Development of an O3-assisted photocatalytic water-purification unit by using a TiO2 modified titanium mesh filter
Tsuyoshi
Ochiai
*ab,
Hayato
Nanba
ac,
Touko
Nakagawa
d,
Ken
Masuko
d,
Kazuya
Nakata
ab,
Taketoshi
Murakami
a,
Ryuichi
Nakano
ae,
Masayuki
Hara
e,
Yoshihiro
Koide
c,
Tomonori
Suzuki
d,
Masahiko
Ikekita
bd,
Yuko
Morito
bf and
Akira
Fujishima
ab
aKanagawa Academy of Science and Technology, KSP East 421, 3-2-1 Sakado, Takatsu-ku, Kawasaki, Kanagawa, 213-0012, Japan. E-mail: pg-ochiai@newkast.or.jp; Fax: +81-44-819-2070; Tel: +81-44-819-2040
bDivision of Photocatalyst for Energy and Environment, Research Institute for Science and Technology, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo, 162-8601, Japan
cDepartment of Material and Life Chemistry, Faculty of Engineering, Kanagawa University, 3-27-1 Rokkakubashi, Yokohama, Kanagawa, 221-8686, Japan
dDepartment of Applied Biological Science, Tokyo University of Science, Yamazaki 2641, Noda, Chiba, 278-8510, Japan
eKitasato Research Center for Environment Science, 1-15-1, Kitasato, Minami-ku, Sagamihara, Kanagawa, 252-0329, Japan
fU-VIX Corporation, 2-14-8 Midorigaoka, Meguro-ku, Tokyo, 152-0034, Japan
First published on 5th October 2011
Abstract
An ozone-assisted photocatalytic water-purification unit using a TiO2 modified titanium-mesh sheet (TMiP) was investigated. Significant decomposition of biological and chemical contaminants has been achieved by highly active intermediates formed by catalytic decomposition and photocatalysis.
Water purification is one of the most important technologies for human life. The strong oxidation ability of TiO2 has received growing attention.1–3TiO2 generates hydroxyl radicals and superoxide ions by UV light irradiation. These are highly reactive with organic compounds. Thus, a TiO2 photocatalyst is expected to be effective in a water purification system. However, there are several limitations such as electron–hole recombination, low efficiency, and difficulty in decomposition of large amounts of pollutants. In the previous work, the fabrication method of easy-to-handle photocatalytic filter material, TiO2 nanoparticles impregnated titanium mesh (TMiPTM), and its ability for air- and water-purification were investigated.4 Based on these results, here we reported an ozone-assisted photocatalytic water-purification unit using a TMiP. Biological and chemical purification efficiencies of the reactor were examined by a decomposition test of waterborne pathogens and phenol, respectively.
Fig. 1 shows the schematic illustration of a water-purification system which consists of a water-purification unit, a reservoir, a pump, and an O3 production unit. For comparison of the effect of UV-C, TMiP, and O3 bubbling, four types of units were investigated (Fig. 2): (a) UV-C + TMiP + O3, (b) O3 only, (c) UV-C + TMiP, and (d) UV-C only. An acrylic tube was equipped with two ports for water flow inlet and outlet. The irradiation was provided by a UV-C lamp (Cnlight, 18 W) in the tube. The UV-C lamp was wrapped using a corrugated TMiP sheet. An O3 production unit consists of the following parts: oxygen source, flow meter, ozone generator using a discharge cell, and bubbler. One litre of an aqueous solution containing a large number of waterborne pathogens or phenol was circulated through the units by a pump at a flow rate of 1 L min−1 and was treated under each condition. The experiments were carried out at room temperature and atmospheric pressure.
 | ||
| Fig. 1 Schematic view of a water-purification system using the ozone-assisted photocatalytic water-purification unit. | ||
 | ||
| Fig. 2 Schematic illustrations of water-purification units (inside the broken rectangle in Fig. 1). (a) UV-C + TMiP + O3, (b) O3 only, (c) UV-C + TMiP, (d) UV-C only. Sample vol.: 1 L, flow: 1 L min−1, UV intensity: 15 mW cm−2 at 254 nm. | ||
E. coli (NBRC 13965) and Qβ phage were used as the main test waterborne pathogens to assess the biological purification efficiency of the units. E. coli and Qβ phage used in this study were obtained from the Biological Resource Center of the National Institute of Technology and Evaluation (NITE-BRC). E. coli was consecutively cultivated on nutrient broth (NB) agar at 37 °C for 18–20 h; then, the live cells were collected and suspended in PBS solution at a bacterial density of approximately 109 colony-forming units (CFU) per mL. The suspensions of E. coli were then used as the biologically contaminated water sample. Qβ phage was prepared following a similar method,5 with a density of approximately 1011 plaque-forming units (PFU) per mL.
The viability of E. coli in the artificially contaminated water samples was analyzed in NB agar medium. The water samples were diluted with PBS in a 10-fold dilution series. One-hundred microlitre aliquots of each dilution stage of the water samples were plated onto NB agar in a 10 cm Petri dish and were incubated at 37 °C for 24 h to determine the number of CFUs. The bacterial survival rate was calculated as: bacterial survival rate (α min) = CFU (α min) × 100/CFU (0 min). Similarly, plaque assays to determine phage titer were performed by individually mixing 100 μL of an overnight culture of E. coli, 900 μL of the water samples at each dilution stage, and 3 mL of top layer agar (Bacto-Agar 0.5%), at 45 °C. The mixture was pour plated onto a NB agar plate and allowed to solidify. The plates were incubated overnight at 37 °C for 24 h to determine the number of PFUs. The survival rate was calculated as: survival rate (α min) = PFU (α min) × 100/PFU (0 min). On the other hand, one litre of an aqueous solution containing 273 mg L−1phenol was supplied to the units and was treated under each condition. The concentration of dissolved phenol as a function of irradiation time was measured with a Digital pack test for phenol (Kyoritsu Chemical-Check Lab., Corp) to assess the chemical purification efficiency of the units.
Fig. 3 shows the time course of the log survival rate of E. coli with the units. The survival rate of E. coli in the artificially contaminated water was decreased by 105–107 (below the detection limits of colony-forming method) for each unit. By using the UV-C only (crosses) or the UV-C + TMiP (solid circles) unit, more than 5 min of treatment time was required for total inactivation of E. coli. In contrast, the O3 only (solid triangles) or the UV-C + TMiP + O3 (open circles) unit takes a shorter treatment time (less than 1 min) for the inactivation. There is no difference between the O3 only unit and the UV-C + TMiP + O3 unit. On the other hand, Qβ phage could not be inactivated by the UV-C + TMiP unit efficiently (Fig. 4, solid circles). The difference of inactivation efficiency between E. coli and Qβ phage by the UV-C + TMiP unit may be due to the size of the waterborne pathogens. Because the size of Qβ phage (ca. 20 nm) is smaller than that of E. coli (c.a. 1–2 μm), it becomes difficult for Qβ phage to contact the TiO2 surface efficiently. However, O3 only (Fig. 4, solid triangles) or UV-C + TMiP + O3 (Fig. 4, open circles) units could inactivate Qβ phage efficiently. Ozone could attack the bacterial cell wall, membrane, and the surface of waterborne pathogens and then disrupt their integrity by its extremely strong oxidation potential.5
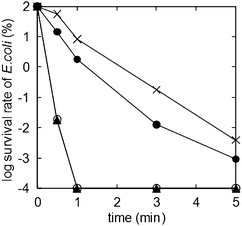 | ||
| Fig. 3 Time course of the log survival rate of E. coli with the units. Crosses: UV-C only (Fig. 2d); solid circles: UV-C + TMiP (Fig. 2c); solid triangles: O3 only (Fig. 2b); open circles: UV-C + TMiP + O3 (Fig. 2a). | ||
 | ||
| Fig. 4 Time course of the log survival rate of Qβ phage with the units. Solid circles: UV-C + TMiP (Fig. 2c); solid triangles: O3 only (Fig. 2b); open circles: UV-C + TMiP + O3 (Fig. 2a). | ||
Interestingly, inactivation efficiency of the UV-C + TMiP + O3 unit (Fig. 4, open circles) is larger than that of the O3 only unit (Fig. 4, solid triangles). The result of a phenol decomposition test shows this tendency more clearly (Fig. 5). The concentration of phenol after 20 min of treatment by the UV-C + TMiP + O3 unit (Fig. 5, the rightmost brack bar) is smaller than that by the O3 only or the UV-C + TMiP unit. This result indicates that the UV-C + TMiP + O3 unit could decompose phenol more efficiently than the other units. On the other hand, O3 concentration in the UV-C + TMiP + O3 unit (Fig. 5, the rightmost gray bar) after 20 min of treatment is smaller than that in the O3 only unit.
 | ||
| Fig. 5 The concentration of phenol (black bar) and ozone (gray bar) after 20 min of treatment by the units. Initial concentration of phenol and ozone were 273, 0 mg L−1, respectively. | ||
The log reduction of E. coli, the log reduction of Qβ phage, and the decomposition amount of phenol by each unit are summarized in Table 1. Purification efficiency of the UV-C + TMiP + O3 unit against both biological and chemical contaminants is the most highest among the units in the present research.
Ozone may play two roles in the system, i.e., to prevent carrier recombination in photocatalysis and to produce oxidative intermediates by photolytic and catalytic decomposition. Fig. 6 shows the schematic illustration of the reaction mechanism on the TiO2 surface. When the TiO2 is irradiated by UV light from a UV-C lamp, excitation of electrons into the conduction band takes place, resulting in formation of holes in the valance band. Both the holes and the electrons migrate to the TiO2 surface, where they either recombine or react with adsorbed species such as H2O and dissolved O2. The holes oxidize adsorbed H2O to ˙OH, which are the potential oxidants in photocatalysis, whereas the electrons reduce O2 to O2˙−.3 Here O3 is a good acceptor of excited electrons, similar to O2. Thus, ˙OH production and photocatalytic oxidation are enhanced by the presence of O3, which prevents carrier recombination in photocatalysis.6–9 Moreover, O3˙− can oxidize organics with a relatively long lifetime.10,11 On the other hand, there are many reports about photolytic/catalytic decomposition of O3.12–15 Highly oxidative intermediates such as atomic oxygen could be produced from O3 by UV-C irradiation and/or the presence of heterogeneous catalyst surfaces. In this case, UV-C irradiation and the large relative surface area of TMiP could decompose O3 effectively.16 A smaller O3 concentration in the UV-C + TMiP + O3 unit in Fig. 5 is an evidence of decomposition of O3. Moreover, a phenol decomposition test was carried out by using an additional unit, i.e., UV-C + O3. The decomposition amount of phenol and ozone concentration after 20 min of treatment were 122 mg and 2.75 mg L−1, respectively. This result indicates that the amount of residual phenol and O3 in the UV-C + O3 unit is larger than in the UV-C + TMiP + O3 unit. Therefore, photolytic and catalytic decomposition of O3 and prevention of career recombination by O3 are critical for the present system. Loading of Pt onto the TiO2 surface for stabilizing oxidative intermediates is worthy of further study.10,11
 | ||
| Fig. 6 Schematic illustration of the reaction mechanism on the TiO2 surface. | ||
In conclusion, an ozone-assisted photocatalytic water-purification unit was investigated. Much higher efficiency for decomposition of biological and chemical contaminants was achieved in the ozone-assisted photocatalytic water-purification unit, compared with the photocatalysis or ozone treatment only. Ozone could prevent carrier recombination in photocatalysis and could produce oxidative intermediates by photolytic and catalytic decomposition. These roles are important for efficient water purification in the present system. Moreover, the purification efficiency of TMiP did not decrease by repeating the test. This result indicates the strong adhesion of TiO2 nanoparticles onto the TMiP surface. Although we used a simple reactor with conventional methods and conditions, for example, atmospheric pressure, it would be attractive to develop a similar continuous-type water purification system for the practical treatment of a highly contaminated environment, such as sewage water.
Acknowledgements
The authors are grateful to Dr Etsuko T. Utagawa (National Institute of Infectious Diseases) and Dr Jitsuo Kajioka (KAST) for helpful discussion.Notes and references
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS.
- A. Fujishima, T. N. Rao and D. A. Tryk, J. Photochem. Photobiol., C, 2000, 1, 1 CrossRef CAS.
- A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515 CrossRef CAS.
- T. Ochiai, T. Hoshi, H. Slimen, K. Nakata, T. Murakami, H. Tatejima, Y. Koide, A. Houas, T. Horie, Y. Morito and A. Fujishima, Catal. Sci. Technol., 2011 10.1039/C1CY00185J.
- Y. Yao, Y. Kubota, T. Murakami, T. Ochiai, H. Ishiguro, K. Nakata and A. Fujishima, J. Water Health, 2011, 9, 534 CrossRef CAS.
- S. Nishimoto, T. Mano, Y. Kameshima and M. Miyake, Chem. Phys. Lett., 2010, 500, 86 CrossRef CAS.
- M. Nicolas, M. Ndour, O. Ka, B. D'Anna and C. George, Environ. Sci. Technol., 2009, 43, 7437 CrossRef CAS.
- F. J. Beltrán, F. J. Rivas, O. Gimeno and M. Carbajo, Ind. Eng. Chem. Res., 2005, 44, 3419 CrossRef.
- F. J. Rivas, F. J. Beltrán, O. Gimeno and M. Carbajo, Ind. Eng. Chem. Res., 2006, 45, 166 CrossRef CAS.
- R. Nakamura and S. Sato, J. Phys. Chem. B, 2002, 106, 5893 CrossRef CAS.
- H. Einaga, A. Ogata, S. Futamura and T. Ibusuki, Chem. Phys. Lett., 2001, 338, 303 CrossRef CAS.
- H. B. Huang, D. Q. Ye and D. Y. C. Leung, J. Environ. Eng., 2010, 136, 1231 CrossRef CAS.
- H. Einaga and S. Futamura, J. Catal., 2004, 227, 304 CrossRef CAS.
- S. Song, Z. Liu, Z. He, A. Zhang, J. Chen, Y. Yang and X. Xu, Environ. Sci. Technol., 2010, 44, 3913 CrossRef CAS.
- C. Guillard, J. Photochem. Photobiol., A, 2000, 135, 65 CrossRef CAS.
- T. Ochiai, K. Nakata, T. Murakami, Y. Morito, S. Hosokawa and A. Fujishima, Electrochemistry, 2011, 79 CAS , in press.
| This journal is © The Royal Society of Chemistry 2012 |
