Peroxidase mimic activity of hematite iron oxides (α-Fe2O3) with different nanostructures†
Kiran N.
Chaudhari
,
Nitin K.
Chaudhari
and
Jong-Sung
Yu
*
Department of Advanced Materials Chemistry, WCU Research Team, Korea University, 208 Seochang, Jochiwon, Chung, N, am 339-700, Republic of Korea. E-mail: jsyu212@korea.ac.kr; Fax: +82-41-867-5396; Tel: +82-41-867-5396
First published on 29th November 2011
Abstract
Enzyme mimics have garnered considerable attention as they can overcome some serious disadvantages associated with the natural enzymes. In recently developed sphere and rod shaped iron oxide peroxidase mimic nanoparticles, the influence of physical parameters such as shape, size and surface area on the catalytic performance was not clearly demonstrated. In order to better understand the influence of physical parameters on the enzyme mimic activity of iron oxide nanoparticles, the present study was initiated using three different shaped hematiteα-Fe2O3 nanostructures, particularly hexagonal prism, cube-like and rods as model systems. A comparative account of kinetic parameters (Km, Vmax and Kcat) of the peroxidase mimic activity by the various α-Fe2O3 nanostructures indicated that the enzymatic potential of these nanoparticles increased from hexagonal prism to rods, via cube-like, suggesting that one-dimensional particles act as a more efficient enzyme mimic system compared to their multi-dimensional counterparts. Surface area is likely to be a key physical aspect responsible for the enzyme mimic activity. Interestingly, however, particles with lower surface area showed better catalytic performance in the case of one-dimensional rod structure. Upon further analysis of the one-dimensional rods, additional physical properties such as porosity and pore shape also seem to have a significant contribution to their catalytic activity.
1. Introduction
Chemical or artificial synthesis of enzymes is a rapidly developing field due to the drawbacks associated with the natural enzymes such as denaturation by proteases, requirement of special storage conditions and the cost factor involved. Some level of success has been achieved in this direction with the development of chemically synthesized alternatives for serine proteases,1hematin,2 and superoxide dismutase.3 In addition, a recent finding demonstarted that iron oxide nanoparticles have the ability to catalyze oxidation reactions, similar to that of natural peroxidase enzyme,4 giving rise to new possibilities in the field of enzyme mimics or artificial enzymes.Shape has been recognized as an important parameter influencing nanoparticle properties. Particularly, one-dimensional (1D) structures such as nanorods and nanowires possess unique properties that are different from their bulk materials and even the corresponding zero-dimensional counterparts (spherical nanocrystals).5,6 This was also seen in the recently developed peroxidase mimic nanoparticles where the peroxidase mimic activity of rod type Fe3O4 dominated over the spheres which were much smaller in size.7 It raised an interesting question over the involvement of various physical parameters like shape, size, surface area and dimensionality in the enzyme mimic activity of nanoparticles. This inspired us to carry out a systematic study that may help in identifying a possible correlation between peroxidase mimic activity and the physical properties of iron oxide nanoparticles. In order to do so, stable hematiteα-Fe2O3 nanoparticles of various shapes (hexagonal prism, cube-like and rod) were chosen as model systems.
Hematite (α-Fe2O3), an n-type semiconductor with 2.1 eV band gap, is the most stable, non-toxic, corrosion-resistant iron oxide under ambient conditions. Due to its low cost, high resistance to corrosion and being environmentally safe, this metal oxide has been used in cellular delivery,8 imaging,9cell separation,10 biosensing11 and for evaluation of cellular response towards the nanomaterials.12Hematiteα-Fe2O3 nanoparticles used in this work are different from previously used Fe3O4 nanoparticles in terms of chemical composition, structure, size and synthesis route. Although both Fe3O4 and α-Fe2O3 are ferromagnetic in nature, they differ on the basis of net magnetic moment and magnetic crystal structure.13,14α-Fe2O3 nanoparticles of varying shape (hexagonal prisms, cube-like and rods) used for this study are synthesized through simple, aqueous and surfactant-free routes.15,16 Such synthesis procedures utilizing green approaches17–19 yield biocompatible iron oxides with much lesser toxicity,20 which facilitates their direct use for biological and biochemical application. In this study we analyzed the influence of various physical parameters like shape, size, surface area and dimensionality on the enzyme mimic properties of α-Fe2O3 nanomaterial. Kinetic parameters (Vmax and Km) of all the nanoparticles were assessed, and a correlative investigation between the various physical features of α-Fe2O3 and its enzyme mimicking property is presented. It was shown for the first time that α-Fe2O3 nanoparticles possess peroxidase-like activity and also serve as model systems to give understanding of the influence of various physical parameters on the catalytic activity of the nanoparticles.
2. Experimental section
2.1. Synthesis of α-Fe2O3 nanostructures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5. After addition of iron chloride, the colour of the solution changed to brown. The solution was stirred for 12 h at the same temperature and the resulting nanostructures were isolated by centrifugation with repeated washings.
7.5. After addition of iron chloride, the colour of the solution changed to brown. The solution was stirred for 12 h at the same temperature and the resulting nanostructures were isolated by centrifugation with repeated washings.
In addition, cube-like α-Fe2O3 particles were synthesized using 0.06 mol of iron chloride (FeCl3·6H2O) and 0.03 mol of caffeine instead of hydrochloric acid or urea in aqueous solution. The flask was magnetically stirred for 24 h at 90 °C. The mixture was allowed to cool naturally to room temperature, and the resultant brown product, also indicative of formation of hematite, was isolated through centrifugation with successive washings by deionized water and ethanol and dried at 80 °C overnight.
2.2. Characterization of α-Fe2O3 nanostructures
The as-synthesized α-Fe2O3 nanostructures were characterized by using various analytical techniques. The microscopic features of the samples were elucidated with a transmission electron microscope (TEM, EM 912 Omega) operated at 120 kV and a scanning electron microscope (SEM) (SEM, LEO 1455VP, Hitachi S-4700) operated at an acceleration voltage of 25 kV. X-Ray diffraction (XRD) patterns of the samples were obtained with a Rigaku 1200 diffractometer with CuKα radiation using a Ni β-filter at a scan rate of 4° min−1. The X-ray source was operated at 40 kV and 30 mA.Biocatalytic and enzyme mimic activities of all iron oxide nanoparticles are considered to be mostly a surface governed phenomenon.4 Hence, surface area is an important aspect for analyzing the enzyme mimic activity of the biocatalytic nanomaterials. N2 adsorption–desorption isotherms were conducted at 77 K on a KICT SPA-3000 gas adsorption analyzer after the test materials were degassed at 423 K to 20 μTorr for 12 h. Surface areas of the various materials were determined from the adsorption branch of nitrogen isotherms in a relative pressure range from 0.05 to 0.2 using the Brunauer–Emmett–Teller (BET) equation. Total pore volumes were determined from the amount of gas adsorbed at the relative pressure of 0.99. Any apparent physical changes in the α-Fe2O3 nanorods after the isotherm measurements were not detected and were confirmed on the basis of the microscopic analysis of the samples by SEM and TEM.
2.3. Peroxidase mimic activity of α-Fe2O3 nanostructure
3. Results and discussion
3.1. Structural parameters of α-Fe2O3 nanoparticles
Hexagonal prisms have average parallel and diagonal sides measuring around 80–90 nm and 60–70 nm along with width and thickness ranging between 105–110 nm and 85–95 nm, respectively. Cube-like nanoparticles have an average size of 100 nm, whereas rod shaped particles have size varying between 250–400 nm in length and 30–35 nm in diameter. The N2 adsorption–desorption isotherms for the hexagonal prism, cube-like, and rod shaped α-Fe2O3 are provided in the ESI† (Fig. S1–S3), and the surface area details are presented in Table 1. The XRD patterns of all the iron oxide nanostructures are shown in ESI† (Fig. S4 to S6). Despite variations in shape, all the α-Fe2O3 nanostructures reveal similar XRD patterns, confirming that all the as-synthesized iron oxide nanostructures correspond to the crystalline hematite α-Fe2O3 phase with lattice parameters a = 5.037 Å and c = 13.75 Å. These values are well in agreement with the literature values (JCPDS 89-0597) for hexagonal prism and cube-like nanoparticles along with the values (JCPDS 33-0664) for nanorods. All the reflection peaks are sharp and readily indexed to a pure rhombohedral phase of α-Fe2O3, suggesting that the particles are highly crystalline. It can be seen that these samples have a pure phase since no other peaks are observed for the presence of impurities. Length distribution was estimated by measuring lengths of particles from randomly selected regions of the respective TEM images. Distribution plots for R-1, R-2 and R-3 are provided in Fig S7, ESI.† Most of the particles were found to be of average length as mentioned in Table 1.| Morphology | Size/nm | Surface area/m2 g−1 | K m/mM | V max/nM s−1 | K cat/s−1 |
|---|---|---|---|---|---|
| Hexagonal prism | 110 ± 10 | 43 ± 4 | 0.768 ± 0.091 | 5 ± 0.44 | 1.66 |
| Cube-like | 100 ± 7 | 47 ± 5 | 0.957 ± 0.085 | 6.3 ± 0.35 | 2.2 |
| Rod shaped (R-1) | 31 ± 5 (diameter) | 55 ± 5 | 0.257 ± 0.089 | 16.5 ± 2.1 | 5.5 |
| 380 ± 20 (length) | |||||
| Rod shaped (R-2) | 30 ± 3 (diameter) | 82 ± 8 | 0.317 ± 0.071 | 15.3 ± 1.81 | 5.1 |
| 330 ± 20 (length) | |||||
| Rod shaped (R-3) | 30 ± 3 (diameter) | 111 ± 10 | 0.518 ± 0.047 | 10.60 ± 1.38 | 3.53 |
| 278 ± 20 (length) |
3.2. Peroxidase mimic activity analysis by steady state kinetic assay of α-Fe2O3 nanoparticles
The current study began with a correlative assessment of various physical parameters such as shape, size, and surface area, and their influence on peroxidase mimic activity of α-Fe2O3 nanoparticles. The enzyme like activity of α-Fe2O3 originates because of the presence of ferrous ions at the surface of the nanoparticles. The ferrous ions interact with the substrate in the presence of peroxide, resulting in a coloured reaction product. The mechanism most likely follows Fenton’s reaction21 and can be written as follows:| Fe2+ + H2O2 → Fe3+ + OH˙ + OH− | (1) |
| Fe3+ + H2O2 → Fe2+ + OOH˙ + H+ | (2) |
| Kcat = Vmax/[E] | (3) |
 | ||
| Fig. 1 Peroxidase enzyme-like activity of α-Fe2O3 hexagonal prism (first row), cube-like (second row), and rod-shaped nanoparticles (third row), showing (1) blue colour development after 30 min, and (2) yellow colour development after stopping the reaction with 2.0 M sulfuric acid. (A/A1, D/D1, G/G1) TMB in citrate buffer with α-Fe2O3 nanoparticles and no H2O2 (control-1), (B/B1, E/E1, H/H1) TMB in citrate buffer with both α-Fe2O3 and H2O2, and (C/C1 F/F1, I/I1) TMB in citrate buffer with H2O2 and no α-Fe2O3 (Control-2). | ||
The respective TEM images of the three different shaped nanoparticles and corresponding Lineweaver–Burk plots are provided in Fig. 2. Additional SEM images of hexagonal prism and cube-like α-Fe2O3 are presented in Fig. S8, ESI.† We witnessed a rise in Vmax values from hexagonal prism (5.0 ± 0.44 nM s−1) (Fig. 2A and B) through cube-like (6.3 ± 0.35 nM s−1) (Fig. 2C and D) to rod (R-1) (Fig. 2E and F), showing the highest maximal reaction velocity (16.5 ± 2.1 nM s−1). On the other hand, the lowest Km value was shown by the rods (0.257 ± 0.089 mM) followed by the hexagonal prisms (0.768 ± 0.091 mM) and then the cube-like nanoparticles (0.957 ± 0.085 mM). In context to the natural enzymes, Vmax and Km values are the indicators of maximal reaction velocity (i.e. the rate of reaction when an enzyme is saturated with the substrate) and affinity (ability to bind with the substrate. The lower the Km values, the greater the affinity).
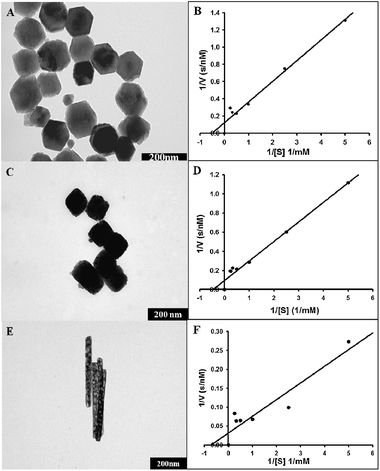 | ||
| Fig. 2 TEM images and Lineweaver–Burk plots of α-Fe2O3 hexagonal prism (A and B), cube-like (C and D), and rod shaped (E and F) nanoparticles used for peroxidase enzyme mimic studies. | ||
When these two traits of the enzyme activity were extrapolated for these three types of α-Fe2O3 nanoparticles, it was seen that the Vmax increased with increase in the surface area. As the surface area increases from hexagonal prism to rod via cube-like particles, Vmax values also increase, showing the highest values for rod and the lowest values for hexagonal prism, suggesting a sound correlation between maximal reaction velocity and surface area. As expected, Km values showed an inverse relationship with surface area. However, hexagonal prisms showed slightly enhanced affinity with lower Km value than that of cube-like nanoparticles for unknown reasons, but in terms of activity, cube-like nanoparticles were slightly better. From the results discussed above, it can be concluded that the peroxidase mimic activity of all the three α-Fe2O3 nanoparticles was mainly influenced by their surface area. Fig. 3 represents a graphical plot of Km and Vmax values plotted as a function of surface area. The respective Vmax, Km and Kcat values for all the nanoparticles studied are summarized in Table 1, providing a collective account of correlation between shape, size, surface area and the kinetic parameters representing enzyme mimic activity.
 | ||
| Fig. 3 Correlation plot showing Vmax and Km values as a function of surface area for hexagonal prism, cube-like and rod shaped nanoparticles. | ||
Till this point of investigation we observed a sound correlation between enzyme mimic activity and the surface area of nanoparticles. However, the difference in the surface area of rods when compared with the hexagonal prisms and cube-like nanoparticles is not significant enough to explain its exceptionally high activity, i.e. ca. 2–3 times higher activity than those of the cube-like and hexagonal prisms. This clearly indicates that not only the surface area, but other factors also should be responsible for the higher catalytic activity of the rods. This was the most intriguing part of the study, which forced us to further examine the peroxidase mimic activity of rod shaped nanoparticles in detail. To do so, in addition to the already analyzed rod R-1 we chose two more rods (R-2 and R-3) having different surface areas and slight variations in their length. The size of these three rods varied between 250–400 nm in length, and the diameters ranged between 30–35 nm. Surface area increased in the order of R-1 (55 ± 5 m2 g−1) < R-2 (82 ± 8 m2 g−1) < R-3 (111 ± 10 m2 g−1) with R-3 having the highest surface area, while R-1 having the lowest one. In order to verify the ability of R-2 and R-3 to catalyze the peroxidase substrate TMB, they were subjected to similar experimentation as that of R-1. The data obtained were processed as described in Section 3.2. Coloured images of peroxidase mimic activity for all the three rods are provided in Fig. S9, ESI.†TEM images for all the three rods with respective Lineweaver–Burk plots are provided in Fig. 4. The Vmax values of R-1, R-2 and R-3 are in the following order: R-1 > R2 > R-3, while the Km values display a reverse trend: R-1 < R-2 < R-3 with R-1 showing the maximum enzyme mimic activity followed by R-2 and R-3. It is very interesting to note that the Vmax and Kcat values of the rods increase with decrease in Km values and the surface area. Correlation of Vmax and Km values as a function of surface area is plotted in Fig. 5, indicating a deviation in the sequel of the study from earlier outcomes, where the peroxidase mimetic activity was mainly governed by the surface area of nanoparticles. Results shown by our hexagonal prism and cube-like α-Fe2O3 nanoparticles were similar to those of the Fe3O4 nanoparticles published earlier.4 In contrast, the rods having lower surface area (R-1 and R-2) performed as a better peroxidase mimic than the one with higher surface area (R-3), which was truly surprising and “off the trend”.
 | ||
| Fig. 4 TEM images and Lineweaver–Burk plots of α-Fe2O3 rod shaped R-3 (A and B), R-2 (C and D), and R-1(E and F) nanoparticles used for peroxidase enzyme mimic studies. | ||
 | ||
| Fig. 5 Correlation plot showing Vmax and Km values as a function of surface area for R-1, R-2 and R-3 nanorods. | ||
Although a conclusive reason is yet to be established for such ‘off the trend’ activity shown by the α-Fe2O3 nanorods, other physical features like porosity and pore shape which had not been considered yet, could explain this exceptional trend in catalytic performance to some extent. Generally particle morphology, surface roughness, and surface porosity are the main features influencing the surface area. In the case of α-Fe2O3 nanorods, the surface area is mostly credited to the pore shape and number of pores on the particles. An earlier work22 reports that the presence of slit shaped pores along the C-axis results in a larger surface area, while isolated circular pores result in a smaller surface area. If we look at the enlarged TEM images of the rods presented in Fig. 6, it is clearly visible that R-2 and R-3 mainly have slit shaped pores and both of them have a higher surface area compared to R-1, which has large circular pores (4–20 nm) and a lower specific surface area. The reason for R-1 with lower surface area being more active may be related to the circular pore shape, which would provide a favourable space for higher ferrous ions–substrate interaction, resulting in much higher catalytic activity compared to the other two rods with slit shaped pores. In the cases of R-2 and R-3, the difference in length between R-2 and R-3 is not significant but there is a considerable difference in the surface area and catalytic activity. The most likely reason for such behaviour can be explained on the basis of enlarged images of R-2 and R-3 in Fig. 6. Both of the rods mainly possess slit shaped pores, and there are reasonable chances that the number of pores present on R-3 may be higher compared to that of R-2, but the pore size (less than 1 nm) may not be wide enough to facilitate good substrate–ferrous ion interactions. Whereas R-2 may have lesser number of pores, the higher catalytic performance suggests that the pore structure may be wide enough to facilitate better substrate–ferrous ion interactions than that of R-3. From the above mentioned comparison of physical parameters with respect to enzyme mimic activity, it can be speculated that pore shape and structure may also be a contributing factor to the peroxidase mimic activity of rod type materials.
 | ||
| Fig. 6 Enlarged TEM images of R-3 (right), R-2 and R-1 (left) showing the slit and circular shaped pores. | ||
Results from our and earlier studies suggest that if similar investigations are protracted in other enzyme mimicking nanomaterials such as FeS,23cerium oxide,3 a better understanding of the relation between physical parameters and enzyme mimic activities of nanoparticles may be obtained that may act as guidelines for development of effective nanoparticle-based enzyme mimics. Further study in this regard is under consideration.
4. Conclusions
Current work attempts to explore various facets of hematite α-Fe2O3 nanomaterials as an enzyme mimic. The α-Fe2O3 nanoparticles with different nanostructures such as hexagonal prism, cube-like, and rod have been demonstrated for the first time as enzyme mimics that possess peroxidase-like activity. The Vmax, Km and Kcat values of the α-Fe2O3 reveal that the peroxidase-like activity is mostly governed by the surface area, structure and pore shape of the nanomaterials. Surface area must be a key physical aspect responsible for the enzyme mimic activity. Hematiteα-Fe2O3 nanoparticle enzyme mimics having one-dimensional nature act as a more efficient enzyme mimic system than zero or multi-dimensional counterparts. On the other hand, amongst α-Fe2O3 nanorods, the peroxidase mimic activity increases with decrease in surface area, which still needs further work for a better understanding. Surface features like porosity and pore shape are also involved in contributing towards enzyme mimic activity in the case of one-dimensional rods. Similar studies on other enzyme mimicking nanomaterials will certainly help in establishing a set of principles which may act as guidelines for development of effective nanoparticle-based enzyme mimetics.Acknowledgements
This work was supported by Korea University (2011). The authors would also like to thank the Korean Basic Science Institute at Jeonju and Chuncheon for SEM and TEM analyses.Notes and references
- M. Ghosh, J. L. Conroy and C. T. Seto, Angew. Chem., Int. Ed., 1999, 38, 514–516 CrossRef CAS.
- Z. Genefa and P. K. Dasgupta, Anal. Chem., 1992, 64, 517–522 CrossRef.
- C. Korsvik, S. Patil, S. Seal and W. T. Self, Chem. Commun., 2007, 1056–1058 RSC.
- L. Z. Gao, J. Zhuang, L. Nie, J. B. Zhang, Y. Zhang, N. Gu, T. H. Wang, J. Feng, D. L. Yang, S. Perrett and Y. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS.
- X. Peng, L. Manna, W. Yang, J. Wickham, E. Scher, A. Kadavanich and A. P. Alivisatos, Nature, 2000, 404, 59–61 CrossRef CAS.
- B. Fang, M.-S. Kim, J. H. Kim, M. Y. Song, Y.-J. Wang, H. Wang, D. P. Wilkinson and J.-S. Yu, J. Mater. Chem., 2011, 21, 8066–8073 RSC.
- S. Nath, C. Kaittanis, V. Ramachandran, N. S. Dalal and J. M. Perez, Chem. Mater., 2009, 21, 1761–1767 CrossRef CAS.
- Y. Hu, X. T. Zheng, J. S. Chen, M. Zhou, C. M. Lib and X. W. Lou, J. Mater. Chem., 2011, 21, 8052–8056 RSC.
- N. Chekina, D. Horák, P. Jendelová, M. Trchová, M. J. Beneš, M. Hrubý, V. Herynek, K. Turnovcová and E. Syková, J. Mater. Chem., 2011, 21, 7630–7639 RSC.
- D. Wang, J. He, N. Rosenzweig and Z. Rosenzweig, Nano Lett., 2004, 4, 409–413 CrossRef CAS.
- A. Liu, Biosens. Bioelectron., 2008, 24, 167–177 CrossRef CAS.
- J. Meng, Y. Zhang, X. Qi, H. Kong, C. Wang, Z. Xu, S. Xie, N. Gu and H. Xu, Nanoscale, 2010, 2, 2565–2569 RSC.
- T. J. Bastow and A. Trinchi, Solid State Nucl. Magn. Reson., 2009, 35, 25–31 CrossRef CAS.
- M. Gotic and S. Music, Eur. J. Inorg. Chem., 2008, 966–973 CrossRef CAS.
- N. K. Chaudhari and J.-S. Yu, J. Phys. Chem. C, 2008, 112, 19957–19962 CAS.
- N. K. Chaudhari, H. C. Kim, D. Son and J.-S. Yu, CrystEngComm, 2009, 11, 2264–2267 RSC.
- J. A. Dahl, B. L. S. Maddux and J. E. Hutchison, Chem. Rev., 2007, 107, 2228–2269 CrossRef CAS.
- N. K. Chaudhari, B. Fang, T. S. Bae and J.-S. Yu, J. Nanosci. Nanotechnol., 2011, 11, 4457–4462 CrossRef CAS.
- M. N. Nadagouda and R. S. Varma, Green Chem., 2008, 10, 859–862 RSC.
- P. Dua, K. N. Chaudhari, C. H. Lee, N. K. Chaudhari, S. W. Hong, J.-S. Yu, S. Y. Kim and D. K. Lee, Bull. Korean Chem. Soc., 2011, 32, 2051–2057 CrossRef CAS.
- W. P. Kwan and B. M. Volker, Environ. Sci. Technol., 2003, 37, 1150–1158 CrossRef CAS.
- L. A. Pérez-Maqueda, J. M. Criado, J. Subrt and C. Real, Catal. Lett., 1999, 60, 151–156 CrossRef.
- Z. Dai, S. Liu, J. Bao and H. Ju, Chem.–Eur. J., 2009, 15, 4321–4326 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: N2 isotherms, XRD patterns, length distribution, SEM images and peroxidase enzyme-like activity of different α-Fe2O3. See DOI: 10.1039/c1cy00124h |
| This journal is © The Royal Society of Chemistry 2012 |
