Urine utilisation by microbial fuel cells; energy fuel for the future
Ioannis
Ieropoulos
*ab,
John
Greenman
ab and
Chris
Melhuish
a
aBristol Robotics Laboratory, Environment & Technology Faculty, University of the West of England, Bristol, UK. E-mail: ioannis.ieropoulos@brl.ac.uk; Fax: +441173283960; Tel: +441173286319
bSchool of Life Sciences, Health & Life Sciences Faculty, University of the West of England, Bristol, UK
First published on 19th October 2011
Abstract
This communication reports for the first time the direct utilisation of urine in MFCs for the production of electricity. Different conversion efficiencies were recorded, depending on the amount treated. Elements such as N, P, K can be locked into new biomass, thus removed from solution, resulting in recycling without environmental pollution.
Introduction
Alternative energy sources have been the focus of global interest, as perhaps one viable solution to the growing problem of fossil fuel depletion. Promising technologies such as photovoltaics, wind-turbines and wave-generators, dominate the field of natural energy harnessing for electricity and indeed provide practical solutions in areas where solar radiation, wind force and wave power are abundant. One other type of alternative energy source that has been receiving increased attention, is biomass and its conversion to electricity via Microbial Fuel Cells (MFCs).1 This offers the unique advantage of converting “too-wet-to-burn” low-grade organic matter directly into electricity at high efficiencies and for long periods of time. A wide range of substrates have been reported as suitable fuels for MFCs,2 but one potential fuel, which has so far been neglected—and is therefore underexploited—is urine.Urine is an abundant waste product with an estimated annual global production of ∼6.4 trillion litres (based on a world population of 6.97 billion and average daily urine production of 2.5 litres/adult human). This short communication for the first time describes the direct conversion of urine into electricity through MFCs.
The specific aims of this study were: (i) to investigate whether untreated urine can produce electricity through MFCs; (ii) to compare the responses of a re-circulation MFC system to the addition of large urine volumes into a reservoir or injection of small urine volumes directly into the MFC inlet; (iii) to evaluate the sensitivity of the established MFC anodic community to the addition of neat urine; (iv) to calculate the energy yield from urine when utilised in MFCs.
Materials and methods
Re-circulating MFCs
Three analytical type MFCs made from acrylic with 25 mL anode and cathode chambers (50 mL in total) were used (see Fig. 1). The two half-cells were separated by a cation exchange membrane (VWR, UK), and each contained carbon veil electrodes with 270 cm2 total surface area (PRF Composite Materials, UK). Both anode and cathode half-cells were connected via small pumps, to 1 L reservoir bottles. The anolyte recirculation was 4 mL min−1 facilitated by a single channel peristaltic pump (WELCO Co. Ltd, Japan) and the catholyte recirculation was 30 mL min−1 using a single channel diaphragm pump (KNF Neuberger, Germany).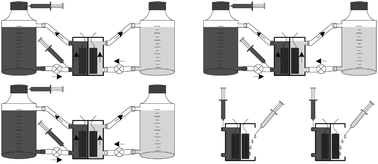 | ||
| Fig. 1 MFC setup for urine experiments showing three replicate assemblies with anolyte (left/dark coloured) and catholyte [right/light coloured) re-circulation. Pumps are shown as ⊗ and the injection ports are shown as syringes for the MFC chambers as well as for the bottles. | ||
Batch mode MFCs
Two analytical type MFCs made from the same acrylic material with a 25 mL anode chamber and an open to the air cathode half-cell (25 mL in total), were also used in the experiments. The same 270 cm2 area of carbon veil electrode was used for both half-cells and parafilm was wrapped around the open-to-air cathode to minimise evaporative losses.Inoculum, anolyte and catholyte
The initial inoculum was microflora from activated sludge, collected from the Cam Valley wastewater treatment works, Wessex Water. All 5 MFCs had been running for at least 1 year, prior to the addition of urine. During this initial enrichment period, every time the electrical current from the MFCs would reach the pre-set baseline of 1 μA, 700 mL of fresh activated sludge was added into the 1 L bottles. The pH of activated sludge prior to its addition into the recirculation system was 6.8 and by the time the sludge was depleted, the pH was increased to 8.7. After 1 year of periodic (8 week) replenishments for enrichment and maturing, untreated urine was used as the pure ‘fuel’ added through the injection ports (Fig. 1). The catholyte was 700 mL of tap water for the three re-circulation MFCs and 7.5 mL (periodic hydrations) for the two open-to-air batch mode MFCs.Urine samples
Neat (unprocessed) urine was added either as (i) large volumes (ranging between 25–300 mL) into the re-circulation reservoir bottle or (ii) small volumes (0.1 mL–10 mL) as injections directly into the anode inlets. Urine was used fresh or within 1 week from donation. Its pH prior to addition into the MFCs ranged between 6–6.5. The volume per donation for use in experiments was between 400–500 mL. Urine samples were taken from a single healthy volunteer (average height and weight for a young adult) on a normal diet with no prior history of urinary tract or renal disease. Samples were stored at 4 °C and allowed 2–3 h to re-equilibrate to ambient temperature prior to usage.Energy extrapolation
For energy extrapolation of experimental data into global figures, the population of the planet was taken to be 6.97 billion,3 and the amount of daily urine production per adult human to be ∼2.5 L.4 According to the UN FAO statistics,5 the world population of farm livestock (cattle, sheep, goats and pigs) is 4.15 billion.Urine non-mineral composition
The composition of excreted normal human urine, is:4urea 6–18 g day−1, uric acid 1.8 g day−1, creatinine 0.5–0.8 g day−1, amino acids 0.12 g day−1 and peptides 0.5 g day−1. Variable amounts of lactic acid, citric acid, bilirubin and porphyrins, ketone bodies (aceto-acetic acid; β-hydroxybutyrate; acetone), and small amounts of hexose (glucose) and pentose (arabinose) sugars may also be present in normal urine. When considering the bio-available organic content of excreted urine, compounds such as urea and uric acid are not included, since they cannot be utilised as carbon-energy (C/E) sources by the microbial community inside an MFC. In total, the dry weight content of metabolisable organic substrates, within excreted urine, was estimated to be 0.78 g/human/day.4 Due to the insignificant amount of lipid in urine, this has also been ignored. Therefore, the mean calorific value of 1 g of carbohydrates, peptides, proteins or amino-acids (as metabolisable substrates within urine) has been estimated to be 2.08 kcal.6Determination of biomass
Bacterial dry weight was used as a measure of the biomass. Cells, harvested in 15 mL samples from the re-circulating MFCs during peak power production, were centrifuged, re-suspended in de-ionised water and deposited by negative pressure filtration on to pre-dried and pre-weighed cellulose acetate filters (25 mm diameter, 0.45 μm porosity; Oxoid). The filters were then dried for 30 min under an infrared lamp, cooled to room temperature and re-weighed. The bacterial dry weight was obtained from the difference in the weights.7Data acquisition and calculations
Data collection and current, power and normalised energy (per m2) calculations were performed as previously described.8 The resistive load connected for the MFCs varied between 1.3–2.7 kΩ. The calorific yield of urine samples added to the MFCs was calculated as follows:1. Energy yield: AUC of MFC power output over time = E [J]
2. Calorie conversion: E [J] × 0.2389 = E [cal].
3. Calorific yield of added urine: [1] × [2] above. This was compared against 2.08 kcal g−1 ≈ 1.62 kcal day−1 for urine.6
Results and discussion
Typical power output
Fig. 2 shows a typical response and performance from an MFC fed with fresh neat urine (25 mL, pH 6.03), injected directly into the anode inlet as indicated by the arrow. Prior to this injection the MFC was producing 0.9 mA m−2, which increased to 2.9 mA m−2 after 1 h from the point of injection. The initial rate of increase at the point of injection was 0.2 mA m−2 min−1, which decreased to approx. 0.02 mA m−2 min−1 during the first hour. The power output continued to increase until it reached the peak value of 8 mA m−2 ± 0.5 mA m−2 (diurnal variation). The absolute (non-normalised) current output from this single MFC fed with the urine sample was 0.25 mA. This amount of urine was sufficient for continuous energy generation over 3 days, at which point the performance began to plateau and returned to the power output level that this MFC was producing prior to the injection. The data in Fig. 2 are represented in terms of the actual (non-normalised) current output as well as the normalised current density according to the (i) total and (ii) projected electrode macro surface area. This was done in an attempt to more comprehensively demonstrate how the same data set can be represented in various ways, with orders of magnitude difference.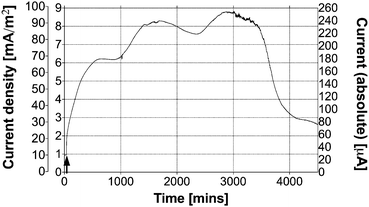 | ||
| Fig. 2 Temporal profile of MFC performance when fed with 25 mL of neat urine. The graph shows three different y-axes for the same data set. The two on the left represent the normalised current density for the projected (outside left) and total (inside left) electrode macro surface area, whereas the y-axis on the right shows the absolute current values. | ||
Dose response: low volumes/high dilution
Fig. 3, shows a detailed profile of a series of direct urine injections in the anode, but of very low volumes ranging between 0.1 mL–0.4 mL (giving neat urine dilutions of between 0.0001–0.0004 v/v). These and data from higher concentrations were used to construct a dose response curve (see inset) based on the area under curve. As can be seen, the average amount of current produced was directly proportional to the dose amount.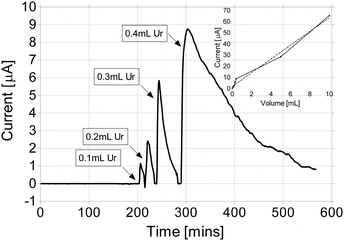 | ||
| Fig. 3 Dose response profile with volumes ranging from 0.1 mL–0.4 mL for a re-circulating MFC. Inset shows the resultant dose response curve from this and further experiments at higher volumes. | ||
As can be further seen from Fig. 3, steady-state MFCs that have exhausted their carbon-energy substrate (zero output), remain extremely sensitive to the addition of small amounts (<1 mL) of fresh urine. The response is rapid and the area under the curve is proportional to the amount of fresh urine added.
Dose response: high volumes/low dilution
For larger but still rate-limiting injections of urine (see Fig. 4), the initial rate of response is equally rapid, but the duration and decay are much slower due to the re-circulatory rate of supply that allows un-utilised substrate—albeit more dilute—to return to the MFC through the reservoir bottle input. Fig. 4 illustrates the response from both re-circulation and batch mode MFCs when fed with different concentrations of fresh neat urine. The first response is from 5 mL of fresh urine (dilution factor 0.007, pH 6.05) injected into the re-circulation system of the MFC. The second response follows an injection of 10 mL of fresh urine (dilution factor 0.014, pH 6.1), into the same MFC. The inset shows the responses and performances from (i) a re-circulation MFC and (ii) a batch mode MFC, fed with a high volume of urine. The higher urine volume addition directly into the anode inlet of the recirculation MFC, gave the fastest response of 1 μA min−1, followed by the same system but fed with half the volume, which gave half the rate of initial response (0.5 μA min−1) (main graph). Both these rates of response were between 2.5 and 5-fold higher respectively, than those recorded from the same re-circulation MFC (see inset, dotted line), fed with a 10-fold higher concentration. The lowest rate of initial response was always recorded from the batch mode MFCs (see inset solid line), even though the injection was directly into the anode. In both these latter 2 cases, the slower response can be attributed to the sub-optimum carbon-energy excess coupled with less dynamic states and lower growth rates of the constituent microbial biofilms.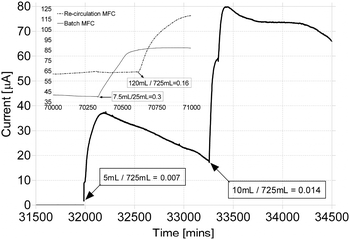 | ||
| Fig. 4 Response curves from re-circulating and batch mode MFC. The main graph shows the fast response from direct injections in re-circulation mode, at carbon-energy limited concentrations. The inset shows the response curves from the same re-circulation mode MFC as well as from a batch mode MFC, when fed with high doses of urine. | ||
From the data shown in Fig. 2, an addition of 25 mL of fresh urine took 3 days to be completely utilised in a single 25 mL volume MFC. For a stack of 10 MFCs, the same sample would require 8 h to be entirely utilised. Based on a daily urine production of 2.5 L/human, it would require a stack consisting of approximately 300 MFCs to utilise the daily urine production of an average human being. This heavily depends on affinity and efficiency of conversion, which is optimal at the smaller scale and thus at a smaller stack footprint.
Longevity
Fig. 5 shows one example of the typical long-term behaviour of these MFCs. The experiments have been running for over 2 years and the response to the addition of fresh urine (fuel) is shown to be consistent throughout. The difference in magnitude can be attributed to differences in volume and composition in urine (morning sample being more concentrated than those later in the day depending on diet). Fig. 5 shows the temporal profile, after the first 300 days, when the system has acclimated and matured. The frequency of peaks reflected the frequency and volume of feeding, with the linear regression fit illustrating stable longevity over 250 days that still continues beyond these recordings.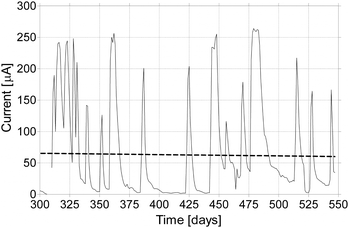 | ||
| Fig. 5 Temporal profile of energy production from the re-circulation MFCs following periodic additions of fresh urine over 250 days. The dotted line is the linear regression that shows the long-term response of the biofilm electrode is not diminished. | ||
Energy balance
Production of new cells in response to the addition of a known volume of urine was measured by dry weight determinations. These have given 1.0 mg ± 0.1 mg of cell dry weight from 15 mL sample volumes of perfusate taken from the outflow of the re-circulating MFC that was fed with fresh urine and allowed to reach steady state. This is the equivalent of 0.067 mg mL−1 or 20 mg from the whole sample. The flow rate was 4 mL min−1 (240 mL h−1) therefore the dry weight removed per minute is 0.28 mg, which is the equivalent of 16.8 mg h−1 or on average 302.4 mg day−1.For the single MFCs employed in this study, the efficiency of conversion was shown to have an inverse relationship to the amount of urine added as fuel. For volumes of up to 25 mL (i.e. equal to the anodic volume) of added urine, the efficiency of direct conversion to electricity was between 60–70%, whereas for higher volumes <700 mL, the efficiency ranged between 22–30%. This indicates an optimum volume and hydraulic retention time for a given anodic volume/electrode surface area and strengthens the case for collectives of MFCs configured as cascades for a more efficient treatment of larger urine quantities. Dry weight biomass was measured in addition to the electrical output and it was found to be ∼40% of the total system efficiency. This was particularly true for the larger volume additions, at which the MFC system was running under urine-replete conditions. These findings suggest that the amount of urine could be used as a control mechanism for defining the amount of electricity or biomass that a urine-treating stack can produce.
Elemental balance
In many areas of the World, the intensification of animal numbers and increasing urbanization, have resulted in damaging impacts on the environment.9,10 Application of excessive quantities of nutrients on land is subject to surface run-off and leaching with concomitant contamination of ground or surface waters. N-pollution (nitrate leaching, ammonia toxicity to fish and altered effectiveness of chlorination) is of major concerns around livestock farms. Phosphorus (P) entering surface waters from neat urine, can stimulate growth of algae and aquatic plants. This can subsequently decompose, resulting in an increased oxygen demand (high BOD), which may be destructive to fish and water-life. Furthermore, volatilization of ammonia into gas (NH3) contributes significantly to acid rain.11 The elemental composition of microbial dry weight biomass is C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K (1
K (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.28
0.28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06
0.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.02) with the remainder consisting of trace amounts of elements such as sulphur, magnesium, sodium and calcium.12 This is in contrast with urine, which has a composition ratio of C
0.02) with the remainder consisting of trace amounts of elements such as sulphur, magnesium, sodium and calcium.12 This is in contrast with urine, which has a composition ratio of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K (1
K (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.5
9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.82
0.82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.24), showing that it is unbalanced with excessive amounts of N, P and K and too little amounts of C4. The imbalance between the elemental composition of microbial biomass and urine, indicates that under normal conditions, there will always be excess amounts of N, P and K in a urine stream; hence the existing energy intensive treatment to remove these elements. If however, extra carbon energy (e.g. in the form of acetate) can be added to offset this imbalance, then the MFC process can ‘absorb’ the excessive concentrations in the build-up of new biomass, thus removing N, P, K entirely from the effluent.
1.24), showing that it is unbalanced with excessive amounts of N, P and K and too little amounts of C4. The imbalance between the elemental composition of microbial biomass and urine, indicates that under normal conditions, there will always be excess amounts of N, P and K in a urine stream; hence the existing energy intensive treatment to remove these elements. If however, extra carbon energy (e.g. in the form of acetate) can be added to offset this imbalance, then the MFC process can ‘absorb’ the excessive concentrations in the build-up of new biomass, thus removing N, P, K entirely from the effluent.
Recently, the production of hydrogen from the electrolysis of urea in urine has been reported.13 The process produces hydrogen, nitrogen and carbon dioxide and requires a small amount of external energy to occur. The work described in the present study, is a direct conversion of the organic constituents of urine into electricity via the MFC (single-stage process), with efficiencies of >50% from a single MFC. This approach does not utilise urea since it is not metabolisable into electricity (although it is hydrolysed into NH3 and CO2viaurease activity).
On a global scale and on the assumption that the daily average farm animal urine production is 2–3 times that of humans, the total daily amount of urine produced from humans and farm animals is estimated to be 38 billion litres per day. From the data presented above, derived from a single MFC, it is possible to project that 25 PJ of energy can be produced per year. Although this is not sufficient to match the current global energy production from biofuels (2.48 EJ)14 and assuming that the MFC energy generation increases for the same amount of fuel in an MFC stack, as has been previously reported,15 this relationship can only improve. Furthermore, the efficient utilisation of urine through MFC stacks will no longer require conventional energy intensive treatment by the wastewater companies and result in a better-balanced fertiliser. These may be assumptions that will need to be further examined in future work, but they do strongly suggest that this is a worthwhile pursuit. With the recent demonstration that scale-up of power output is feasible by miniaturisation and multiplication of MFC units,16 the possibility of developing large-scale treatment systems for practical applications now draws closer.
Conclusions
The present study describes a way of directly producing electricity from urine. This is not another MFC study that simply demonstrates the utilisation of a substrate, but it is the first report that urine can be an abundant fuel for electricity generation. The impact from this could be enormous, not only for the wastewater treatment industry, but also for people as a paradigm shift in the way of thinking about waste. With an annual global production rate of trillions of litres, this is a technology that could help change the world.Acknowledgements
This work is funded by the UK Engineering & Physical Sciences Research Council (EPSRC), Grants EP/I004653/1, EP/H019480/1.Notes and references
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schroder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Environ. Sci. Technol., 2006, 40, 5181 CrossRef CAS.
- D. Pant, G. V. Bogaert, L. Diels and K. Vanbroekhoven, Bioresour. Technol., 2010, 101, 1533 CrossRef CAS.
- US Census Bureau, URL: http://www.census.gov/ (accessed 22/07/2011).
- A. White, P. Handler and E. L. Smith, Principles of Biochemistry, McGraw-Hill Inc, New York, 4th edn, 1968 Search PubMed.
- UN Food and Agriculture Organisation, http://faostat.fao.org (accessed 23/07/2011).
- L. P. Rodriguez, G. J. Padovan, V. M. M. Suen and J. S. Marchini, Salusvita, Bauru, 2005, 24, 227 Search PubMed.
- J. Greenman, K. T. Holland and W. J. Cunliffe, Microbiology, 1981, 121, 371 Search PubMed.
- I. Ieropoulos, J. Greenman and C. Melhuish, Int. J. Energy Res., 2008, 32, 1228 CrossRef CAS.
- D. Morse, J. Anim Sci. (Champaign IL. U. S.), 1995, 73, 2733 CAS.
- P. E. V. Williams, Anim. Feed Sci. Technol., 1995, 32, 106 Search PubMed.
- H. M. ApSimon, M. Kruse and J. N. B. Bell, Atmos. Environ., 1987, 21, 1939 CrossRef CAS.
- M. H. Gerardi, Wastewater Bacteria, John Wiley & Sons Inc, New Jersey, 2006 Search PubMed.
- B. K. Boggs, R. L. King and G. G. Botte, Chem. Commun., 2009, 4859 RSC.
- BP Statistical Review of World Energy, London, June 2011.
- I. A. Ieropoulos, J. Winfield, J. Greenman and C. Melhuish, ECS Trans., 2010, 28, 1 CrossRef CAS.
- I. Ieropoulos, J. Greenman, C. Melhuish and I. Horsfield, Proceedings of the 12th International Conference on the Synthesis and Simulation of Living Systems, Odense, 2010.
| This journal is © the Owner Societies 2012 |
