A magnetically recoverable γ-Fe2O3 nanocatalyst for the synthesis of 2-phenylquinazolines under solvent-free conditions†
Narani
Anand
a,
Kannapu Hari Prasad
Reddy
a,
Tirumalasetty
Satyanarayana
b,
Kamaraju Seetha Rama
Rao
a and
David Raju
Burri
*a
aCatalysis Laboratory, Indian Institute of Chemical Technology, Hyderabad-500607, India. E-mail: david@iict.res.in; Fax: +91-40-27160921; Tel: +91-40-27191712
bDepartment of Chemistry, PG College, Osmania University, Mirzapur-502249, India. E-mail: tirumalasettysatya@gmail.com; Tel: +91-8541-285762
First published on 6th December 2011
Abstract
A magnetically recoverable iron oxide (γ-Fe2O3) nanocatalyst (MRIONC) has been synthesized and characterized by XRD, FT-IR, XPS and TEM techniques and it was found that MRIONC is a new and highly efficient green catalyst for the synthesis of 2-phenylquinazolines from 2-aminoarylketones and benzyl amines under solvent free conditions, and in addition MRIONC could be easily recovered by a simple magnetic separation and recycled at least 5 times without significant loss in catalytic activity.
Introduction
Of late, magnetic iron nanoparticles have received a great deal of attention because of their unique physicochemical characteristics1 and wide range of applications in catalysis,2,3 protein separations,4 magnetic resonance imaging (MRI),5,6 drug delivery,7,8 magnetic sensors9 and magnetic refrigeration.10 Albeit the nanocatalysts are highly effective in the organic reactions, their wider applications have been hindered due to two important barriers such as (i) difficulty in recovery of the catalyst and (ii) safeguarding of the particle dimension for repeated use. Conventionally, recovery of the nanocatalyst is being done either by filtration or by centrifugation, wherein loss of a substantial amount of nanocatalyst is inevitable. At this juncture, recovery of the catalyst from the product mixture using an external magnet has been emerging recently as a new and effective technique.11As stated earlier, the second aspect that has to be borne in mind is the prevention of nanoparticle agglomeration either by depositing the nanoparticles on a suitable support or pursuing milder operating conditions. Another aspect that is to be taken care of is the protection of environment, which can be achieved to some extent by avoiding the solvents. Recently, the use of magnetic iron nanoparticles as catalysts for various organic transformations has been under intensive investigation.12,13Currently, quinazoline and its derivatives have drawn much interest in organic and medicinal chemistry because of their biological activities like anti-malarial agent,14 anti-cancer agent,15,16 anti-viral,17 anti-bacterial,18 and anti-tubercular agents.19 For the synthesis of biologically important quinazolines, several methods are available. Bischler cyclization is one of the conventional methods, in which reaction takes place between dicarbonyl compounds and diamines (2-aminobenzonitriles or anthranilic acids as well as N-arylbenzamides etc.).20–22 Kotsuki et al. developed a method for the synthesis of quinazolines by the condensation of cyano and nitro activated o-fluorobenzaldehyde with amidines via intramolecular nucleophilic aromatic substitution.23 Truong and Marrow reported a method for synthesizing quinazolines by the condensation of o-iodobenzaldehydes with amidine hydrochlorides under ligand-free copper catalysed Ullmann N-arylation conditions.24 Wang et al. developed an efficient method to synthesize 2-phenylquinazolines using molecular iodine/TBHP via SP3 C–H functionalization.25 Other pioneering recent reports of Wang et al. reveal the successful synthesis of quinazolines using supported copper oxide nanoparticles and metal-free intramolecular oxidative decarboxylative amination of primary α-amino acids under mild and neutral conditions.26,27 Recently, the synthesis of 2-phenylquinazolines has been achieved using ceric ammonium nitrate (CAN) and 4-hydroxy-TEMPO as catalysts.28,29 However, synthesis of these biologically important quinazolines using eco-friendly and reusable catalysts under solvent free conditions is still a thrust area in the chemical sector.
Hence, herein, we report the environmentally friendly MRIONC as a highly efficient heterogeneous catalyst for the synthesis of 2-phenylquinazolines from 2-aminoarylketones and benzyl amines under solvent free conditions for the first time. Ease of separation, solvent free operation and sustainable activity are the credentials of the MRIONC.
Experimental
Preparation of MRIONC
The magnetic Fe3O4 nanocatalyst was synthesized by a co-precipitation technique using Fe2+ and Fe3+ in accordance with the literature reports.30 In a typical procedure FeCl2·4H2O and FeCl3·6H2O (mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were dissolved in distilled water under a nitrogen atmosphere. After slow addition of 25% NH4OH, the solution turned into dark black colour (pH = 10). The resultant black coloured solution was stirred for 1 h at room temperature and heated to 60 °C and the stirring was continued for 1 h at this temperature. Later on, the solution was cooled down to room temperature under constant stirring. The precipitated Fe3O4 magnetic nanoparticles were washed with distilled water and ethanol. The obtained Fe3O4 nanoparticles were collected with the help of an external magnet and dried at 60 °C for 2 h under vacuum and subjected to thermal treatment at 150 °C for 2 h in air which resulted in the formation of MRIONC.31
2) were dissolved in distilled water under a nitrogen atmosphere. After slow addition of 25% NH4OH, the solution turned into dark black colour (pH = 10). The resultant black coloured solution was stirred for 1 h at room temperature and heated to 60 °C and the stirring was continued for 1 h at this temperature. Later on, the solution was cooled down to room temperature under constant stirring. The precipitated Fe3O4 magnetic nanoparticles were washed with distilled water and ethanol. The obtained Fe3O4 nanoparticles were collected with the help of an external magnet and dried at 60 °C for 2 h under vacuum and subjected to thermal treatment at 150 °C for 2 h in air which resulted in the formation of MRIONC.31
Characterization of MRIONC
X-Ray diffraction (XRD) patterns of catalysts were obtained on a Bruker D8 Advance X-Ray Powder Diffractometer using Ni filtered Cu Kα radiation at a scan speed of 2° min−1. X-Ray photoelectron spectroscopy (XPS) analysis of the catalyst was carried out by a Kratos analytical spectrophotometer, with Mg Kα monochromated excited radiation (1253.6 eV). The residual pressure in the analysis chamber was around 10−9 mbar. The binding energy (BE) measurements were corrected for charging effects with reference to the C 1s peak of the adventitious carbon (284.6 eV). Infrared spectra were recorded on a Bruker Alpha-T, FT-IR system, in the scan range of 4000–400 cm−1. A Philips Tecnai F12 FEI transmission electron microscope (TEM) operating at 80–100 kV was used to record TEM images.Catalytic reaction
A mixture of 2-aminoacetophenone (1 mmol), benzyl amine (1.2 mmol) and catalyst (25 mg) was taken in a 25 ml round bottom flask. Then 70% tert-butyl hydrogen peroxide in water (2.5 mmol) was added slowly to the reaction mixture. The reaction mixture was stirred at 85 °C for a given reaction time under solvent free conditions. After completion of the reaction (monitored by TLC), the reaction mixture was cooled to room temperature and diluted with ethyl acetate, the catalyst was separated from reaction mixture using an external magnet. The crude residue was washed with brine solution, dried over anhydrous Na2SO4, evaporated to get the crude product and purified by column chromatography to obtain the desired product. All the products were characterized by 1H NMR and 13C NMR and the data were compared with existing literature.Results and discussion
The XRD patterns of MRIONC (a) before and (b) after the reaction are displayed in Fig. 1. The main characteristic peaks of γ-Fe2O3 appeared around 18.32°, 30.14°, 35.73°, 43.51°, 53.76°, 57.1°, 62.94° etc., which correspond to (111), (220), (311), (400), (422), (511), and (440) planes.32,33 The observed diffraction peaks well matched with the cubic structure of maghemite γ-Fe2O3 (JCPDS file 39-1346). No substantial variation was observed in the XRD patterns of fresh and used catalysts, revealing the retention of the original structure of maghemite γ-Fe2O3 in the used catalyst.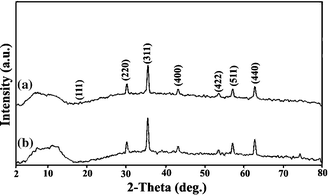 | ||
| Fig. 1 X-Ray diffraction patterns of (a) fresh (b) used MRIONC. | ||
The XPS spectrum of MRIONC is depicted in Fig. 2. The binding energies of 711.43 and 723.86 eV correspond to Fe2p3/2 and Fe2p1/2 of Fe3+ of γ-Fe2O3. Another peak that appeared at a binding energy of 718.75 eV of Fe2p3/2 is the characteristic satellite peak of Fe3+ species. The Fe2p3/2 satellite peak supposed to appear at 716 eV is the characteristic peak of Fe+2 species, which is absent, suggesting the absence of Fe3O4 in the catalyst.34,35
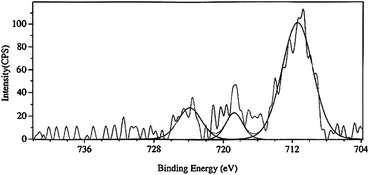 | ||
| Fig. 2 X-Ray photoelectron spectrum of MRIONC. | ||
FT-IR spectra of fresh and used catalysts are shown in Fig. 3. The IR bands at 3438 cm−1 and 1638 cm−1 appeared in both fresh and used catalysts which match with the stretching and deformation vibration of –OH groups on the surface of γ-Fe2O3. The strong bands at 634 and 583 cm−1 and a weak band at 443 cm−1 refer to Fe–O vibration of γ-Fe2O3 nanoparticles found in both catalyst samples.36,37 The FT-IR results are in agreement with the XRD and XPS for the characterization of the maghemite phase of γ-Fe2O3 nanoparticles.
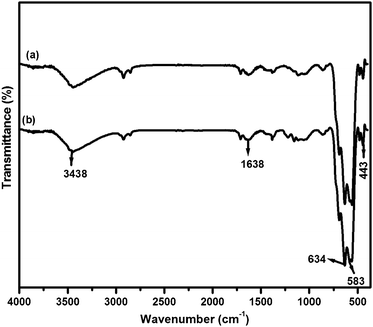 | ||
| Fig. 3 FT-IR spectra of (a) fresh (b) used MRIONC. | ||
The TEM images of (a) fresh and (b) used MRIONC are displayed in Fig. 4, which reveal that the iron oxide nanoparticles are spherical in nature and are present in the form of loosely bound nanoaggregates. The particle size distribution graphs of fresh and used catalysts are shown in Fig. 4 as insets. After 5 times of repeated use, the TEM image for MRIONC was recorded (Fig. 4b), which is more or less similar to that of the fresh MRIONC (Fig. 4a).
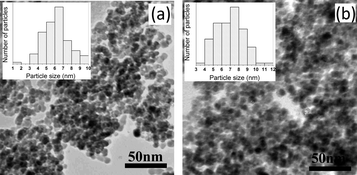 | ||
| Fig. 4 TEM images of (a) fresh (b) used MRIONC. | ||
Initially, the catalytic performance of MRIONC was tested for the synthesis of 2-phenylquinazolines by reacting 2-aminoacetophenone (1 mmol) with benzyl amine (1 mmol) using MRIONC (13 mg) and TBHP (2 mmol) under solvent free conditions at 85 °C for 7 h, in which the yield of the desired product is found to be 59%. These results encouraged us to optimize the reaction conditions. In the subsequent study, different oxidants such as molecular oxygen, 90% urea hydrogen peroxide in water (UHP), 30% hydrogen peroxide in water (H2O2) and 70% tert-butyl hydrogen peroxide in water (TBHP) are examined and the results are summarized in Table 1. Except TBHP none of the oxidants is found to be suitable. To find out the appropriate solvent, the reaction was conducted using different solvents such as toluene, DMF, DMSO, acetonitrile, water and neat system. The results are shown in Table 2, which unveil that the solvent-free (neat) system is superior to the solvent systems. When the reaction was conducted using 1 mmol of 2-aminoacetophenone, 1.2 mmol of benzyl amine, 25 mg of catalyst and 2.5 mmol of TBHP at 85 °C for 7 h, the yield of 2-phenylquinazoline improved to 90%. Unless otherwise specified the above reaction conditions are optimal.
| Entry | Oxidant | Yield (%) |
|---|---|---|
| Reaction conditions: 2-aminoacetophenone (1 mmol), benzylamine (1.2 mmol), catalyst, (25 mg), TBHP (2.5 mmol), 85 °C, 7 h. | ||
| 1 | O2 | — |
| 2 | Urea hydrogen peroxide | Trace |
| 3 | 30% hydrogen peroxide in water | 3 |
| 4 | 70% tert-butyl hydrogen peroxide in water (TBHP) | 90 |
| Entry | Solvent | Yield (%) |
|---|---|---|
| Reaction conditions: 2-aminoacetophenone (1 mmol), benzyl amine (1.2 mmol), catalyst, (25 mg), TBHP (2.5 mmol), 85 °C, 7 h. | ||
| 1 | Toluene | 59 |
| 2 | DMF | 28 |
| 3 | DMSO | 20 |
| 4 | Acetonitrile | 15 |
| 5 | Water | Trace |
| 6 | Neat | 90 |
To compare the catalytic performance of MRIONC with bulk iron oxide (Fe2O3) and its homogeneous counterparts (FeCl3·6H2O and Fe(NO3)·9H2O) different experiments were conducted under optimized conditions. When the commercial Fe2O3 powder is used as a catalyst the yield of 2-phenylquinazoline is only 28%. With FeCl3·6H2O the yield of 2-phenylquinazoline is 48%, whereas with Fe(NO3)3·9H2O the yield of 2-phenylquinazoline is 17%. The presence of Fe3+ species in the reaction mixture alone cannot drive the reaction adequately to produce 2-phenylquinazoline; it should be in a definite structure, shape and size (MRIONC). No products were observed without using MRIONC or TBHP, which implies that the oxidative coupling of 2-aminoacetophenone with benzyl amine to yield 2-phenylquinazoline is exclusively catalytic and the usage of oxidant (TBHP) is obvious.
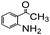
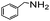
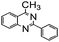
Using the optimized reaction conditions, various 2-aminoacetoophenone/2-benzophenones are coupled with benzyl amines and the results are summarized in Table 3. Wherein, the yields of desired products are good to excellent (Table 3, entries 1 and 5), functional groups present on the benzyl amines played a significant role. When the electron donating groups are present on benzyl amines, the product yields are slightly lowered (Table 3, entries 2 and 6). Contrarily, electron withdrawing groups favour the reaction (Table 3, entries 3, 4, 7 and 8). When the electron withdrawing groups such as chloro, bromo, nitro are present on the aniline ring of the 2-aminobenzophenone, the yields are higher (Table 3, entries 11–13) compared to electron donating groups (Table 3, entries 9 and 10).
| Entry | 2-Aminobenzoketone | Benzylamines | 2-Phenylquinazolines | Yield (%) |
|---|---|---|---|---|
| Reaction conditions: 2-aminoacetophenone (1 mmol), benzyl amine (1.2 mmol), catalyst (25 mg), TBHP (2.5 mmol), 85 °C, 7 h. | ||||
| 1 |
|
|
|
90 |
| 2 |
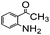
|
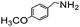
|
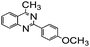
|
87 |
| 3 |
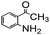
|
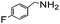
|
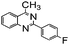
|
92 |
| 4 |
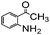
|
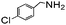
|
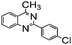
|
91 |
| 5 |
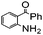
|
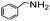
|
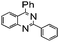
|
96 |
| 6 |
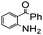
|
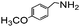
|
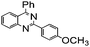
|
89 |
| 7 |
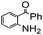
|
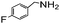
|
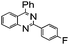
|
93 |
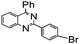
| 8 |
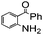
|
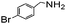
|
|
92 |
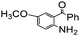
| 9 |
|
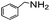
|
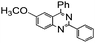
|
88 |
| 10 |
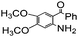
|
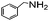
|
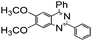
|
82 |
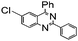
| 11 |
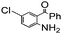
|
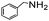
|
|
90 |
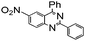
| 12 |
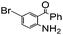
|
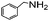
|
|
91 |
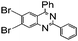
| 13 |
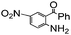
|
|
|
93 |
To evaluate the catalyst stability in repeated use, the used catalyst was recovered with an external magnet from the reaction mixture, washed with ethyl acetate and ethanol and dried at 50 °C for 4 h and reused, and the data obtained are displayed in Fig. 5, from which it is clear that the catalytic activity is constant at least for five repeated cycles. The reason is that the characteristics obtained from XRD, FT-IR and TEM of fresh and used catalysts are similar, which suggest the retention of structure and morphology of MRIONC after repeated use as catalyst except a minute change in the particle size distribution.
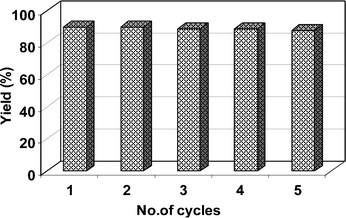 | ||
| Fig. 5 Recyclability of the catalyst for the synthesis of 2-phenylquinazolines. | ||
However in the present context the exact mechanism is not clear; on the basis of present results and literature reports a plausible mechanism is proposed as shown in Scheme 1, initially tert-butyl hydrogen peroxide forms a highly valent Fe4+,38 which reacts with imine (I) to form a nano γ-Fe2O3 stabilized imine cation (II), then it readily undergoes intramolecular cyclization to form III, which undergoes further oxidation to yield the desired product.
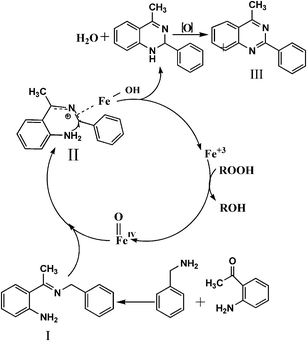 | ||
| Scheme 1 Plausible mechanism for the MRIONC catalysed synthesis of 2-phenylquinazolines. | ||
Conclusions
In conclusion, MRIONC is a versatile and efficient heterogeneous catalyst for the preparation of 2-phenylquinazolines from 2-aminoarylketones and benzyl amines under solvent-free conditions. The catalyst is capable of catalysing quinazoline derivatives in good to excellent yields. The preparation of the catalyst is simple, inexpensive, non-corrosive and environmentally benign. The catalyst can be easily separated and reused without substantial loss in activity.Acknowledgements
Anand and Hari Prasad Reddy are thankful to Council of Scientific and Industrial Research (CSIR) New Delhi, for awarding research fellowships.Notes and references
- A. H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef CAS.
- R. A. Reziq, D. Wang, M. Post and H. Alper, Chem. Mater., 2008, 20, 2544 CrossRef.
- C. H. Jun, Y. J. Park, Y.-R. Yeon, J. R. Choi, W. R. Lee, S. J. Ko and J. Cheon, Chem. Commun., 2006, 1619 RSC.
- J. Bao, W. Chen, T. Liu, Y. Zhu, P. Jin, L. Wang, J. Liu, Y. Wei and Y. Li, ACS Nano, 2007, 1, 293 CrossRef CAS.
- L. Wang, K. G. Neoh, E. T. Kang, B. Shuter and S. C. Wang, Biomaterials, 2010, 31, 3502 CrossRef CAS.
- F. Hu, L. Wei, Z. Zhou, Y. Ran, Z. Li and M. Gao, Adv. Mater., 2006, 18, 2553 CrossRef CAS.
- N. Nasongkla, E. Bey, J. Ren, H. Ai, C. Khemtong, J. S. Guthi, S. F. Chin, A. D. Sherry, D. A. Boothman and J. Gao, Nano Lett., 2006, 6, 2427 CrossRef CAS.
- C. Sun, J. S. H. Lee and M. Zhang, Adv. Drug Delivery Rev., 2008, 60, 1252 CrossRef CAS.
- S. G. Grancharov, H. Zeng, S. Sun, S. X. Wang, S. O'Brien, C. B. Murray, J. R. Kirtley and G. A. Held, J. Phys. Chem. B, 2005, 109, 13030 CrossRef CAS.
- O. Tegus, E. Bruck, K. H. J. Buschow and F. R. de Boer, Nature, 2002, 415, 150 CrossRef CAS.
- D. Guin, B. Baruwati and S. V. Manorama, Org. Lett., 2007, 9, 1419 CrossRef CAS.
- T. Zeng, W. W. Chen, C. M. Cirtiu, A. Moores, G. Song and C. J. Li, Green Chem., 2010, 12, 570 RSC.
- B. Sreedhar, A. Suresh Kumar and P. Surendra Reddy, Tetrahedron Lett., 2010, 51, 1891 CrossRef CAS.
- W. T. Ashton and J. B. Hynes, J. Med. Chem., 1973, 16, 1233 CrossRef CAS.
- E. A. Henderson, V. Bavetsias, D. S. Theti, S. C. Wilson, R. Clauss and A. L. Jackman, Bioorg. Med. Chem., 2006, 14, 5020 CrossRef CAS.
- B. Baruah, K. Dasu, B. Vaitilingam, P. Mamnoor, P. P. Venkata, S. Rajagopal and K. R. Yeleswarapu, Bioorg. Med. Chem., 2004, 12, 1991 CrossRef CAS.
- T. C. Chien, C. S. Chen, F. H. Yu and J. W. Chern, Chem. Pharm. Bull., 2004, 52, 1422 CrossRef CAS.
- H. Kakuta, A. Tanatani, K. Nagasawa and Y. Hashimoto, Chem. Pharm. Bull., 2003, 51, 1273 CrossRef CAS.
- K. Waisser, J. Gregor, H. Dostal, J. Kunes, L. Kubicova, V. Klimesova and J. Kaustova, Farmaco, 2001, 56, 803 CrossRef CAS.
- J. R. Li, X. Chen, D. X. Shi, S. L. Ma, Q. Li, Q. Zhang and J. H. Tang, Org. Lett., 2009, 11, 1193 CrossRef CAS.
- D. J. Connolly, D. Cusack, T. P. OSullivan and P. J. Guiry, Tetrahedron, 2005, 61, 10153 CrossRef CAS.
- A. Witt and J. Bergman, Curr. Org. Chem., 2003, 7, 659 CrossRef CAS.
- H. Kotsuki, H. Sakai, H. Morimoto and H. Suenaga, Synlett, 1999, 1993 CrossRef CAS.
- V. L. Truong and M. Marrow, Tetrahedron Lett., 2010, 51, 758–760 CrossRef CAS.
- J. Zhang, D. Zhu, C. Yu, C. Wan and Z. Y. Wang, Org. Lett., 2010, 12, 2841 CrossRef CAS.
- J. Zhang, C. Yu, S. Wang, C. Wan and Z. Y. Wang, Chem. Commun., 2010, 46, 5244 RSC.
- Y. Yan and Z. Y. Wang, Chem. Commun., 2011, 47, 9513 RSC.
- K. Karnakar, J. Shankar, S. Narayana Murthy, K. Ramesh and Y. V. D. Nageswar, Synlett, 2011, 1089 CAS.
- B. Han, C. Wang, R.-F. Han, W. Yu, X.-Y. Duan, R. Fang and X.-L. Yang, Chem. Commun., 2011, 47, 7818 RSC.
- V. Polshettiwar, B. Baruwati and R. S. Varma, Green Chem., 2009, 11, 127 RSC.
- F. Jiao, J. C. Jumas, M. Womes, A. V. Chadwick, A. Harrison and P. G. Bruce, J. Am. Chem. Soc., 2006, 128, 12905 CrossRef CAS.
- K. Woo, J. Hong, S. Choi, H. W. Lee, J.-P. Ahn, C. S. Kim and S. W. Lee, Chem. Mater., 2004, 16, 2814 CrossRef CAS.
- C. Pereira, A. M. Pereira, P. Quaresma, P. B. Tavares, E. Pereira, J. P. Araujob and C. Freire, Dalton Trans., 2010, 39, 2842 RSC.
- D. Ma, T. Veres, L. Clime, F. Normandin, J. Guan, D. Kingston and B. Simard, J. Phys. Chem. C, 2007, 111, 1999 CAS.
- T. Fujii, F. M. F. de Groot, G. A. Sawatzky, F. C. Voogt, T. Hibma and K. Okada, Phys. Rev. B: Condens. Matter, 1999, 59, 3195 CrossRef CAS.
- R. de Palma, J. Trekker, S. Peeters, M. J. Van Bael, K. Bonroy, R. W. Speetjens, G. Reekmans, W. Laureyn, G. Borghs and G. Maes, J. Nanosci. Nanotechnol., 2007, 7, 4626 CAS.
- T. Belin, N. G. Millot, T. Caillot, D. Aymes and J. C. Niepce, J. Solid State Chem., 2002, 163, 459 CrossRef CAS.
- W. Nam, H. J. Han, S. Y. Oh, Y. J. Lee, M. H. Choi, S. Y. Han, C. Kim, S. K. Woo and W. Shin, J. Am. Chem. Soc., 2000, 122, 8677 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1cy00341k |
| This journal is © The Royal Society of Chemistry 2012 |