol. Other solvents, such as ethyl ether, toluene, and hexane were also good solvents, but methanol, THF, ethyl acetate were less effective solvents for the transformation (Table 1, entries 1–7). The hydrogenation of nitrobenzene also afforded aniline in good yield without a solvent (Table 1, entry 8). Since some nitroarenes and anilines were solid, a solvent was necessary, and thus ethanol was selected as solvent for the hydrogenation of nitroarenes. Moreover, the commercially available Rh/C, Pt/C and Pd/C were tested for comparison, and aniline was obtained in relatively low yields (Table 1, entries 9–11). And the hydrogenation of nitrobenzene gave only trace aniline without catalyst under similar reaction conditions (Table 1, entry 12). The Rh/PICP catalyzed hydrogenation of nitrobenzene with H2 gas (1.0 atm.) as reducer instead of hydrazine gave trace aniline under similar reaction conditions, which implied the interaction of the nitro group with the hydrazine (Table 1, entry 13).
| Entry | Catalyst | Solvent | Conv./yield (%) | TOF d |
|---|---|---|---|---|
| Reaction conditions: nitrobenzene, 1.0 mmol; Rh/PICP, 0.1 mol%; N2H4·H2O, 2.0 mmol; reaction temperature: 60 °C; in 1 hour; the conversions and yields were determined by GC (C16H34 used as internal standard).a In 1.5 hours.b Rh/C (5.0 wt% Rh contained), Pt/C (5.0 wt% Pt contained) and Pd/C (5.0 wt% Pd contained) used as catalysts with 0.1 mol% metal loadings.c H2 (1.0 atm) used instead of N2H4·H2O.d TOF = turnover frequency, mol aniline/mol catalyst h−1. | ||||
| 1 | Rh / PICP | Ethanol | 100/99 | 990 |
| 2 | Rh/PICP | THF | 100/70 | 700 |
| 3 | Rh/PICP | Methanol | 100/86 | 860 |
| 4 | Rh/PICP | Ethyl ether | 100/99 | 990 |
| 5 | Rh/PICP | Toluene | 100/99 | 990 |
| 6 | Rh/PICP | Ethyl acetate | 76/40 | 400 |
| 7 | Rh/PICP | Hexane | 100/99 | 990 |
| 8a | Rh/PICP | Solvent free | 100/99 | 660 |
| 9b | Rh/C | Ethanol | 68/54 | 540 |
| 10b | Pt/C | Ethanol | 34/33 | 330 |
| 11b | Pd/C | Ethanol | 49/44 | 440 |
| 12 | No catalyst | Ethanol | 2/1 | |
| 13c | Rh/PICP | Ethanol | 1/1 | 10 |
The Rh/PICP catalyst was easily recovered and reused for the next reaction cycle, and the performance of the recycled Rh/PICP catalyst is shown in Table 2. The Rh/PICP can be reused at least 4 times without evident loss of activity, and then the yield decreased slightly (Table 2). The 4th time recovered Rh/PICP was analyzed by TEM also and the mean diameter of the recovered Rh nanoparticles was not increased evidently (Fig. 1, right). After removal of the Rh/PICP nanocatalyst by filtration, the filtrate was investigated by ICP-OES (inductively coupled plasma optical emission spectroscopy), and no Rh was detected (below the detection limit 7 ppb), which suggested that the Rh/PICP was a heterogeneous catalyst and no Rh was leaked into the solution.
| Recycled | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Reaction conditions were the same as entry 1 in Table 1. | ||||||
| Conv./yield (%) | 100/99 | 100/99 | 100/99 | 100/96 | 100/94 | 95/92 |
The chemoselective reduction of various functional nitroarenes was studied with hydrazine monohydrate, and the results are summarized in Table 3. For the hydrogenation of nitro aromatics with halides (F, Cl, Br and I), the Rh/PICP catalyst showed excellent activity and selectivity, and the corresponding anilines were obtained in high yields (Table 3, entries 1–7). The Rh/C, Pd/C and Pt/C catalysts were less active or less selective for the hydrogenation of nitro aromatics with halides under similar reaction conditions, and some aniline (dehalogenation side reaction) by-product was observed.
| Entry | Catalyst | Hydrazine equiv. | R | Reaction time/h | Conv./yield (%) |
|---|---|---|---|---|---|
| Reaction conditions: nitrobenzene, 1.0 mmol; Rh/PICP, 0.1 mol%; N2H4·H2O, 2.0 mmol; reaction temperature: 60 °C; the conversions and yields were determined by GC (C16H34 used as internal standard).a 1 mol% metal used; at 80 °C.b 0.5 mol% noble metal used.c 3-Vinylaniline yield. | |||||
| 1 | Rh / PICP | 4 | 4-F | 1 | 100/99 |
| Rh/C | 82/65 | ||||
| Pd/C | 30/14 | ||||
| Pt/C | 40/30 | ||||
| 2 | Rh / PICP | 4 | 2-F | 1 | 100/99 |
| Rh/C | 90/50 | ||||
| Pd/C | 65/48 | ||||
| Pt/C | 72/58 | ||||
| 3 | Rh / PICP | 4 | 4-Cl | 1 | 100/99 |
| Rh/C | 100/94 | ||||
| Pd/C | 65/62 | ||||
| Pt/C | 100/81 | ||||
| 4 | Rh / PICP | 4 | 2-Cl | 1 | 100/99 |
| Rh/C | 99/93 | ||||
| Pd/C | 95/82 | ||||
| Pt/C | 98/92 | ||||
| 5 | Rh / PICP | 4 | 3-Cl | 1 | 100/99 |
| Rh/C | 100/87 | ||||
| Pd/C | 100/82 | ||||
| Pt/C | 100/86 | ||||
| 6 | Rh / PICP | 4 | 2-Br | 1 | 100/99 |
| Rh/C | 97/95 | ||||
| Pd/C | 81/73 | ||||
| Pt/C | 98/94 | ||||
| 7a | Rh / PICP | 6 | 3-I | 10 | 100/99 a |
| Rh/C | 98/85a | ||||
| Pd/C | 97/68a | ||||
| Pt/C | 96/90a | ||||
| 8b | Rh / PICP | 4 | 4-CN | 10 | 100/99 b |
| Rh/C | 96/95b | ||||
| Pd/C | 92/88b | ||||
| Pt/C | 96/90b | ||||
| 9 | Rh / PICP | 4 | 4-NH2 | 1 | 100/99 |
| Rh/C | 95/94 | ||||
| Pd/C | 49/48 | ||||
| Pt/C | 90/89 | ||||
| 10 | Rh / PICP | 4 | 2-NH2 | 1 | 100/99 |
| Rh/C | 63/63 | ||||
| Pd/C | 51/50 | ||||
| Pt/C | 93/91 | ||||
| 11 | Rh / PICP | 4 | 2-OH | 1 | 100/99 |
| Rh/C | 95/94 | ||||
| Pd/C | 94/93 | ||||
| Pt/C | 97/96 | ||||
| 12 | Rh / PICP | 4 | 4-OH | 1 | 100/99 |
| Rh/C | 94/93 | ||||
| Pd/C | 94/93 | ||||
| Pt/C | 96/95 | ||||
| 13 | Rh / PICP | 4 | 3-OH | 1 | 100/99 |
| Rh/C | 97/96 | ||||
| Pd/C | 96/95 | ||||
| Pt/C | 97/96 | ||||
| 14 | Rh / PICP | 4 | 2,6-Dimethyl | 5 | 100/99 |
| Rh/C | 91/90 | ||||
| Pd/C | 10/9 | ||||
| Pt/C | 40/40 | ||||
| 15 | Rh / PICP | 4 | 4-H2NCO | 2 | 100/99 |
| Rh/C | 99/67 | ||||
| Pd/C | 99/72 | ||||
| Pt/C | 98/66 | ||||
| 16 | Rh / PICP | 4 | 4-H3COOC | 1 | 100/99 a |
| Rh/C | 76/75a | ||||
| Pd/C | 70/68a | ||||
| Pt/C | 71/70a | ||||
| 17 | Rh / PICP | 2 | 3-CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
5 | 100/98 c |
| Rh/C | 18/16c | ||||
| Pd/C | 15/12c | ||||
| Pt/C | 64/60c |
The Rh/PICP catalyzed hydrogenation of nitroarenes with CN, NH2 and OH groups gave corresponding anilines quantitatively, but the reduction of 4-nitrobenzonitrile required more Rh catalyst and longer reaction time (Table 3, entries 8–13). With Rh/C, Pd/C and Pt/C catalysts, the activity was not good enough, but the selectivity was good also for the reduction of nitroarenes with CN, NH2 and OH groups. The hydrogenation of 2,6-dimethylnitrobenzene by Rh/PICP afforded 2,6-dimethylaniline quantitatively, and good yield was obtained also with the Rh/C catalyst. But Pd/C and Pt/C exhibited the steric effect for the hydrogenation of 2,6-dimethylnitrobenzene, and 2,6-dimethylaniline was obtained in low yield (Table 3, entry 14). The hydrogenation of 4-nitrobenzamide and methyl 4-nitrobenzoate by Rh/PICP gave 4-aminobenzamide and methyl 4-aminobenzoate in excellent yields, respectively, which were much better than the yields using Rh/C, Pd/C and Pt/C catalysts (Table 3, entries 15 and 16). Moreover, the catalysts (Rh/PICP, Rh/C, Pd/C and Pt/C) were used for the hydrogenation of 3-nitrostyrene with 2 equivalents of hydrazine monohydrate, and the results showed that all the catalysts were selective for the transformation. The Rh/PICP catalyzed hydrogenation of 3-nitrostyrene gave 100% conversion and >98% selectivity to 3-vinylaniline, whereas low conversions were achieved with Rh/C, Pd/C and Pt/C catalysts (Table 3, entry 17).
Additionally, the mixture of styrene and nitrobenzene was reduced selectively with Rh/PICP and the results are shown in Fig. 2. Nitrobenzene was reduced preferentially (the first 10 min), and then the reduction of styrene was started and completed finally (5 h). The Rh/PICP catalyst showed high selectivity for the hydrogenation of nitro and alkene groups, and aniline and ethyl benzene were formed in excellent yields sequentially.
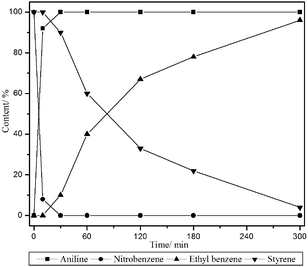 | ||
| Fig. 2 Selective hydrogenation of the mixture of styrene and nitrobenzene. | ||
Although hydrazine monohydrate was ready to be decomposed to N2 and H2, the formation of anilines was not through the decomposition–hydrogenation way, as the reduction of nitrobenzene with H2 (1.0 atm) was much slower than with hydrazine monohydrate using the same Rh/PICP catalyst (Table 1, entry 13). The mechanism of the reduction of nitrobenzene by hydrazine has not been proposed in the present case, but we suggest that nitro groups should react with hydrazine firstly, and subsequently, Rh/PICP catalyzed the N2 emission and aniline formation from the related intermediates. The hydrogenation of alkenes is easy, but the reduction of alkenes requires the decomposition of hydrazine monohydrate and the H2 formation, which explains the preferential selectivity of the reduction of nitroarenes over alkenes. Moreover, the Rh nanoparticles were protected at around 2–5 nm by the PICP material, which allowed the high performance of the rhodium catalyst for the transformations.
Conclusions
In summary, we demonstrated a highly active and selective Rh/PICP nanocatalyst for the reduction of nitroarenes into corresponding anilines with hydrazine monohydrate under mild conditions. Nitroarenes with halides (F, Cl, Br and I) were reduced selectively, and the corresponding anilines were obtained in excellent yields. Functional groups such as CN, NH2, OH, alkene, ester and amide groups were untouched during the hydrogenation of the nitro compounds, and the corresponding anilines were obtained quantitatively. Moreover, Rh/C, Pd/C and Pt/C catalysts were studied and compared for the selective hydrogenation of the nitroarenes with hydrazine monohydrate under the same reaction conditions.Experimental section
Typical procedure for the reduction of nitroarenes: a nitroarene (1.0 mmol), hydrazine monohydrate (4.0 mmol) and ethanol (2.0 mL) were added to a Schlenk tube which contained Rh/PICP (Rh: 0.1 mol%) catalyst and a stir bar under argon. The Schlenk tube was kept at 60 °C with stirring for 1 hour. After the reaction mixture was cooled, the Rh/PICP was separated by filtration, and the products (in the filtrate mixture) were analyzed by GC/MS and GC.Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 20802008, 20636020) and the foundation from State Key Laboratory of Materials-Oriented Chemical Engineering (grant no. ZK201003, ZK201011).References
- (a) A. M. Tafesh and J. Weiguny, Chem. Rev., 1996, 96, 2035–2052 CrossRef CAS; (b) H. Blaser, C. Malan, B. Pugin, F. Spindler, H. Steiner and M. Studer, Adv. Synth. Catal., 2003, 345, 103–151 CrossRef CAS; (c) H. Blaser, H. Steiner and M. Studer, ChemCatChem, 2009, 1, 210–221 CrossRef CAS; (d) G. Booth, Ullmanns Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag, Weinheim, Germany, 2002 Search PubMed.
- Pt catalysts, see: (a) P. Maity, S. Basu, S. Bhaduri and G. K. Lahiri, Adv. Synth. Catal., 2007, 349, 1955–1962 CrossRef CAS; (b) M. Takasaki, Y. Motoyama, K. Higashi, S. H. Yoon, I. Mochida and H. Nagashima, Org. Lett., 2008, 10, 1601–1604 CrossRef CAS; (c) Y. Motoyama, K. Kamo and H. Nagashima, Org. Lett., 2009, 11, 1345–1348 CrossRef CAS; (d) S. Shironita, T. Takasaki, T. Kamegawa, K. Mori and H. Yamashita, Top. Catal., 2010, 53, 218–223 CrossRef CAS; (e) Z. Sun, Y. Zhao, Y. Xie, R. Tao, H. Zhang, C. Huang and Z. Liu, Green Chem., 2010, 12, 1007–1011 RSC; (f) M. Li, L. Hu, X. Cao, H. Hong, J. Lu and H. Gu, Chem.–Eur. J., 2011, 17, 2763–2768 CrossRef CAS; (g) X. Lin, M. Wu, D. Wu, S. Kuga, T. Endoe and Y. Huang, Green Chem., 2011, 13, 283–287 RSC; (h) V. Pandarus, R. Ciriminna, F. Beland and M. Pagliaro, Adv. Synth. Catal., 2011, 353, 1306–1316 CrossRef CAS.
- Pd catalysts, see: (a) A. Mori, T. Mizusaki, M. Kawase, T. Maegawa, Y. Monguchi, S. Takao, Y. Takagi and H. Sajiki, Adv. Synth. Catal., 2008, 350, 406–410 CrossRef CAS; (b) M. L. Kantam, R. Chakravarti, U. Pal, B. Sreedhar and S. Bhargava, Adv. Synth. Catal., 2008, 350, 822–827 CrossRef CAS; (c) J. Toubiana, M. Chidambaram, A. Santo and Y. Sasson, Adv. Synth. Catal., 2008, 350, 1230–1234 CrossRef CAS; (d) H. Wu, L. Zhuo, Q. He, X. Liao and B. Shi, Appl. Catal., A, 2009, 366, 44–56 CrossRef CAS; (e) A. J. Amali and R. K. Rana, Green Chem., 2009, 11, 1781–1786 RSC; (f) J. Li, X. Shi, Y. Bi, J. Wei and Z. Chen, ACS Catal., 2011, 1, 657–664 CrossRef CAS.
- Ru catalysts, see: (a) S. Zhao, H. Liang and Y. Zhou, Catal. Commun., 2007, 8, 1305–1309 CrossRef CAS; (b) K. V. R. Chary and C. S. Srikanth, Catal. Lett., 2009, 128, 164–170 CrossRef CAS.
- Au catalysts, see: (a) A. Corma and P. Serna, Science, 2006, 313, 332–334 CrossRef CAS; (b) A. Corma, P. Serna and H. Garcia, J. Am. Chem. Soc., 2007, 129, 6358–6359 CrossRef CAS; (c) D. He, H. Shi, Y. Wu and B. Xu, Green Chem., 2007, 9, 849–851 RSC; (d) Y. Chen, J. Qiu, X. Wang and J. Xiu, J. Catal., 2006, 242, 227–230 CrossRef CAS; (e) A. Corma, C. Gonzalez-Arellano, M. Iglesias and F. Sanchez, Appl. Catal., A, 2009, 356, 99–102 CrossRef CAS; (f) A. Corma, P. Concepcion and P. Serna, Angew. Chem., Int. Ed., 2007, 46, 7266–7269 CrossRef CAS; (g) M. Boronat, P. Concepcion, A. Corma, S. Gonzalez, F. Illas and P. Serna, J. Am. Chem. Soc., 2007, 129, 16230–16237 CrossRef CAS; Ag catalysts, see: (h) Y. Chen, C. Wang, H. Liu, J. Qiu and X. Bao, Chem. Commun., 2005, 5298–5300 RSC; (i) K. I. Shimizu, Y. Miyamoto and A. Satsuma, J. Catal., 2010, 270, 86–94 CrossRef CAS; Cu catalysts, see: (j) S. Diao, W. Qian, G. Luo, F. Wei and Y. Wang, Appl. Catal., A, 2005, 286, 30–35 CrossRef CAS; (k) U. Sharma, P. Kumar, N. Kumar, V. Kumar and B. Singh, Adv. Synth. Catal., 2010, 352, 1834–1840 CrossRef CAS; (l) A. Sama and B. Ranu, J. Org. Chem., 2008, 73, 6867–6870 CrossRef.
- (a) L. He, L. C. Wang, H. Sun, J. Ni, Y. Cao, H. Y. He and K. N. Fan, Angew. Chem., Int. Ed., 2009, 48, 9538–9541 CrossRef CAS; (b) K. Junge, B. Wendt, N. Shaikh and M. Beller, Chem. Commun., 2010, 46, 1769–1771 RSC; (c) X. B. Lou, L. He, Y. Qian, Y. M. Liu, Y. Cao and K. N. Fan, Adv. Synth. Catal., 2011, 353, 281–286 CrossRef CAS; (d) U. Sharma, P. K. Verma, N. Kumar, V. Kumar, M. Bala and B. Singh, Chem.–Eur. J., 2011, 17, 5903–5907 CrossRef CAS; (e) P. K. Mandal and J. S. McMurray, J. Org. Chem., 2007, 72, 6599–6601 CrossRef CAS; (f) Q. Shi, R. Lu, L. Lu, X. Fu and D. Zhao, Adv. Synth. Catal., 2007, 349, 1877–1881 CrossRef CAS; (g) M. Paul, N. Pal and A. Bhaumik, Eur. J. Inorg. Chem., 2010, 5129–5134 CAS; (h) Y. K. Park, S. B. Choi, H. J. Nam, D. Jung, H. C. Ahn, K. Choi, H. Furukawa and J. Kim, Chem. Commun., 2010, 46, 3086–3088 RSC; (i) L. Liu, B. Qiao, Z. Chen, J. Zhang and Y. Deng, Chem. Commun., 2009, 653–655 RSC.
- (a) T. Hu, X. Chen, J. Wang and J. Huang, ChemCatChem, 2011, 3, 661–665 CrossRef CAS; (b) Y. Yu, T. Hu, X. Chen, K. Xu, J. Zhang and J. Huang, Chem. Commun., 2011, 47, 3592–3595 RSC.
- K. Xu, Y. Zhang, X. Chen, L. Huang, R. Zhang and J. Huang, Adv. Synth. Catal., 2011, 353, 1260–1264 CrossRef CAS.
- Y. Jang, S. Kim, S. W. Jun, B. H. Kim, S. Hwang, I. K. Song, B. M. Kim and T. Hyeon, Chem. Commun., 2011, 47, 3601–3603 RSC.
- (a) S. K. Singh, X. B. Zhang and Q. Xu, J. Am. Chem. Soc., 2009, 131, 9894–9895 CrossRef CAS; (b) S. K. Singh and Q. Xu, J. Am. Chem. Soc., 2009, 131, 18032–18033 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c1cy00358e |
| This journal is © The Royal Society of Chemistry 2012 |