Recovered LiCoO2 as anode materials for Ni/Co power batteries
Dawei
Song
b,
Yanan
Xu
a,
Cuihua
An
a,
Qinghong
Wang
a,
Yaping
Wang
a,
Li
Li
a,
Yijing
Wang
*a,
Lifang
Jiao
a and
Huatang
Yuan
a
aInstitute of New Energy Material Chemistry, Key Laboratory of Advanced Energy Materials Chemistry (MOE), TKL of Metal and Molecule Based Material Chemistry, Nankai University, Tianjin 300071, P.R. China. E-mail: wangyj@nankai.edu.cn; Fax: +86 22 23503639; Tel: +86 22 23503639
bSchool of Chemistry and Chemical Engineering, Tianjin University of Technology, Tianjin 300384, P.R. China
First published on 9th November 2011
Abstract
LiCoO2 material is recovered from spent lithium-ion batteries and investigated as anode materials for Ni/Co power batteries for the first time. LiCoO2 electrodes with a small amount of S-doping display excellent electrochemical properties. The electrochemical reactions occurring on M0 electrodes during the first several cycles and after being activated are proposed, respectively. A function mechanism of S powder on M10 electrode is also proposed.
Alkaline rechargeable batteries are considered to be one of the most promising power sources for electric tools such as drills, saws, etc. and electric vehicles (EV) and hybrid electric vehicles (HEV). Owing to the performance amelioration of lead-acid batteries and cost reduction of lithium-ion secondary batteries, alkaline rechargeable Ni/MH batteries have been withdrawn from the power battery market gradually. The features of high energy density, high power, and low price are crucial for alkaline rechargeable batteries to be widely used as the power batteries of electric vehicles. Among the components of alkaline rechargeable batteries, the anode material is one of the important factors that determine the battery performances. Therefore, it is of practical significance to develop advanced anode materials possessing features of excellent cycle stability, high discharge capacities, high-rate discharge capability and low cost.
Recently, the papers on Co-based materials as anode materials for alkaline rechargeable batteries were reported.1–21 Gao et al. systematically proposed the new type Ni/Co batteries system for the first time in 2009, which employed Ni(OH)2 as cathode materials and Co-based materials as anode materials in KOH aqueous solution.22 The new system displays excellent cycle stability, high capacities and environmental friendly properties. However, the reported Co-based materials anodes are still of high cost and poor rate discharge capability. Therefore, for the Co-based material anodes, it is essential to further improve the rate discharge capability and reduce the cost.
Though various new generation cathode materials are developed at present, LiCoO2 is still the leading cathode materials for commercialized Li-ion batteries that power most of the portable electronic devices: cellular phones, laptops, digital cameras, etc.23 Therefore, a large number of spent lithium-ion batteries are produced everyday, and they will cause serious environmental contamination and represent a waste of materials if they are discarded. Recently, much effort has been focused on the recovery of valuable materials (especially Co and Li) from spent lithium-ion batteries. Many recovery and recycling systems of Co and Li are developed, but most of these systems are still complex, environmentally unfriendly and of low profit.24–35 So, it is of great practical importance to look for a simple, green and high profit recovery and recycle systems.
Similar to other Co-based materials, recovered LiCoO2 has the possibility to be used in Ni/Co power batteries. In addition, according to our previous research, the discharge capacity and rate discharge capability of Co-based materials anodes can be improved significantly after a small amount of S-doping.38 Thus, in this work, recovered LiCoO2 is reported for the first time as anode materials for Ni/Co power batteries, and a small amount of S is doped to further improve the electrochemical performances of LiCoO2 electrode.
The spent lithium-ion batteries are from Tianjin Lishen Battery Joint-Stock Co. Ltd. LiCoO2 material is recovered from the spent lithium-ion batteries. A typical recovery process is as follows: First, the lithium-ion batteries are dismantled and the anodes are separated from other parts. Then the anodes are crushed and the powders are fully soaked in KOH alkaline solution in a fume cupboard to remove the aluminum foil (aluminum foil can react with KOH alkaline solution to release H2 gas). Finally, the material is obtained after filtering, washing with distilled water and drying in a vacuum at 50 °C for 10 h. The above obtained material is designated as M0. Moreover, the materials doped with 1–15 wt% S powder by direct mixing are designated as M1–M15, respectively.
The crystal structure and surface configuration of the materials are characterized by X-ray diffraction (XRD, Rigaku D/Max-2500 with Cu Kα radiation) and scanning electron microscopy (SEM, SUPRA 55VP Field Emission). The elemental composition is measured by inductive coupled plasma atomic emission spectroscopy (ICP-AES) on a USA Themo Jarrel-Ash Corp.
Negative electrodes are fabricated with the smearing method. They are constructed by mixing the prepared material with carbonyl nickel powders and PTFE in a weight ratio of 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to form a paste and coated on a piece of Ni-foam. Electrochemical measurements are conducted in a three compartment cell using a Land battery test instrument (CT2001A). The NiOOH/Ni(OH)2 electrode and Hg/HgO electrode in a 6 M KOH aqueous solution serve as the counter electrode and the reference electrode, respectively. The electrodes are charged at 500 mA g−1 for 80 min, and discharged at 500 mA g−1 to −0.5 V (vs.Hg/HgO) after resting for 5 min. Zahner IM6e electrochemical workstation is used for cyclic voltammetry (scan rate: 0.2 mV s−1; potential interval: −1.3 V to −0.4 V vs.Hg/HgO). All the tests are performed at room temperature.
4 to form a paste and coated on a piece of Ni-foam. Electrochemical measurements are conducted in a three compartment cell using a Land battery test instrument (CT2001A). The NiOOH/Ni(OH)2 electrode and Hg/HgO electrode in a 6 M KOH aqueous solution serve as the counter electrode and the reference electrode, respectively. The electrodes are charged at 500 mA g−1 for 80 min, and discharged at 500 mA g−1 to −0.5 V (vs.Hg/HgO) after resting for 5 min. Zahner IM6e electrochemical workstation is used for cyclic voltammetry (scan rate: 0.2 mV s−1; potential interval: −1.3 V to −0.4 V vs.Hg/HgO). All the tests are performed at room temperature.
Fig. 1 shows the X-ray diffraction (XRD) pattern and SEM image of the M0 material. All the diffraction peaks are attributed to LiCoO2 (space groupR![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, JCPDS no. 77-1370). No peaks of other impurities are observed, implying that the aluminium foil in the LiCoO2 electrode has been completely removed. Inductively coupled plasma (ICP) results demonstrate that the mass contents of LiCoO2 in M0 material is 94.1%. It can be inferred that a small amount of conductive agent and binder still remain in M0 material, which are insensitive to alkali treatment. The SEM image illustrates that M0 material is composed of uniform particles with size of about 2–8 μm. This is similar to LiCoO2 materials in industry. Thus, it is concluded that the particle size of LiCoO2 does not change in the process of alkali treatment.
m, JCPDS no. 77-1370). No peaks of other impurities are observed, implying that the aluminium foil in the LiCoO2 electrode has been completely removed. Inductively coupled plasma (ICP) results demonstrate that the mass contents of LiCoO2 in M0 material is 94.1%. It can be inferred that a small amount of conductive agent and binder still remain in M0 material, which are insensitive to alkali treatment. The SEM image illustrates that M0 material is composed of uniform particles with size of about 2–8 μm. This is similar to LiCoO2 materials in industry. Thus, it is concluded that the particle size of LiCoO2 does not change in the process of alkali treatment.
 | ||
| Fig. 1 XRD pattern and SEM image of M0 material. | ||
The cycle performances of M0-M15 electrodes is illustrated in Fig. 2. All the electrodes have an activation process of several cycles. It is shown that the M0 electrode displays a low capacity of about 320 mA h g−1 and it is greatly improved after S-doping. Among these electrodes, the M10 electrode displays the highest discharge capacity of 455 mA h g−1, while the M2 electrode shows the most outstanding cycle performance with a capacity retention rate of more than 80% after 200 cycles. Compared with those Co-based materials anodes reported, the discharge capacities and rate capabilities are attractive.
 | ||
| Fig. 2 Cycle performances of M0–M15 electrodes (discharge current density is 500 mA g−1). | ||
To further investigate the charge–discharge reaction mechanism of the M0 electrodes, the phase change of the M0 electrode at different charge–discharge states is carefully determined using XRD patterns. In order to avoid the influence of the Ni diffraction peaks, the electrodes for XRD measurements are prepared with acetylene black as conductive agent.
As shown in Fig. 3a, all peaks of the initial M0 electrode can be attributed to LiCoO2. After the 10th charged, only Co and Co(OH)2 diffraction peaks can be observed, demonstrating that LiCoO2 in the M0 electrode has completely transformed into Co and Co(OH)2. After the 10th discharged, the diffraction peaks of Co become weaker, while those of Co(OH)2 become stronger, illustrating that some Co transforms into Co(OH)2.
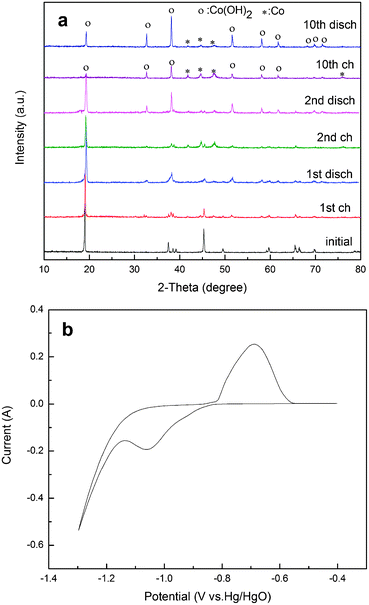 | ||
| Fig. 3 (a) XRD patterns of M0 electrode at different charge–discharge states, (b) cyclic voltammetry (CV) of M0 electrode after the 10th charge–discharge cycle at a scan rate of 0.2 mV s−1. | ||
To further confirm the electrochemical reaction process of the M0 electrode after the 10th cycle, a cyclic voltammetry (CV) curve at a scan rate of 0.2 mV s−1 is presented in Fig. 3b. A pair of obvious redox peaks are detected, indicating that the reversible capacity is mainly based on the faradaic redox mechanism. The curve shape and peak voltage are very similar to those of metallic Co powder electrode, confirming that the same reversible reaction occurs on M0 electrode, Co(OH)2 electrode and metallic Co electrode.36–38 Based on the XRD patterns after charge and discharge (Fig. 3a), the reduction peak is due to the reduction of Co(OH)2 to metallic Co while the oxidation peak should be attributed to the oxidation of metallic Co to Co(OH)2. The reversible electrochemical reaction occurring on the electrode after the 10th cycle is only the conversion between Co and Co(OH)2, which can be expressed as follows:
 | (1) |
| Charge: LiCoO2 + 2H2O + e → Co(OH)2 + 2OH− + Li+ | (2) |
| Co(OH)2 + 2e → Co + 2OH− | (3) |
| Discharge: Co + 2OH− → Co(OH)2 + 2e | (4) |
 | ||
| Fig. 4 (a) and (b) XRD patterns of the M0 electrode at different charge–discharge states, (c) charge–discharge curves of the M0 electrode for different cycles. | ||
Charge–discharge curves of M0 electrode for different cycles, illustrated in Fig. 4c, are also in good agreement with the above proposed reaction mechanism. There is one potential plateau in the discharge curves and two potential plateaus in the charge curves. With the cycle number increasing, the discharge potential plateaus hardly change, while the first charge potential plateaus shift positively and the second ones shift negatively. Both the discharge reactions in the initial four cycles and in the 10th cycle are attributed to the transformation from Co to Co(OH)2, so all the discharge potential plateaus appear at around −0.78 V (vs.Hg/HgO). The first charge potential plateaus in the initial four cycles correspond to reaction (2) and reaction (3) (around −0.88 V), and the second charge potential plateaus correspond to reaction (2) and the electrolysis reaction of water (around −1.1 V). With the cycle number increasing, the amount of reaction (2) decreases, so the first charge potential plateaus shift positively and the second ones shift negatively. In the 10th cycle, there is no reaction (2) occurring, so the first charge potential plateau appearing at around −0.88 V only corresponds to reaction (3), and the second one appearing at around −1.1 V only corresponds to electrolysis reaction of water, and there are no longer changes in the later cycles.
The above reaction mechanism can also illustrate why the M0 electrode needs an activation in charge–discharge cycles. The discharge capacity is attributed to the transformation from Co to Co(OH)2. Because only a small amount of Co is produced during the 1st charge process, a small amount of active material Co participates in the 1st discharge process, displaying low discharge capacity. In the following cycles, the discharge capacity increases gradually with the increasing content of Co in the electrode. After 10 cycles, LiCoO2 completely transforms into Co and Co(OH)2. The reversible reaction (1) is dominant during the charge–discharge process and the discharge capacity is stable.
From Fig. 2, M1–M15 electrodes with S-doping display better electrochemical properties than the M0 electrode. In order to evaluate the effects of S powder on M1–M15 electrodes, the S elemental content in KOH aqueous solution and in M10 electrode are measured by ICP during the initial four charge–discharge cycles. Elemental S in KOH aqueous solution is discovered after the 1st cycle and increases gradually during the following cycles, while elemental S content in the M10 electrode decreases gradually, indicating S powder in M10 electrode is gradually dissolved into KOH aqueous solution. The elemental S content in KOH aqueous solution remains stable and no elemental S can be detected in the electrode after the 4th cycles, indicating that S powder has absolutely dissolved into the electrolyte.
Based on our previous research, the function mechanism of S powder on M10 electrode can be described as Scheme 1.38 The electrode is fabricated by coating M10 material on a piece of Ni-foam, then pressed between two pieces of nickel foam, forming a sandwich-structure. The structure is favorable for the sufficient contact between the M10 material and alkaline solution, which is in favor of the redox conversion among the active materials (LiCoO2, Co(OH)2 and Co). Moreover, the dissolution of S powder in the M10 electrode brings new interspaces among the active materials and greatly increases the contact between the active materials and alkaline solution. Thus, the capacity utilization of the active materials is enhanced and higher discharge capacities and better rate capabilities are obtained. But the discharge capacity does not always increase with the increase of S powder, because too much S-doping will create many interspaces among the active materials, leading to poor electrical conductivity.
 | ||
| Scheme 1 The function schematic representation of S powder on M10 electrode. | ||
In summary, LiCoO2 material is recovered from spent lithium-ion batteries and investigated as anode materials for Ni/Co power batteries for the first time. The discharge capacity of the M0 electrode is about 320 mA h g−1 at the current density 500 mA g−1. The electrochemical reactions occurring on M0 electrodes during the first several cycles and after activation are proposed, respectively. S powder is doped into M0 electrode to further improve its electrochemical properties. Electrochemical measurements show that the M10 electrode displays a high reversible discharge capacity of 455 mA h g−1, and the capacity retention rate of the M2 electrode is over 80% after 200 cycles. A function mechanism of S powder on the M10 electrode is also proposed. This work is of great practical importance, which provides a simple, green and high value-added recovery and recycle system for Co and Li from spent lithium-ion batteries.
This work was financially supported by MOST project (2010CB631303), NSFC (50971071, 51071087), MOE (IRT-0927) and Nature Science Foundation of Tianjin (11JCYBJC07700, 10SYSYJC27600).
Notes and references
- S. R. Chung, K. W. Wang and T. P. Perng, J. Electrochem. Soc., 2006, 153, A1128 CrossRef CAS.
- S. R. Chung, K. W. Wang and M. H. Teng, Int. J. Hydrogen Energy, 2009, 34, 1383 CrossRef CAS.
- X. Y. Zhao, L. Q. Ma, Y. Yao, M. Yang, Y. Ding and X. D. Shen, Electrochim. Acta, 2010, 55, 169 Search PubMed.
- Y. Wang, J. M. Lee and X. Wang, Int. J. Hydrogen Energy, 2010, 35, 1669 CrossRef CAS.
- D. S. Lu, W. S. Li, X. Jiang, C. L. Tan and R. H. Zeng, J. Alloys Compd., 2009, 485, 621 CrossRef CAS.
- S. M. Han, Z. H. Feng, L. Hu, Y. Li, J. S. Hao and J. W. Zhang, Mater. Chem. Phys., 2010, 24, 17 CrossRef.
- Y. D. Wang, X. P. Ai and H. X. Yang, Chem. Mater., 2004, 16, 5194 CrossRef CAS.
- D. S. Lu, W. S. Li, F. M. Xiao and R. H. Tang, Electrochem. Commun., 2010, 12, 362 CrossRef CAS.
- D. S. Lu, W. S. Li, C. L. Tan and R. H. Zeng, Electrochim. Acta, 2009, 55, 171 CrossRef CAS.
- C. Wu, Y. Bai, F. Wu, L. W. Dong, X. Wang, L. X. Yang and C. Z. Zhang, Electrochim. Acta, 2008, 53, 4715 CrossRef CAS.
- G. He, L. F. Jiao, H. T. Yuan, Y. Y. Zhang and Y. J. Wang, Electrochem. Commun., 2006, 8, 1633 CrossRef CAS.
- Y. L. Cao, W. Z. Zhou, X. Y. Li, X. P. Ai, X. P. Gao and H. X. Yang, Electrochim. Acta, 2006, 51, 4285 CrossRef CAS.
- Y. Liu, Y. J. Wang, L. L. Xiao, D. W. Song, L. F. Jiao and H. T. Yuan, Electrochem. Commun., 2007, 9, 925 CrossRef CAS.
- Y. Liu, Y. J. Wang, L. L. Xiao, D. W. Song, Y. P. Wang, L. F. Jiao and H. T. Yuan, Electrochim. Acta, 2008, 53, 2265 CrossRef CAS.
- D. W. Song, Y. J. Wang, Y. P. Wang, L. F. Jiao and H. T. Yuan, Electrochem. Commun., 2008, 10, 1486 CrossRef CAS.
- Q. H. Wang, L. F. Jiao, H. M. Du, D. W. Song, W. X. Peng, Y. J. Wang and H. T. Yuan, Electrochim. Acta, 2010, 55, 7199 CrossRef CAS.
- Y. Han, Y. J. Wang, Y. P. Wang, L. F. Jiao and H. T. Yuan, Int. J. Hydrogen Energy, 2010, 35, 8177 CrossRef CAS.
- Q. H. Wang, L. F. Jiao, H. M. Du, W. X. Peng, S. C. Liu, Y. J. Wang and H. T. Yuan, Int. J. Hydrogen Energy, 2010, 35, 8357 CrossRef CAS.
- Q. H. Wang, L. F. Jiao, H. M. Du, W. X. Peng, D. W. Song, Y. J. Wang and H. T. Yuan, Electrochim. Acta, 2011, 56, 1106 CrossRef CAS.
- D. W. Song, Y. P. Wang, Q. H. Wang, Y. J. Wang, L. Li, G. Liu, L. F. Jiao and H. T. Yuan, J. Power Sources, 2010, 195, 7462 CrossRef CAS.
- H. M. Du, L. F. Jiao, Q. H. Wang, W. X. Peng, D. W. Song, Y. J. Wang and H. T. Yuan, J. Power Sources, 2011, 196, 5751 CrossRef CAS.
- X. P. Gao, S. M. Yao, T. Y. Yan and Z. Zhou, Energy Environ. Sci., 2009, 2, 502 CAS.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243 CAS.
- J. Q. Xu, H. R. Thomas, R. W. Francis, K. R. Lum, J. W. Wang and B. Liang, J. Power Sources, 2008, 177, 512 CrossRef CAS.
- J. Kang, J. Sohn, H. Chang, G. Senanayake and S. M. Shin, Adv. Powder Technol., 2010, 21, 175 CrossRef CAS.
- L. Li, J. Ge, R. J. Chen, F. Wu, S. Chen and X. X. Zhang, Waste Manage., 2010, 30, 2615 CrossRef CAS.
- C. K. Lee and K. I. Rhee, J. Power Sources, 2002, 109, 17 CrossRef CAS.
- L. Li, R. J. Chen, F. Sun, F. Wu and J. R. Liu, Hydrometallurgy, 2011, 108, 220 CrossRef CAS.
- Y. Pranolo, W. Zhang and C. Y. Cheng, Hydrometallurgy, 2010, 102, 37 CrossRef CAS.
- C. Lupi, M. Pasquali and A. Dell'Era, Waste Manage., 2005, 25, 215 CrossRef CAS.
- S. M. Shin, N. H. Kim, J. S. Sohn, D. H. Yang and Y. H. Kim, Hydrometallurgy, 2005, 79, 172 CrossRef CAS.
- L. Li, J. Ge, F. Wu, R. J. Chen, S. Chen and B. R. Wu, J. Hazard. Mater., 2010, 176, 288 CrossRef CAS.
- J. F. Paulino, N. G. Busnardo and J. C. Afonso, J. Hazard. Mater., 2008, 150, 843 CrossRef CAS.
- R. C. Wang, Y. C. Lin and S. H. Wu, Hydrometallurgy, 2009, 99, 194 CrossRef CAS.
- J. M. Nan, D. M. Han and X. X. Zuo, J. Power Sources, 2005, 152, 278 CrossRef CAS.
- Z. W. Lu, S. M. Yao, G. R. Li, T. Y. Yan and X. P. Gao, Electrochim. Acta, 2008, 53, 2369 CrossRef CAS.
- S. M. Yao, K. Xi, G. R. Li and X. P. Gao, J. Power Sources, 2008, 184, 657 CrossRef CAS.
- D. W. Song, Q. H. Wang, Y. P. Wang, Y. J. Wang, L. F. Jiao and H. T. Yuan, J. Power Sources, 2010, 195, 7115 CrossRef CAS.
| This journal is © the Owner Societies 2012 |
