Amination and dehydration of 1,3-propanediol by hydrogen transfer: reactions of a bio-renewable platform chemical†
Sophie D.
Lacroix
,
Annie
Pennycook
,
Shifang
Liu
,
Thomas T.
Eisenhart
and
Andrew C.
Marr
*
School of Chemistry and Chemical Engineering, Queen's University Belfast, David Keir Building, Belfast, UK BT9 5AG. E-mail: a.marr@qub.ac.uk; Fax: +44 (0)28 9097 6524; Tel: +44 (0)28 9097 4442
First published on 17th October 2011
Abstract
1,3-propanediol was subjected to a range of amination conditions. The N-heterocyclic carbene piano stool complex [Cp*IrCl2(bmim)] was found to be a good catalyst for amination and dehydration in toluene or ionic liquid; product compositions could be tuned by altering the ratio of diol to amine.
Chemicals that can be derived from biomass will be of increasing importance as oil resources become stretched over an ever expanding market. The efficient conversion of biomass to commercial chemicals is the key to a more sustainable chemical industry. We have been investigating the chemo-catalytic conversion of chemicals that can be derived from biomass by fermentation.1,21,3-Propanediol (1,3-PDO) is one such intermediate, for example the fermentation of glycerol from biodiesel production by Clostridium butyricum affords 1,3-propanediol with good productivity.2 This diol typifies the products of fermentation as it is aliphatic and highly oxygenated.
Organometallic hydrogen transfer catalysts have great potential to transform bio-renewable alcohols into value added products.3 One such class of catalyst has the general formula [Cp*IrCl2(NHC)] (NHC = N-heterocyclic carbenes). The high hydrogen transfer activity of these complexes has been demonstrated.2,4–7 Previously we showed that complex 1 (Fig. 1) performed better than leading hydrogen transfer catalysts for the amination of 1,3-PDO and furthermore the complex exhibited activity in an ionic liquid at low temperatures.2Catalyst 1 has also shown good potential in the chemoenzymic dynamic kinetic resolution of secondary alcohols to enantiopure esters, although this activity depends on the batch of enzyme employed.1,5 In this communication we report the testing of related catalyst 2, active in ionic liquid methyl-trioctylammonium bis(trifluoromethylsulfonyl)imide (N1,8,8,8NTf2), that enables better control over the selectivity of the reaction. In addition, both 1 and 2 show an unprecedented tendency to dehydrate 1,3-PDO. It has been shown, for catalyst 2, that dehydration occurs in the absence of added amine.
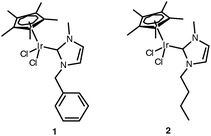 | ||
| Fig. 1 Hydrogen transfer catalysts. | ||
N-alkylation of aniline using 1,3-PDO may be expected to generate mono or di-amines or quinolines (particularly at higher temperatures),8–13 Unexpected, was the observation of the product of concurrent dehydration (5 in Scheme 1).2
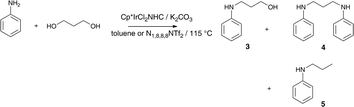 | ||
| Scheme 1 The reaction of aniline with 1,3-PDO promoted by hydrogen transfer. | ||
In the reaction of aniline with 1,3-propanediol catalyzed by 1 in toluene in a sealed tube at 115 °C, diamination and dehydration were observed, 1,3-PDO was converted to yield products 4 and 5 (65% and 35% respectively by 1H NMR).2
In order to make the catalyst more accessible, we attempted to prepare a catalyst from 1-butyl-3-methylimidazolium halide [(bmim)X]; these salts are readily available, primarily due to application in the synthesis of ionic liquids.14 When the imidazolium bromide salt was employed in the synthetic procedure that yielded 1,15 namely the reaction with silver oxide followed by [Cp*IrCl2]2, 2 was obtained. After repeated filtering through celite to remove silver contaminating the product, crystals of sufficient quality were grown by diffusion of 1-hexane into a dichloromethane solution of 2 and the structure was solved by single crystal X-Ray diffraction (Fig. 2).
![Structure of [Cp*IrCl2(bmim)]. Thermal ellipsoids are at the 50% level. Hydrogen atoms are omitted for clarity. Further details are included in the ESI.](/image/article/2012/CY/c1cy00339a/c1cy00339a-f2.gif) | ||
| Fig. 2 Structure of [Cp*IrCl2(bmim)]. Thermal ellipsoids are at the 50% level. Hydrogen atoms are omitted for clarity. Further details are included in the ESI.† | ||
The N-alkylation activities of 1 and 2 were compared (Table 1). 1,3-PDO and aniline were dissolved in N1,8,8,8NTf2 or toluene in the presence of 1 or 2 and K2CO3 and heated to 115 °C in a sealed tube. Ionic liquids have great potential as a solvent for N-alkylation by hydrogen transfer.2,16 Under these conditions the activity of both catalysts was greater in N1,8,8,8NTf2 than toluene and full conversion could be achieved at higher concentration and lower concentration of 1,3-PDO after 24 and 48 h respectively.
| a | Cat. | Solvent | Time/ h | 1,3-PDO/mmol | Aniline/mmol | Conv’n/%b |
|---|---|---|---|---|---|---|
| a Solvent (1 mL), catalyst (0.02 mmol), K2CO3 (0.10 mmol). b By 1H NMR. | ||||||
| 1 | 1 | toluene | 24 | 2.0 | 2.0 | 24 |
| 2 | 2 | toluene | 24 | 2.0 | 2.0 | 71 |
| 3 | 1 | N1,8,8,8NTf2 | 24 | 2.0 | 2.0 | >99 |
| 4 | 2 | N1,8,8,8NTf2 | 24 | 2.0 | 2.0 | >99 |
| 5 | 2 | N1,8,8,8NTf2 | 48 | 0.21 | 0.42 | >99 |
| 6 | 2 | N1,8,8,8NTf2 | 48 | 0.21 | 2.0 | >99 |
Catalyst
2 was tested under a range of conditions in order to control the product distribution (Table 2). With equimolar amounts of aniline and 1,3-PDO, for catalyst 1amination was found to be dominant in toluene, as noted previously.2 While dehydration becomes highly significant operating in N1,8,8,8NTf2 (entry 3), this effect was not so marked for catalyst 2 (entry 4) at higher concentrations of 1,3-PDO, and this reflects the higher rates of amination for this catalyst (compare entries 1 and 2, Table 1). At lower concentrations of 1,3-PDO the product distribution could be tuned by changing the ratio of aniline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,3-PDO. With a ratio of 2
1,3-PDO. With a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 the product was predominantly 5, the product of amination of one hydroxyl and dehydration and hydrogenation at the other. At 10
1 the product was predominantly 5, the product of amination of one hydroxyl and dehydration and hydrogenation at the other. At 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 di-amination dominates producing 4 as the major product.
1 di-amination dominates producing 4 as the major product.
N-allyl aniline was detected in product solutions by GC-MS revealing that one route to N-propyl aniline was viaamination to 3 followed by dehydration to N-allyl aniline and subsequent hydrogenation. It seemed unlikely, however, that this was the only route, as the primary product of dehydrogenation 3-hydroxypropaldehyde has a documented dehydration chemistry.17 To test the dehydration behaviour of 1,3-PDO in the presence of 2 and absence of amine, a sealed tube was prepared containing the catalyst 1,3-PDO, K2CO3 and 4 Å molecular sieves in toluene. After 24 h at 115 °C the product mixture was analysed by GC-MS and found to contain 2-methyl-2-pentenal as a major and acrolein dimer as a minor product (Scheme 2).
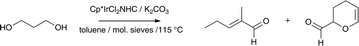 | ||
| Scheme 2 Products of Dehydration of 1,3-PDO Catalyzed by 2. | ||
In both cases the diol has been dehydrogenated, dehydrated and dimerized. Major product 2-methyl-2-pentenal is the formed in a ‘modified Guerbet reaction’, dehydrogenation is followed by aldol condensation and dehydration, but, in contrast to conventional Guerbet chemistry,18,19 the subsequent α,β-unsaturated aldehyde is not reduced. Acrolein is formed when the product of dehydrogenation 3-hydroxypropaldehyde is dehydrated then dimerizes. Initial results suggest that the relative amounts of these products are catalyst dependent.
A range of products can be prepared from the bio-renewable platform chemical 1,3-propanediol, by hydrogen transfer employing organometallic catalysts. Amination and dehydration reactions have been demonstrated, catalyzed by the piano stool complex 2. The relative rate of these reactions can be manipulated to generate five different products in highly atom efficient reactions that only generate H2O and/or H2 as side products; thus demonstrating the enormous synthetic potential of applying hydrogen transfer catalysts to renewable alcohols.
Funded by EPSRC life science interface grant EP/E010636/1 and Queen's University Belfast. Thanks to Dr John Briggs at Dow; ASEP (in particular Dr Robin Patrick) at Queen's University Belfast and Dr Peter Horton at the EPSRC National Crystallography Service, University of Southampton.
Notes and references
- A. C. Marr and S. F. Liu, Trends Biotechnol., 2011, 29, 199–204 CrossRef CAS.
- S. F. Liu, M. Rebros, G. Stephens and A. C. Marr, Chem. Commun., 2009, 2308–2310 RSC.
- A. C. Marr, Catal. Sci. Technol. 10.1039/c1cy00338k.
- F. Hanasaka, K. Fujita and R. Yamaguchi, Organometallics, 2004, 23, 1490–1492 CrossRef CAS.
- A. C. Marr, C. L. Pollock and G. C. Saunders, Organometallics, 2007, 26, 3283–3285 CrossRef CAS.
- A. Prades, R. Corberán, M. Poyatos and E. Peris, Chem.–Eur. J., 2008, 14, 11474–11479 CrossRef CAS.
- A. Pontes da Costa, M. Viciano, M. Sanaú, S. Merino, J. Tejeda, E. Peris and B. Royo, Organometallics, 2008, 27, 1305–1309 CrossRef CAS.
- Y. Tsuji, K.-T. Huh and Y. Watanabe, J. Org. Chem., 1987, 52, 1673–1680 CrossRef CAS.
- C. S. Cho, H. Y. Lim, S. C. Shim, T. J. Kim and H.-J. Choi, Chem. Commun., 1998, 995–996 RSC.
- R. N. Monrad and R. Madsen, Org. Biomol. Chem., 2011, 9, 610–615 CAS.
- H. Aramoto, Y. Obora and Y. Ishii, J. Org. Chem., 2009, 74, 628–633 CrossRef CAS.
- Y. Obora and Y. Ishii, Synlett, 2011, 30–51 CrossRef CAS.
- R. Yamaguchi, K. Fujita and M. W. Zhu, Heterocycles, 2010, 81, 1093–1140 CrossRef CAS.
- J. D. Holbrey and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133–2140 RSC.
- R. Corberán, M. Sanaú and E. Peris, J. Am. Chem. Soc., 2006, 128, 3974–3979 CrossRef.
- O. Saidi, A. J. Blacker, G. W. Lamb, S. P. Marsden, J. E. Taylor and J. M. J. Williams, Org. Process Res. Dev., 2010, 14, 1046–1049 CrossRef CAS.
- R. H. Hall and E. S. Stern, J. Chem. Soc., 1950, 490–498 RSC.
- M. Guerbet, C. R. Acad. Sci., 1899, 128, 1002–1004 Search PubMed.
- M. Guerbet, C. R. Acad. Sci., 1909, 149, 129–132 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic and catalytic procedures, analytical data, X-Ray structure data and report. CCDC reference number 846741. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1cy00339a |
| This journal is © The Royal Society of Chemistry 2012 |
