Enzymatic isomerization of glucose and xylose in ionic liquids†
Tim
Ståhlberg
a,
John M.
Woodley
b and
Anders
Riisager
*a
aCentre for Catalysis and Sustainable Chemistry, Department of Chemistry, Technical University of Denmark, DK-2800 Kgs. Lyngby, Denmark. E-mail: ar@kemi.dtu.dk
bCentre for Process Engineering and Technology, Department of Chemical and Biochemical Engineering, Technical University of Denmark, DK-2800 Kgs. Lyngby, Denmark
First published on 29th November 2011
Abstract
Glucose isomerase has been found for the first time to catalyze the isomerization of glucose to fructose in the ionic liquid N,N-dibutylethanolammonium octanoate (DBAO). Isomerization was achieved at temperatures of 60–80 °C although a substantial amount of mannose was formed at elevated temperatures via the Lobry-de Bruyn–van Ekenstein transformation. Complete recovery of the sugars after reaction was achieved by extraction with aqueous HCl, thus making the protocol attractive for continuous operation.
Introduction
The increased demand for sustainable and environmentally benign chemical processes makes enzymes attractive alternatives as catalysts in industrial processes. Enzymes are by virtue most effective in aqueous environments where many chemical substrates and products have low solubility. Therefore the search for alternative solvents in which enzymes have retained activity is of the essence.Ionic liquids (ILs) are interesting alternatives to conventional organic solvents due to their negligible vapor pressure, non-flammability and unique dissolving abilities for polar compounds and polymers.1 The study of enzymes in ILs has intensified over recent years and many interesting examples are covered in two excellent reviews by Sheldon and coworkers.2,3 In particular lipases have proven to be stable and have retained or improved activity in ILs.4–15Other noteworthy examples are the synthesis of aspartame with thermolysin in 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIm][PF6])16 and the oxidation of cellobiose with cytochrome c in hydrated choline dihydrogen phosphate ([Choline][dhp]).17,18 An additional advantage with ILs in combination with enzymes is the possibility for two-phase systems with supercritical fluids. Combining IL/enzyme mixtures with supercritical CO2 enables a dynamic system where products or reactants can be selectively removed from the reaction mixture.4
Glucose isomerase (GI) catalyzes the isomerization of glucose to fructose and is used for the production of high-fructose corn syrup (HFCS) which is currently one of the most important industrial bioprocesses (Scheme 1).19 The process is limited by the poor yield of fructose and in spite of significant research on enriching fructose, the process currently requires an expensive chromatographic step to achieve the desired fructose concentration.19–21 The process usually works in water and examples in the literature of GI in alternative solvents are scarce, nevertheless successful isomerization has been achieved in aqueous ethanol, indicating that GI can maintain activity at reduced water concentrations.22 Finding an IL system where GI could exhibit activity would be of significant interest, not only for the food industry, but also since it potentially make one-pot reactions for future platform chemicals such as 5-hydroxymethyl furfural (HMF) possible. HMF is formed via the dehydration of hexoses and is readily obtained from fructose while the conversion from glucose requires special catalysts.23 HMF and its derivatives are believed to be among the most important platform chemicals of the future biopetrochemical industry.24 Given the high solubility of carbohydrates in ILs25 and the irreversible hydrolysis of HMF in aqueous solutions leading to levulinic acid and formic acid, a direct process from glucose to HMF in ILs has the potential to become an important industrial process in a post-petrochemical world.26,27
 | ||
| Scheme 1 Isomerization of glucose to fructose and its further derivatization to biopetrochemicals. | ||
To the best of our knowledge, no successful enzymatic isomerization of glucose to fructose in ILs has been reported. We present here a study on GI in different ILs and show for the first time an IL/GI system that converts glucose to fructose enzymatically.
Results and discussion
Screening of GI activity in various ILs
GI is in industrial applications primarily used in its immobilized form. Sweetzyme® is a GI derived from a strain of the bacterium Streptomyces murinus and immobilized by the crosslinking of whole cells with glutaraldehyde.28 The activity of GI was first screened in several ILs using the immobilized GI Sweetzyme®. Examples of anions and cations of the ILs screened are depicted in Scheme 2. Experiments without water and addition of 100 and 200 μL of water were made. Since the ILs used had a different water content prior to the addition of water, the final water content varied in the final solution depending on the liquid. A complete list of the water content by weight before and after water addition is available as ESI† (Tables S1 and S2). | ||
| Scheme 2 Examples of different ions of the ionic liquids used in the initial screening. | ||
Most ILs had a detrimental effect on the enzyme activity whereas others simply did not dissolve glucose in reasonable amounts. In Table 1 the results of the ILs that gave conversion of glucose to fructose after 24 hours are shown. In all cases, the isomerization rate was significantly slower than in water, and the minor conversion to fructose observed with the basic acetate ILs was likely associated to direct base catalyzed isomerization rather than enzymatic activity. The two ILs most commonly employed for biomass conversion, 1-ethyl-3-methylimidazolium chloride ([EMIm]Cl) and 1-butyl-3-methylimidazolium chloride ([BMIm]Cl) both had a detrimental effect on enzyme activity and afforded no fructose. Most promising results were found using ammonium carboxylates, where 0.8 mL N,N-dibutylethanolammonium octanoate (DBAO) together with 200 μL H2O gave a fructose yield of 52% after 24 hours. The addition of 200 μL H2O to DBAO corresponded to a final water content of 21 wt%. In addition to fructose, 2% mannose was formed, most likely from fructosevia the Lobry-de Bruyn–van Ekenstein transformation.29 Notably, no mannose was formed using any of the other ILs.
| Ionic liquid |
Glucose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fructose ratio (mol fructose ratio (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) mol) |
||
|---|---|---|---|
| No H2O | 100 μL H2O | 200 μL H2O | |
| a Reaction conditions: 100 mg glucose, 0.8–1.0 g IL, 0–0.2 mL H2O, 30 mg Sweetzyme® and 3.3 mg MgSO4·7H2O, 60 °C, 24 h. b 2% mannose present. | |||
| N,N-Dimethylethanolammonium formate | N/A | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| N,N-Dimethylethanolammonium propionate | N/A | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| N,N-Dimethylethanolammonium decanoate | N/A | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 14 |
| Choline propionate | N/A | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| N,N-Dimethylbutylammonium propionate | N/A | N/A | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| N-Methylpiperidinium acetate | N/A | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| DBAO | N/A | N/A | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52b 52b |
| N,N-Dimethylisopropanolammonium propionate | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| [Choline][OAc] | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| [EMIm][OAc] | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| [BMIm][OAc] | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
Glucose isomerization in DBAO
In Fig. 1 the results from the isomerization of glucose in DBAO over time are shown. After 1 hour around 25% fructose had formed, which was significantly slower than in water where equilibrium was reached already after 0.5 hours using the same batch of Sweetzyme®. After 4 hours in DBAO a glucose to fructose molar ratio of 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 was reached after which the fructose amount was stabilized at 52% while the mannose part increased at the expense of glucose over time. The pH of the IL/H2O-solution was neutral, suggesting that the isomerization of fructose to mannose was a result of a specific base-catalysis.
51 was reached after which the fructose amount was stabilized at 52% while the mannose part increased at the expense of glucose over time. The pH of the IL/H2O-solution was neutral, suggesting that the isomerization of fructose to mannose was a result of a specific base-catalysis.
 | ||
| Fig. 1 Enzymatic conversion of glucose to fructose in DBAO at 60 °C. Reaction conditions: 100 mg glucose, 0.8 g DBAO, 0.2 mL H2O, 30 mg Sweetzyme® and 3.3 mg MgSO4·7H2O. | ||
Increasing the temperature to 70 and 80 °C retained enzyme activity in DBAO and equilibrium was reached both faster and with a greater enrichment of fructose, as expected at elevated temperatures (Fig. 2 and 3).19 At 70 °C a fructose content of 55% was reached after 4 hours and correspondingly an amount of 58% at 80 °C. The amount of mannose was also increased and after 24 hours at 80 °C as much as 11% was reached whereas glucose only amounted to 31%. No mannose was formed after 4 hours in either case which indicated that a certain amount of fructose was formed before the chemical isomerization of fructose to mannose was initiated. However, by-product formation was a problem over longer reaction times and after 48 hours significant caramelization of the sugars had taken place. The initial rate constants for the isomerization at all three temperatures in DBAO as well as the corresponding rates in water are listed in Table 2. The reaction was considered to be first order with respect to glucose concentration.
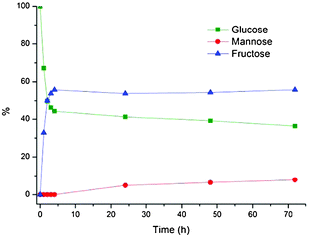 | ||
| Fig. 2 Enzymatic conversion of glucose to fructose in DBAO at 70 °C. Reaction conditions: 100 mg glucose, 0.8 g DBAO, 0.2 mL H2O, 30 mg Sweetzyme® and 3.3 mg MgSO4·7H2O. | ||
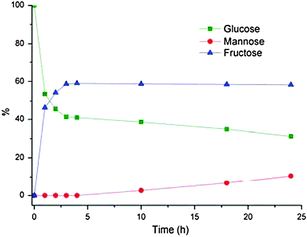 | ||
| Fig. 3 Enzymatic conversion of glucose to fructose in DBAO at 80 °C. Reaction conditions: 100 mg glucose, 0.8 g DBAO, 0.2 mL H2O, 30 mg Sweetzyme® and 3.3 mg MgSO4·7H2O. | ||
| Temperature/°C | Rate const. DBAO/h−1 | Rate const. H2O/h−1 |
|---|---|---|
| 60 | 0.27 | 0.71 |
| 70 | 0.35 | 1.14 |
| 80 | 0.80 | 1.46 |
Work-up after complete isomerization was done by extracting the IL with 1 M HCl. A single extraction with an equal volume of acid solution to IL resulted in complete recovery of the sugars to the aqueous phase. Water and DBAO formed a homogeneous clear solution when using 200 μL of water and 0.0135 mmol of MgSO4 at temperatures of 60 °C and above, but formed a clear two phase system with 1 M HCl. This provides a separation method that can be suitable for large scale production of these sugar mixtures.
In order to verify that the observed glucose isomerization in DBAO was enzymatically catalyzed, glucose was left to stir under normal reaction conditions without the addition of Sweetzyme®. This resulted in 8% fructose after 72 hours, confirming that the high fructose yields in the former experiments were a result of catalysis by the active enzyme (Fig. 4).
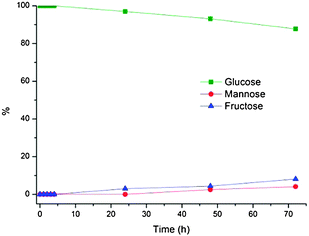 | ||
| Fig. 4 Conversion of glucose to fructose in DBAO at 60 °C without enzyme. Reaction conditions: 100 mg glucose, 0.8 g DBAO, 0.2 mL H2O, and 3.3 mg MgSO4·7H2O. | ||
Xylose isomerization in DBAO
The natural substrate of GI is xylose which is isomerized catalytically in nature to xylulose. The isomerization of xylose to xylulose exhibits a different product distribution upon reaching equilibrium than that of glucose to fructose. The thermodynamic equilibrium between the two pentoses is 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14, respectively.30–32
14, respectively.30–32
When using the DBAO system equilibrium was reached already after two hours as would be expected since it is the natural substrate of the enzyme (Fig. 5).19 After 24 hours significant degradation had occurred indicated by an increase of several unidentified peaks during HPLC analysis, suggesting that the xylose and xylulose were less stable in the IL system compared to the hexoses.
 | ||
| Fig. 5 Enzymatic conversion of xylose to xylulose in DBAO at 60 °C. Reaction conditions: 100 mg xylose, 0.8 g DBAO, 0.2 mL H2O, 30 mg Sweetzyme® and 3.3 mg MgSO4·7H2O. | ||
Experimental
Materials
All chemicals were used as received. D-glucose (99.5%) and xylose (99%) were purchased from Aldrich. D-fructose (puriss) was purchased from Riedel-de Haën. [EMIm]Cl and [BMIm]Cl were obtained from BASF while all other ILs were purchased from CLEA Technologies. Sweetzyme® was obtained from Novozymes A/S.All experiments were performed using a Radley Carousel 12 Plus Basic System with temperature control (±1 °C). Samples were analyzed by HPLC (Agilent 1200 series, Phenomenex Rezex RCM-Monosaccharide Ca2+ (%), 300 × 7.8 mm pre-packed column, MilliQ water as mobile phase, 80 °C, 0.6 mL min−1). Peaks were identified from standards of all products and substrates.
General procedure for isomerization
Ionic liquid (0.8–1.0 g), MgSO4·7H2O (3.3 mg, 0.0135 mmol), water (0–0.2 mL) and sugar (100 mg, 9 wt%) were stirred for 5 minutes and a clear homogeneous solution was obtained. Sweetzyme® (30 mg) was added and the reaction was allowed to stir for a maximum of 72 hours. Samples were taken out during the reaction and analyzed by HPLC.Conclusions
The first successful enzymatic conversion of glucose to fructose as well as xylose to xylulose in ILs is reported. During isomerization of glucose the formed fructose was converted to mannosevia the Lobry-de Bruyn–van Ekenstein transformation leading to an accumulation of fructose and mannose at the expense of glucose over time. The activity of the enzyme was lower in ILs in comparison to water resulting in slower reaction rates. Xylose isomerization was, however, faster in comparison to glucose as would be expected being the natural substrate of the enzyme. It is reasonable to believe that the surface of the enzyme is hydrated and hence facilitates activity even in the presence of the IL. Analogous to the previous report on ethanol22 21 wt% of water was used for the reactions. In this study, however, solubility of the sugar and MgSO4—not enzyme activity—was the main reason for using this particular solvent composition.The conversion of biomass to commodity chemicals is alongside the production of sweeteners the most important future application of this catalytic system. Hence, our work will continue with studies of fructose dehydration to HMF using Lewis and Brønsted acid catalysts like, e.g.WCl6,33CrCl3,34,35 or HCl36 in DBAO at elevated temperatures.
We believe that this work provides interesting discoveries concerning the compatibility of GI and ILs and could initiate further studies on GI in alternative solvents. Furthermore, it facilitates opportunities to make a one-pot synthesis of platform chemicals from glucose or its natural polymers.
Acknowledgements
The reported work was supported by the Danish National Advanced Technology Foundation in cooperation with Novozymes A/S. Special gratitude to BASF for providing the ionic liquids.Notes and references
- P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH Verlag GmbH & Co. KGaA, 2008 Search PubMed.
- R. A. Sheldon, R. M. Lau, M. J. Sorgedrager, F. van Rantwijk and K. R. Seddon, Green Chem., 2002, 4, 147–151 RSC.
- F. van Rantwijk and R. A. Sheldon, Chem. Rev., 2007, 107, 2757–2785 CrossRef CAS.
- R. Bogel-Łukasik, V. Najdanovic-Visak, S. Barreiros and M. Nunes da Ponte, Ind. Eng. Chem. Res., 2008, 47, 4473–4480 CrossRef.
- R. M. Lau, F. van Rantwijk, K. R. Seddon and R. A. Sheldon, Org. Lett., 2000, 2, 4189–4191 CrossRef.
- M. Eckstein, P. Wasserscheid and U. Kragl, Biotechnol. Lett., 2002, 24, 763–767 CrossRef CAS.
- P. Lozano, T. de Diego, D. Carrie, M. Vaultier and J. L. Iborra, Chem. Commun., 2002, 692–693 RSC.
- M. T. Reetz, W. Wiesenhofer, G. Francio and W. Leitner, Chem. Commun., 2002, 992–993 RSC.
- R. M. Lau, M. J. Sorgedrager, G. Carrea, F. van Rantwijk, F. Secundo and R. A. Sheldon, Green Chem., 2004, 6, 483–487 RSC.
- T. De Diego, P. Lozano, S. Gmouh, M. Vaultier and J. L. Iborra, Biomacromolecules, 2005, 6, 1457–1464 CrossRef CAS.
- F. van Rantwijk, F. Secundo and R. A. Sheldon, Green Chem., 2006, 8, 282–286 RSC.
- H. Shan, Z. Li, M. Li, G. Ren and Y. Fang, J. Chem. Technol. Biotechnol., 2008, 83, 886–891 CrossRef CAS.
- R. Bogel-Łukasik, N. M. T. Lourenço, P. Vidinha, M. D. R. Gomes da Silva, C. A. M. Afonso, M. Nunes da Ponte and S. Barreiros, Green Chem., 2008, 10, 243–248 RSC.
- Z. Guo and X. Xu, Org. Biomol. Chem., 2005, 3, 2615–2619 CAS.
- M. Adamczak and U. T. Bornscheuer, Process Biochem., 2009, 44, 257–261 CrossRef CAS.
- M. Erbeldinger, A. J. Mesiano and A. J. Russell, Biotechnol. Prog., 2000, 16, 1129–1131 CrossRef CAS.
- K. Fujita, D. R. MacFarlane, M. Forsyth, M. Yoshizawa-Fujita, K. Murata, N. Nakamura and H. Ohno, Biomacromolecules, 2007, 8, 2080–2086 CrossRef CAS.
- K. Fujita, N. Nakamura, K. Igarashi, M. Samejima and H. Ohno, Green Chem., 2009, 11, 351–354 RSC.
- S. Bhosale, M. Rao and V. Deshpande, Microbiol. Rev., 1996, 60, 280–300 CAS.
- S. A. Barker, H. Pelmore and P. J. Somers, Enzyme Microb. Technol., 1983, 5, 121–124 CrossRef CAS.
- Y. Takasaki, Agric. Biol. Chem., 1971, 35, 1371–1375 CrossRef CAS.
- K. Visuri and A. M. Klibanov, Biotechnol. Bioeng., 1987, 30, 917–920 CrossRef CAS.
- J. Lewkowski, ARKIVOC, 2001, 17, 54 Search PubMed.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Energy Fuels, 2010, 24, 737–745 CrossRef CAS.
- T. Ståhlberg, W. Fu, J. M. Woodley and A. Riisager, ChemSusChem, 2011, 4, 451–458 CrossRef.
- M. E. Zakrzewska, E. Bogel-Łukasik and R. Bogel-Łukasik, Chem. Rev., 2011, 111, 397–417 CrossRef CAS.
- O. B. Jörgensen, L. G. Karlsen, N. B. Nielsen, S. Pedersen and S. Rugh, Starch/Staerke, 1988, 40, 307–313 CrossRef.
- C. A. Lobry de Bruyn and W. Alberda van Ekenstein, Recl. Trav. Chim. Pays-Bas, 1897, 16, 274–281 CAS.
- R. M. Hochster and R. W. Watson, Arch. Biochem. Biophys., 1954, 48, 120–129 CrossRef CAS.
- K. J. Schray and I. A. Rose, Biochemistry, 1971, 10, 1058–1062 CrossRef CAS.
- M. Whitlow, A. J. Howard, B. C. Finzel, T. L. Poulos, E. Winborne and G. L. Gilliland, Proteins: Struct., Funct., Genet., 1991, 9, 153–173 CrossRef CAS.
- J. Y. G. Chan and Y. Zhang, ChemSusChem, 2009, 2, 731–734 CrossRef CAS.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1600 CrossRef CAS.
- G. Yong, Y. Zhang and J. Ying, Angew. Chem., Int. Ed., 2008, 47, 9345–9348 CrossRef CAS.
- L. Lai and Y. Zhang, ChemSusChem, 2010, 3, 1257–1259 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1cy00155h |
| This journal is © The Royal Society of Chemistry 2012 |
