Tetragonal faceted-nanorods of anatase TiO2 with a large percentage of active {100} facets and their hierarchical structure†
Jianming
Li
,
Ke
Cao
,
Qi
Li
and
Dongsheng
Xu
*
Beijing National Laboratory for Molecular Sciences, College of Chemistry and molecular engineering, Peking University, Beijing, 100871, China. E-mail: dsxu@pku.edu.cn; Fax: (+86) 10-62760360; Tel: (+86) 10-62753580
First published on 4th November 2011
Abstract
Highly uniformed tetragonal faceted-nanorods (TFNRs) of anatase TiO2 enclosed by active {100} facets with narrow size distribution are obtained with high-yield (>90%). In addition, hierarchically structured anatase TiO2 which consist of TFNRs are also prepared, and the sizes of the TFNR components could be tuned.
The synthesis of anatase TiO2 nanocrystals with exposed high-energy facets has attracted extraordinary attentions due to the importance of surface structure of TiO2 in determining their properties.1–11 For example, active {001} facets (with 100% five-coordinate Ti (Ti5c) atoms) of anatase TiO2 have shown superior performance over that of lower-energy {101} facets (with only 50% Ti5c atoms) in the fields of dye-sensitized solar cell, photocatalysis, photosplitting water and Li-ion battery.12–17 However, due to the limited synthesis conditions, another important active {100} facets (also with 100% Ti5c atoms), which have been demonstrated more active than {101} facets both from the theoretical18,19 and practical10,11,20,21 studies, has been less reported. Recently, we prepared tetragonal faceted-nanorods (TFNRs) of anatase TiO2 with a large percentage of active {100} facets.10 The Na-titanate nanotubes were chosen as the titanium precursor mainly because of their low reactivities in the basic solution and the slow release of the hydroxyl ions from them. Herein, we further explore the synthetic conditions for the preparation of high uniform TFNRs with high yield, meanwhile, we first report a novel hierarchical structure which is composed of TFNRs with a large percent of high-energy {100} facets.
Fig. 1a and 1b show the scanning electron microscopy (SEM) images of the products obtained by hydrothermal reaction of Na-titanate nanotubes (Na-TNTs) in basic conditions, as described in the Experimental Section in the ESI†. As our previous report,10 most of the particles had well-defined lateral facets with sharp edges and the adjacent facets were perpendicular as well with the same width, indicating a TFNR shape. However, the sizes of the particles were not well uniform. To improve the size distribution of the TFNRs, H2O2 was introduced into the hydrothermal reactions. H2O2 could react with the surface [Ti(OH)n]4−n of Na-TNT to form a yellow colored [Ti(OH)n−x(H2O2)x]4−n complex, which facilitates Na-TNT decomposition. Fig. 1c–d show the SEM images of the products prepared by adding 2 mL H2O2, the yield of TFNRs exceeded 90%. To examine the uniformity of the synthesized particles, we statistically analyzed the sizes of the edges (labeled as D) and the aspect ratios (labeled as L/D) of the TFNRs (Schematic drawing, Fig. 1g). The average size of D is 59.4 nm with a relative standard deviation of 12.6% (Fig. 1e), and the average value of L/D is 8.6 with a relative standard deviation of 11.2% (Fig. 1f). The anatase phase and the exposed lateral active {100} facets of the prepared TFNRs were further confirmed by powder X-ray diffraction (XRD) patterns and transmission electron microscopy (TEM) images (Fig. S1 and S2 in ESI†).
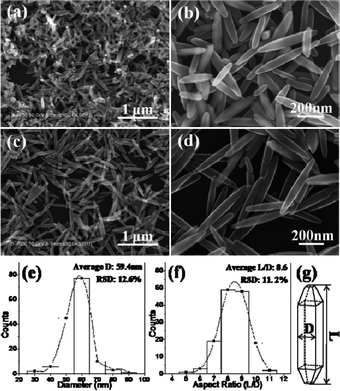 | ||
| Fig. 1 Lower and higher SEM images of tetragonal faceted-nanorods (TFNR) prepared without (a–b) and with (c–d) adding 2 mL H2O2 using Na-TNT as precursor; (e), (f) the distribution of the edge (D) and aspect ratio (L/D) of TFNRs prepared with adding H2O2; (g) schematic drawing of a single TFNR labeled D and L. | ||
Moreover, many researchers focus on the synthesis of TiO2 with different nanostructures. In which, fabrication of complex architectures with three dimensional (3D) or highly ordered nanostructures is very desirable in current materials research.22–24 Due to their unique structure and novel properties, the hierarchically structured anatase TiO2 holds the promise of advanced applications, particularly in the field of advanced photocatalysis, DSSCs and optoelectronic devices. However, the hierarchically structured anatase TiO2 are usually constructed by irregularly shaped particles, and the reported exposed facets are dominated by low-energy {101} facets25,26 or high-energy {001} facets.12–15 The preparation of hierarchically structured anatase TiO2 with large percentage of active {100} facets is a very interesting and important issue from both academic and industrial points of view and has never been reported.
We chose urchin-liked shapes Na-titanates (Na-UTs)27 as a precursor to preparing hierarchically structured anatase TiO2 with a large percentage of active {100} facets, as described in the Experimental Section in the ESI†. XRD patterns indicate that the Na-UT precursor has similar diffraction peaks to that of Na-TNT precursor with a monoclinic (C2/m) crystal system (Fig. 2a). Energy dispersive X-ray spectroscopy reveals that the atomic ratio of sodium to titanium was ca. 16%. After further hydrothermal treatment of the Na-UT precursors in deionized water, a pure anatase phase of TiO2 (tetragonal, I41/amd, JCPDS 21-1272) was obtained (Fig. 2b). Fig. 3a shows the as-prepared titanate precursors with urchin-like shapes ranging from hundreds of nanometers to several micrometers. Fig. 3b–c display the low- and high-resolution SEM images of the hierarchically structured anatase samples prepared by hydrothermal treatment of the Na-UT precursors in deionized water at 200 °C for 24 h. The overall morphologies of the prepared anatase samples were hierarchically spherical structures which consist of interconnected nanocrystals. All the nanocrystals were TFNRs with four well-defined lateral facets (Fig. 3c). The TFNR morphology was further revealed by TEM image. Fig. 4a shows one of the lateral facets of a TFNR. It was flat and the width was constant. By rotating the same particle, four sharp edges with perpendicular adjacent facets were observed when viewing from the top (Fig. 4b–d). The exposed lateral facets were confirmed by selected-area electron diffraction (SAED) patterns and high-resolution TEM (HRTEM) image (Fig. 3d–f). Both TEM image and SAED patterns confirm that the TFNR has a single-crystalline structure of anatase TiO2. Fig. 3f displays the HRTEM image, three sets of lattice fringes with spaces of 0.35, 0.35 and 0.48 nm can be attributed to (101), (10−1) and (001) of anatase phase, respectively. The axis of the rod was parallel to the <001> direction, indicating that the rod grows along the <001> direction. The diffraction spots in the SAED patterns could be indexed into the [010] zone and they indicated that the exposed facet (under electron-beam irradiation) was flat and the thickness of the rod was constant. On the basis of the above observations and structural analysis, we concluded that the exposed lateral facets of the prepared TFNR are mainly the {100} facets. More detailed characterization could be referred to our previous report.10 It is worth noting that this is the first report to prepare hierarchically structured anatase TiO2 which are composed of TFNRs with high-energy {100} facets.
 | ||
| Fig. 2 X-ray diffraction patterns of: (a) the prepared Na-UT precursor and (b) hierarchically structured anatase TiO2. | ||
![(a) SEM image of the prepared urchin-liked Na-titanates (Na-UTs) precursors; (b) and (c) Low- and high-resolution SEM images of hierarchically structured anatase TiO2; (d) Typical TEM image of an individual TFNR viewed along the [010] direction; (e) The corresponding SEAD patterns; (f) HRTEM image taken from (d) indicated by rectangles.](/image/article/2012/CE/c1ce06229h/c1ce06229h-f3.gif) | ||
| Fig. 3 (a) SEM image of the prepared urchin-liked Na-titanates (Na-UTs) precursors; (b) and (c) Low- and high-resolution SEM images of hierarchically structured anatase TiO2; (d) Typical TEM image of an individual TFNR viewed along the [010] direction; (e) The corresponding SEAD patterns; (f) HRTEM image taken from (d) indicated by rectangles. | ||
 | ||
| Fig. 4 TEM image of the same TFNR viewed along different directions by rotating the sample: (a) α = 0°, β = 0°; (b) α = 4.15°, β = 5.88°; (c) α = 8.92°, β = 9.84°; (d) α = 9.05°, β = 12.02°. | ||
As mentioned in our previous report,10 the transformation from a Na-UT precursor to a hierarchical structure TiO2 also followed a “dissolution and nucleation” mechanism. It should be noted that, the resulting hierarchical structure of TiO2 is mainly because of the morphology of the Na-UT precursor, which was important for inducing TiO6 octahedra nucleating on its surface. Meanwhile, the hydroxyl ions gradually released from the Na-UT precursor under hydrothermal conditions would preferentially adsorb onto anatase (100) facets, which lower the surface energy of the (100) facets and thus limited the crystal growth along the a and b-axis, resulting in the {100} facets enclosed TFNRs.
The structure of the Na-UT precursor was important for preparation of TiO2 because it could influence the saturation concentration of Ti(OH)4 fragments and thus the nucleation of TiO2 on its surface. Therefore, we further studied the effect of the Na-UT precursor structure on the morphology of the products. Before hydrothermal treatment, the Na-UT samples were annealed in a muffle furnace at 500 °C (heated in advance) for different times (5, 10 and 20min), and the prepared Na-UT precursors were labeled as Na-UT-c, where c means annealing time. For example, Na-UT-10 denotes Na-UT calcined at 500 °C for 10 minutes while Na-UT-0 denotes Na-UT without annealing. Fig. 5 shows the XRD patterns of Na-UT-c precursors. Obviously, the broadened peak located at 2θ = 9.4° in Na-UT-0 gradually changed to 10.3, 11.8 and 11.9° when the heating time was 5, 10 and 20 minutes, meaning the interlayer distances (d100) of Na-UT-c decreased from 9.4 to 8.6, 7.5 and 7.4nm, respectively. After further hydrothermal treatment of Na-UT-c, they convert to a hierarchical structure of TiO2, as shown in Fig. 6. Compared with the Fig. 3b–c, the hierarchically sphere structures were more compact and the size of the TFNRs were greatly decreased, the average diameters of the TFNRs were decreased from 70 to 30 nm (Fig. 6a–b). The TFNRs sizes were even diminished when the precursor heating time was 10 min (Fig. 6c–d). When the heating time was longer than 10 min, both the structure of Na-UT-c precursors and the morphologies of the TiO2 counterpart remained unchanged. These results indicated that by suitably controlling the Na-UT precursors, such as the morphology and structure, both the shape and size of the hierarchical structure of TiO2 could be tuned.
 | ||
| Fig. 5 X-ray diffraction patterns of: (a) Na-UT-0; (b) Na-UT-5; (c) Na-UT-10; (d) Na-UT-20. | ||
 | ||
| Fig. 6 Lower and higher SEM images of products prepared from Na-UT-c precursors: (a), (b) c = 5; (c), (d) c = 10; (e), (f) c = 20 min. | ||
In summary, by introducing H2O2 into the hydrothermal reactions, highly uniform TFNRs of anatase TiO2 with a narrow size distribution were obtained with high-yield. In addition, hierarchically structured anatase TiO2 which are composed of TFNRs were also prepared, and the sizes of the TFNRs could be controlled by changing the structure of the Na-UT precursors. It should be mentioned that other titanium precursors, such as potassium titanates derived from concentrated KOH treatment of TiO2, which would gradually release hydroxyl ions upon hydrothermal conditions could also be used to prepare anatase TiO2 particles with a large percentage of higher-energy {100} facets.
Acknowledgements
This work was supported by NSFC (Grant Nos. 50821061 and 21133001) and MSTC (Grant Nos. 2007CB936201 and 2011CB808702).Notes and references
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891 CrossRef CAS.
- G. Liu, L. Wang, H. G. Yang, H.-M. Cheng and G. Q. Lu, J. Mater. Chem., 2010, 20, 831 RSC.
- G. Liu, J. C. Yu, G. Q. Lu and H.-M. Cheng, Chem. Commun., 2011, 47, 6763 RSC.
- H. G. Yang, C. H. Sun, S. Z. Qiao, J. Zou, G. Liu, S. C. Smith, H. M. Cheng and G. Q. Lu, Nature, 2008, 453, 638 CrossRef CAS.
- H. G. Yang, G. Liu, S. Z. Qiao, C. H. Sun, Y. G. Jin, S. C. Smith, J. Zou, H. M. Cheng and G. Q. Lu, J. Am. Chem. Soc., 2009, 131, 4078 CrossRef CAS.
- X. Han, Q. Kuang, M. Jin, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2009, 131, 3152 CrossRef CAS.
- Y. Dai, C. M. Cobley, J. Zeng, Y. Sun and Y. Xia, Nano Lett., 2009, 9, 2455 CrossRef CAS.
- X. Y. Ma, Z. G. Chen, S. B. Hartono, H. B. Jiang, J. Zou, S. Z. Qiao and H. G. Yang, Chem. Commun., 2010, 46, 6608 RSC.
- J. Li, Y. Yu, Q. Chen, J. Li and D. Xu, Cryst. Growth Des., 2010, 10, 2111 CAS.
- J. Li and D. Xu, Chem. Commun., 2010, 46, 2301 RSC.
- B. Wu, C. Guo, N. Zheng, Z. Xie and G. D. Stucky, J. Am. Chem. Soc., 2008, 130, 17563 CrossRef CAS.
- J. S. Chen, Y. L. Tan, C. M. Li, Y. L. Cheah, D. Luan, S. Madhavi, F. Y. C. Boey, L. A. Archer and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 6124 CrossRef CAS.
- H. Zhang, Y. Han, X. Liu, P. Liu, H. Yu, S. Zhang, X. Yao and H. Zhao, Chem. Commun., 2010, 46, 8395 RSC.
- W. Yang, J. Li, Y. Wang, F. Zhu, W. Shi, F. Wan and D. Xu, Chem. Commun., 2011, 47, 1809 RSC.
- S. Liu, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2010, 132, 11914 CrossRef CAS.
- M. Liu, L. Piao, L. Zhao, S. Ju, Z. Yan, T. He, C. Zhou and W. Wang, Chem. Commun., 2010, 46, 1664 RSC.
- J. Yu, L. Qi and M. Jaroniec, J. Phys. Chem. C, 2010, 114, 13118 CAS.
- A. Beltrán, J. R. Sambrano, M. Calatayud, F. R. Sensato and J. Andrés, Surf. Sci., 2001, 490, 116 CrossRef.
- M. Calatayud and C. Minot, Surf. Sci., 2004, 552, 169 CrossRef CAS.
- J. Pan, G. Liu, G. Q. Lu and H.-M. Cheng, Angew. Chem., Int. Ed., 2011, 50, 2133 CrossRef CAS.
- J. Pan, X. Wu, L. Wang, G. Liu, G. Q. Lu and H.-M. Cheng, Chem. Commun., 2011, 47, 8361 RSC.
- P. Yang, T. Deng, D. Zhao, P. Feng, D. Pine, B. F. Chmelka, G. M. Whitesides and G. D. Stucky, Science, 1998, 282, 2244 CrossRef CAS.
- L. Zhang and J. C. Yu, Chem. Commun., 2003, 2078 RSC.
- L.-S. Zhong, J.-S. Hu, L.-J. Wan and W.-G. Song, Chem. Commun., 2008, 1184 RSC.
- W.-G. Yang, F.-R. Wan, Q.-W. Chen, J.-J. Li and D.-S. Xu, J. Mater. Chem., 2010, 20, 2870 RSC.
- H.-J. Koo, Y. J. Kim, Y. H. Lee, W. I. Lee, K. Kim and N.-G. Park, Adv. Mater., 2008, 20, 195 CrossRef CAS.
- Y. Mao, M. Kanungo, T. Hemraj-Benny and S. S. Wong, J. Phys. Chem. B, 2006, 110, 702 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, XRD, TEM, and HRTEM figures. See DOI: 10.1039/c1ce06229h |
| This journal is © The Royal Society of Chemistry 2012 |
