A novel T4(1)6(1) water tape encapsulated in a (3,3)-connected 2D copper metal–organic framework†
Xia
Zhu
,
Yun-Fei
Feng
,
Min
Li
,
Bao-Long
Li
* and
Yong
Zhang
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123, China. E-mail: libaolong@suda.edu.cn
First published on 8th November 2011
Abstract
A (3,3)-connected 2D copper coordination polymer {[Cu(abit)(NO3)]·5H2O}n (1) containing a novel T4(1)6(1) water tape consisting of chair-shaped water hexamer with two dangling water molecules and uudd configuration of tetramer water that share one water molecule between the adjacent rings has been synthesized and characterized by single crystal X-ray diffraction analysis.
Water is the most abundant and cheapest solvent available for chemical processes and is essential for life processes. Water has been intensively investigated experimentally and theoretically because of its fundamental importance in biological and chemical processes.1Science ranked the study of water among the top 10 breakthroughs in 2004.2 The study of water clusters is very important to understanding the anomalous behavior of bulk water. The key to understanding the behavior of water is the precise structural data of various hydrogen-bonded water networks in diverse environments.
The discrete small water clusters including tetramer,3hexamer,4–6octamer,6–8 and decamer9–19water morphologies found in the crystalline hosts have been structurally characterized. Among the water clusters, the water hexamer is particularly interesting because it is a dominant form in ice and bulk water.20
One-dimensional water plays an important role in several biological processes.21 The 1D tape water lies between a 2D sheet and 0D cluster and can either edge- or vertex-share. Although a variety of 1D waters have been structurally characterized.22 The tape water clusters have been relatively less studied.23–38 Metal–organic frameworks (MOFs) can provide a void space where discrete water clusters can exist. The interaction between the water clusters and the surroundings play a key role in the crystal lattice.
Our synthetic approach starts by focusing on the construction of new topological frameworks using flexible ligands such as 1,2-bis(1,2,4-triazol-1-yl)ethane (bte),391,3-bis(1,2,4-triazol-1-yl)propane,401,4-bis(1,2,4-triazol-1-yl)- butane (btb)41 and 1,4-bis(1,2,4-triazol-1-ylmethyl)benzene.42 Previously,41a we synthesized a novel 3D coordination polymer [Cd2(btec)(btb)3](H2O)10 containing a discrete water decamer and a thick 2D network [Cd2(phth)2(btb)(H2O)3](H2O)4.5 containing an undulated water chain (btec = 1,2,4,5-benzenetetracarboxylate, phth = 1,2-benzenedicarboxylate).
In the present work, we reported a 2D copper coordination polymer {[Cu(abit)(NO3)]·5H2O}n (1) which contains a novel T4(1)6(1) water tape consisting of chair-shaped water hexamer with two dangling water molecules and tetramer cyclic arrangements (Habit = 4-amino-3,5-bis(imidazol-1-ylmethyl)-1,2,4-triazole).
1 was synthesized by reaction of Cu(NO3)2·3H2O and Habit in a CH3OH/H2O medium.43 X-ray single-crystal diffraction analysis‡ reveals that the structure of 1 is a (3,3)-connected 2D network. The asymmetry unit consists of one Cu(II) atom, one abit, one nitrate and five water molecules. Each Cu1 atom is coordinated to three nitrogen atoms from three abit ligands and two oxygen atoms from a chelating nitrate anion in a distorted trigonal-bipyramidal geometry (Cu1 is 3-connected.) (Fig. S1†). Each abit ligand connects three Cu atoms using its two imidazole nitrogen atoms and one triazole nitrogen atom (The abit is 3-connected) and extends to form a novel 2D network with Cu1⋯Cu1C, Cu1⋯Cu1D and Cu1C⋯Cu1D distances of 8.537(2), 9.792(1), 11.833(2) Å (Fig. 1). The Schläfli symbol for 1 is 4.82 (Fig. 2). The 2D networks parallel pack along the a direction (Fig. S2 and S3†). For comparison, the abit ligands all show bidentate coordination mode using its two imidazole nitrogen atoms in {[Mn(Habit)3](ClO4)2}n, {[Mn(Habit)3](PF6)2}n, [Mn(Habit)(dca)2(H2O)2]n and [Mn(Habit)(dca)2(H2O)2]n.44
 | ||
| Fig. 1 2D coordination network in 1. | ||
 | ||
| Fig. 2 Scheme (3,3)-connected 2D net with a Schläfli symbol (4.82) in 1. | ||
An interesting feature of 1 is that there is T4(1)6(1) water tape (Fig. 3). The tape water consists of chair-shaped water hexamer with two dangling water molecules and tetramer cyclic arrangements that share one water molecule between the adjacent rings. There are five independent lattice water molecules, O4, O5, O6, O7 and O8. Interestingly, three water molecules O5, O6, O7 and symmetry-related O5@, O6@ and O7@ form a cyclic water hexamer through hydrogen bonds (Table 1). The cyclic hexamer assumes a chair conformation. The O5 and O5@ atoms deviate from the O6, O7, O6@ and O7@ plane −0.699(2) and 0.699(2) Å, respectively. The O⋯O distances in the hexamer vary from 2.711(9) to 2.737(9) Å, i.e., 2.715(8) Å for O5⋯O7, 2.711(9) Å for O7@⋯O6, 2.737(9) for O6⋯O5. The average O⋯O separations of 2.721 Å is obvious shorter than the distances of O⋯O in ice Ic (2.75 Å), Ih (2.759 Å) determined at −130 and −90 °C,45 and 2.85 Å in liquid water.46 In the hexamer, each water molecule acts as both single hydrogen bond donor and acceptor. The hydrogen bond length (O6⋯O8 2.834(8) Å) between the hexamer and the dangling water O8 is slightly longer than the average intra-hexameric length (2.721 Å).
| D–H⋯A | d(D–H) | d(H⋯A) | d(D⋯A) | ∠(DHA) |
|---|---|---|---|---|
| a Symmetry transformations used to generate equivalent atoms: * x + 1/2, −y + 1/2, z − 1/2; # −x + 1, −y, −z + 1; @ −x + 2, −y, −z + 1; & x + 1, y, z. | ||||
| O(4)-H1W)⋯O(3)* | 0.69 | 2.16 | 2.762(7) | 147.0 |
| O(4)-H(2W)⋯O(5) | 0.69 | 2.30 | 2.791(9) | 130.4 |
| O(5)-H(3W)⋯O(4)# | 0.65 | 2.14 | 2.785(9) | 171.4 |
| O(5)-H(4W)⋯O(7) | 1.00 | 1.93 | 2.715(8) | 133.5 |
| O(6)-H(5W)⋯O(8) | 0.55 | 2.29 | 2.834(8) | 172.9 |
| O(6)-H(6W)⋯O(5) | 0.82 | 1.94 | 2.737(9) | 164.4 |
| O(7)-H(7W)⋯O(2)* | 0.70 | 2.14 | 2.837(7) | 178.3 |
| O(7)-H(8W)⋯O(6)@ | 0.78 | 1.94 | 2.711(9) | 168.0 |
| O(8)-H(9W)⋯N(2) | 0.72 | 2.16 | 2.838(7) | 157.1 |
| O(8)-H(10W)⋯O(3)& | 0.91 | 1.87 | 2.761(7) | 166.9 |
| N(4)-H(4C)⋯O(3)* | 0.85(3) | 2.05(4) | 2.888(7) | 169(9) |
 | ||
| Fig. 3 A T4(1)6(1) water tape containing the chair conformation of hexamer water and uudd configuration of tetramer water. | ||
The tetramer cyclic (H2O)4 is formed by O4, O5 and symmetry-related O4#, O5# atoms. In the tetramer, four water molecules are fully coplanar and each water molecule acts as both single hydrogen bond donor and acceptor. The remaining four hydrogen atoms in an up–up–down–down (uudd) fashion and the water tetramer adopts the uudd configuration.3 The O⋯O distances in the tetramer vary from 2.785(9) to 2.791(9) Å. The average O⋯O separations of 2.788 Å is slightly longer than the distances of O⋯O in hexamer (2.722 Å), ice Ic (2.75 Å), Ih (2.759 Å) determined at −130 and −90 °C,45 and slightly shorter than the distances of O⋯O 2.85 Å in liquid water.46
O5 water molecule shows both double hydrogen bond donor and acceptor because that O5 water is both shared by adjacent hexamer and tetramer cycles. O4, O6 and O7 water molecules exhibit double hydrogen bond donor and single acceptor because O6 also acts as hydrogen bond donor to form hydrogen bond with dangling water molecule O8, O4 and O7 water molecule both act as hydrogen bond donor and forms hydrogen bond with O atom of nitrate anion (Fig. 4 and Fig. S4†). The dangling water molecule O8 also acts as hydrogen bond donor and forms hydrogen bond with O3 atom of nitrate anion and N2 atom of triazole ring.
 | ||
| Fig. 4 The hydrogen bond interactions between the water tape and the nitrate oxygen/triazole nitrogen atoms. | ||
The hexamer and tetramer are linked alternately by sharing one water molecule O5 between the adjacent rings to extend a novel water tape. The water tape can be represented by a T4(1)6(1). Although a number of water tapes were synthesized, including T4(1),24,25T4(2),26T5(2),27–29T6(0)A(0),30 quasi T6(0)A(0),31T6(2),32,332T6(2),34T7(2),35T4(3)5(0)A(0) and TU.36 Two examples of T4(2)6(2) water tape are also reported.37,38 Mascal and co-workers checked the 17 publications which reported extraordinary or even unprecedented assemblies of water molecules, revised the claims of the water clusters and compared these structures against those already in the CSD.47 Infantes and co-workers reviewed the water tapes and mentioned a T4(1)6(1) water tape in the CSD which was not characterized.23a To the best of our best knowledge, no T4(1)6(1) water tape has been structurally analysed up to now. The T4(1)6(1) water tape in 1 is also different from a simple T4(1)6(1) water tape because it contains two dangling water molecules which connect as a chair-shaped water hexamer.
The O(4)–H⋯O(3), O(7)–H⋯O(2) and O(8)–H⋯N(2) hydrogen bonds anchor the water tapes to the 2D coordination networks (Fig. 5 and Fig. S5†). The 3D hydrogen bond network is formed via these hydrogen bond interactions in 1. For [Cd2(btec)(btb)3](H2O)10,41a a discrete water decamer locates at the voids of the 3D network and joins the 3D coordination network through two O–H⋯O hydrogen bond interactions between its two outer water molecules and two carboxylate oxygen atoms of the 3D coordination network. For [Cd2(phth)2(btb)(H2O)3](H2O)4.5,41a the hydrogen bond interactions between the undulated water chains and the coordinated water molecules or carboxylate oxygen atoms or the triazole nitrogen atoms of 2D thick coordination networks stabilize the water chains.
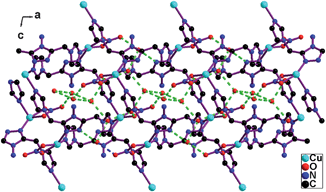 | ||
| Fig. 5 A water tape joins the adjacent 2D coordination networks via the hydrogen bond interactions. | ||
Thermogravimetric analysis showed 19.09% weight loss between room temperature and 116 °C, which corresponds to five molecules. Then the nitrate (13.13%) is lost between 150 and 222 °C (Fig. S6†).
In summary, we synthesized a 2D copper coordination polymer {[Cu(abit)(NO3)]·5H2O}n (1) containing a novel T4(1)6(1) water tape consisting of chair-shaped water hexamer with two dangling water molecules and uudd configuration of tetramer water. This work may be attributed to the prospect of improving understanding the both liquid water and ice.
Acknowledgements
This work is supported by the Natural Science Foundation of China (No.21171126, 20671066), the Priority Academic Program Development of Jiangsu Higher Education Institutions and Key laboratory of Organic Synthesis of Jiangsu Province.Notes and references
- (a) K. Liu, M. G. Brown, C. Carter, R. J. Saykally, J. K. Gregory and D. C. Clary, Nature, 1996, 381, 501 CrossRef CAS; (b) K. Nauta and R. E. Miller, Science, 2000, 287, 293 CrossRef CAS; (c) F. Weinhold, J. Chem. Phys., 1998, 109, 367 CrossRef CAS; (d) J. Sadlej, V. Buch, J. K. Kazimirski and U. Buck, J. Phys. Chem. A, 1999, 103, 4933 CrossRef CAS; (e) J. M. Ugalde, I. Alkorta and J. Elguero, Angew. Chem., Int. Ed., 2000, 39, 717 CrossRef CAS; (f) R. Ludwig, Angew. Chem., Int. Ed., 2001, 40, 1808 CrossRef CAS; (g) B. Sreenivasulu and J. J. Vittal, Angew. Chem., Int. Ed., 2004, 43, 5769 CrossRef CAS; (h) M. H. Mir and J. J. Vittal, Angew. Chem., Int. Ed., 2007, 46, 5925 CrossRef CAS.
- The News Staff, Science, 2004, 306, 2013 CrossRef.
- L. S. Long, Y. R. Wu, R. B. Huang and L. S. Zheng, Inorg. Chem., 2004, 43, 3798 CrossRef CAS.
- J. N. Moorthy, R. Natarajan and P. Venugopalan, Angew. Chem., Int. Ed., 2002, 41, 3417 CrossRef CAS.
- B. K. Saha and A. Nangia, Chem. Commun., 2006, 1825 RSC.
- R. J. Doedens, E. Yohannes and M. I. Khan, Chem. Commun., 2002, 62 RSC.
- J. L. Atwood, L. J. Barbour, T. J. Ness, C. L. Raston and P. L. Raston, J. Am. Chem. Soc., 2001, 123, 7192 CrossRef CAS.
- W. B. Blanton, S. W. Gordon-Wylie, G. R. Clark, K. D. Jordan, J. T. Wood, U. Geiser and T. J. Collins, J. Am. Chem. Soc., 1999, 121, 3551 CrossRef CAS.
- L. J. Barbour, G. W. Orr and J. L. Atwood, Nature, 1998, 393, 671 CrossRef.
- L. J. Barbour, G. W. Orr and J. L. Atwood, Chem. Commun., 2000, 859 RSC.
- M. Yoshizawa, T. kusukawa, M. Kawano, T. Ohhara, I. Tanaka, K. Kurihara, N. Niimura and M. Fujita, J. Am. Chem. Soc., 2005, 127, 2798 CrossRef CAS.
- A. Michaelides, S. Skoulika, E. G. Bakalbassis and J. Mrozinski, Cryst. Growth Des., 2003, 3, 487 CrossRef CAS.
- B. C. R. Sansam, K. M. Anderson and J. W. Steed, Cryst. Growth Des., 2007, 7, 2649 CrossRef CAS.
- Y. Jin, Y. X. Che and J. M. Zheng, Inorg. Chem. Commun., 2007, 10, 514 CrossRef CAS.
- Y. Y. Karabach, A. M. Kirillov, M. F. C. De Silva, M. N. Kopylovich and A. J. L. Pombeiro, Cryst. Growth Des., 2006, 6, 2200 CrossRef CAS.
- S. K. Ghosh and P. K. Bharadwaj, Eur. J. Inorg. Chem., 2006, 1341 CrossRef CAS.
- Y. Li, L. Jiang, X. L. Feng and T. B. Lu, Cryst. Growth Des., 2006, 6, 1074 CrossRef CAS.
- M. Estrader, J. Ribas, V. Tangoulis, X. Solans, M. Font-Bardia, M. Maestro and C. Diaz, Inorg. Chem., 2006, 45, 8239 CrossRef CAS.
- O. Ermer and J. Neudörfl, Chem.–Eur. J., 2001, 7, 4961 CrossRef CAS.
- G. A. Jeffrey, An introduction to hydrogen bonding, Oxford University Press, New York, 1997 Search PubMed.
- (a) K. M. Jude, S. K. Wright, C. Tu, D. N. Silverman, R. E. Viola and D. W. Christianson, Biochemistry, 2002, 41, 2485 CrossRef CAS; (b) K. Tajkhorshid, P. Nollert, M. Jensen, L. J. W. Miercke, J. O'Connell, R. M. Stroud and K. Schulten, Science, 2002, 296, 525 CrossRef CAS.
- (a) K. Raghuraman, K. K. Katti, L. J. Barbour, N. Pillarsetty, C. L. Barnes and K. V. Katti, J. Am. Chem. Soc., 2003, 125, 6955 CrossRef CAS; (b) Q. Y. Liu and L. Xu, CrystEngComm, 2005, 7, 87 RSC; (c) D. L. Reger, R. F. Semeniuc, C. Pettinari, F. Luna-Giles and M. D. Smith, Cryst. Growth Des., 2006, 6, 1068 CrossRef CAS; (d) B. Q. Ma, H. L. Sun and S. Gao, Chem. Commun., 2005, 2336 RSC.
- (a) L. Infantes and S. Motherwell, CrystEngComm, 2002, 4, 454 RSC; (b) L. Infantes, J. Chisholm and S. Motherwell, CrystEngComm, 2003, 5, 480 RSC.
- S. Pal, N. B. Sankaran and A. Samamta, Angew. Chem., Int. Ed., 2003, 42, 1741 CrossRef CAS.
- B. H. Ye, A. P. Sun, T. F. Wu, Y. Q. Weng and X. M. Chen, Eur. J. Inorg. Chem., 2005, 1230 CrossRef CAS.
- R. Carballo, B. Covelo, N. Fernández-Hermida, E. García-Martinez, A. B. Lago and E. M. Vázquez-López, Cryst. Growth Des., 2008, 8, 995 CrossRef CAS.
- B. Q. Ma, H. L. Sun and S. Gao, Chem. Commun., 2004, 2220 RSC.
- J. M. Zheng, S. R. Batten and M. Du, Inorg. Chem., 2005, 44, 3371 CrossRef CAS.
- J. Lu, J. H. Yu, X. Y. Chen, P. Cheng, X. Zhang and J. Q. Xu, Inorg. Chem., 2005, 44, 5978 CrossRef CAS.
- B. Q. Ma, H. L. Sun and S. Gao, Eur. J. Inorg. Chem., 2005, 3902 CrossRef CAS.
- S. K. Ghosh and P. K. Bharadwaj, Eur. J. Inorg. Chem., 2005, 4880 CrossRef CAS.
- X. M. Zhang, R. Q. Fang and H. S. Wu, Cryst. Growth Des., 2005, 5, 1335 CrossRef CAS.
- B. K. Sahn and A. Nangia, Chem. Commun., 2006, 1825 RSC.
- X. F. Shi and W. Q. Zhang, Cryst. Growth Des., 2007, 7, 595 CrossRef CAS.
- D. Sun, H. R. Xu, C. P. Yang, Z. H. Wei, N. Zhang and R. B. Huang, Cryst. Growth Des., 2010, 10, 4642 CrossRef CAS.
- S. P. Chen, G. X. Huang, M. Li, L. L. Pan, Y. X. Yuan and L. J. Yuan, Cryst. Growth Des., 2008, 8, 2824 CrossRef CAS.
- R. Custelcean, C. Afloroaei, M. Vlassa and M. Polverejan, Angew. Chem., Int. Ed., 2000, 39, 3094 CrossRef CAS.
- M. Li, S. Chen, J. Xiang, H. He, L. Yuan and J. Sun, Cryst. Growth Des., 2006, 6, 1250 CrossRef CAS.
- (a) X. Y. Wang, B. L. Li, X. Zhu and S. Gao, Eur. J. Inorg. Chem., 2005, 3277 CrossRef CAS; (b) B. L. Li, X. Y. Wang, X. Zhu, S. Gao and Y. Zhnag, Polyhedron, 2007, 26, 5219 CrossRef CAS.
- J. Wang, X. Zhu, Y. F. Cui, B. L. Li and H. Y. Li, CrystEngComm, 2011, 13, 3342 RSC.
- (a) L. Y. Wang, Y. Yang, K. Liu, B. L. Li and Y. Zhang, Cryst. Growth Des., 2008, 8, 3902 CrossRef CAS; (b) X. G. Liu, L. Y. Wang, X. Zhu, B. L. Li and Y. Zhang, Cryst. Growth Des., 2009, 9, 3997 CrossRef CAS; (c) X. Zhu, X. G. Liu, B. L. Li and Y. Zhang, CrystEngComm, 2009, 11, 997 RSC.
- (a) B. L. Li, Y. F. Peng, B. Z. Li and Y. Zhang, Chem. Commun., 2005, 2333 RSC; (b) Y. F. Peng, H. Y. Ge, B. Z. Li, B. L. Li and Y. Zhang, Cryst. Growth Des., 2006, 6, 994 CrossRef CAS.
- Synthesis of 1: A 8 mL aqueous solution of Cu(NO3)2·3H2O (0.048g, 0.2 mmol) was added to a MeOH solution (8 mL) of Habit (0.048 g, 0.2 mmol) with stirring. The light blue single crystals 1 were obtained after the mixture was allowed to stand at room temperature for two weeks in 43% yield (0.039 g). Anal. Calc. for C10H21CuN9O8: C, 26.17; H, 4.61; N, 27.48. Found: C, 26.08; H, 4.54; N, 27.37%.
- Y. F. Feng, D. Y. Yuan, J. Wang, B. L. Li and H. Y. Li, Chin. J. Chem., 2011, 29, 178 CrossRef CAS.
- D. Eisenberg and W. Kauzmann, The Structure and Properties of Water, Oxford University Press: Oxford, 1969 Search PubMed.
- A. H. Narten, W. E. Thiessen and L. Blum, Science, 1982, 217, 1033 CrossRef CAS.
- M. Mascal, L. Infantes and J. Chisholm, Angew. Chem., Int. Ed., 2006, 45, 32 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional plots of the structures. CCDC reference number 830986. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1ce05866e |
| ‡ Crystal data for C10H21CuN9O8 (1): Mr = 458.90, monoclinic, P21/n, a = 9.6073(11), b = 13.1029(15), c = 15.1440(19) Å, β = 100.668(4)°, V = 1873.4(4) Å3, Z = 4, Dc = 1.627 g cm−3, μ = 1.227 mm−1, F(000) = 948, S = 1.085, R = 0.0610, wR = 0.1462. X-ray single-crystal diffraction data collections for 1 were collected on a Rigaku Saturn CCD at 223(2)K. The structure was solved by direct methods and refined with the full-matrix least-squares technique using the SHELXS-97 and SHELXL-97 programs. CCDC 830986. |
| This journal is © The Royal Society of Chemistry 2012 |
