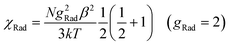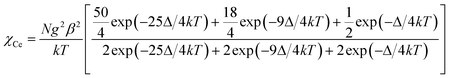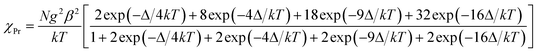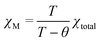DOI:
10.1039/C1CE06016C
(Paper)
CrystEngComm, 2012,
14, 235-239
Linear chain and mononuclear tri-spin compounds based on the lanthanide-nitronyl nitroxide radicals: structural design and magnetic properties†
Received
6th August 2011
, Accepted 1st September 2011
First published on 27th October 2011
Abstract
Four Ln(III) complexes based on two nitronyl nitroxide radicals with different steric hindrance have been synthesized, characterized structurally and magnetically: [Ln(hfac)3(NITPhOEt)]n (Ce (1), Pr (2); hfac = hexafluoroacetylacetonate; and NITPhOEt = 4′-ethoxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide); [Ln(hfac)3(NITPhOCH2Ph)2] (Ce (3), Pr (4); NITPhOCH2Ph = 4′-benzyloxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide). The X-ray crystal structure analyses show that small steric hindrance of the radical makes complexes 1 and 2 show similar linear chain structures composed of Ln(hfac)3 units bridged by NITPhOEt radicals through their N–O groups. While the larger steric hindrance of the radical induces complexes 3 and 4 to be mononuclear tri-spin compounds, in which central Ln(III) ions are coordinated by three hfac and two NITPhOCH2Ph radicals. The variable-temperature magnetic susceptibility studies reveal that there are antiferromagnetic interactions between the paramagenetic ions (Ln(III) and radical) in all four complexes.
Introduction
Nitronyl nitroxides, stable organic radicals, have played a prominent role in the design and construction of molecular magnetic materials.1,2 As they can act as not only spin carriers but also as building blocks, so far they are widely used.3–9 Among the researches, transition metal-radical complexes have been studied greatly, while much less is known for lanthanide-radical complexes.10 This may be attributed to the effective shielding by the outer-shell electrons and the rather large and anisotropic magnetic moments of the rare-earth metal ions, which make their magnetic properties difficult to be treated. However, in recent years, lanthanide compounds involving organic radicals (2p-4f) have attracted much attention,11–14 because several lanthanide compounds involving organic radicals have been reported to exhibit single-molecule and single-chain magnets behavior14a,15–19 owing to the Ising anisotropy of their 4f centers, and the strength of magnetic interaction promoted by organic radicals.2c,3,20 Furthermore, the researches show that the properties and structures of the lanthanide-radical complexes are affected greatly by modifying the radical ligand.14c,d,30
In order to explore how modifying the nitronyl nitroxide radical ligand affects the structure of lanthanide-radical complexes, in this paper, we decided to use two radicals: NITPhOEt and NITPhOCH2Ph (Scheme 1). In NITPhOCH2Ph, the larger aromatic ring has a larger steric hindrance to coordinate with the lanthanide ions than ethyl in NITPhOEt.4,21,22 By using these two different nitronyl nitroxide radical ligands, four new lanthanide-radical complexes were obtained. Among them, two are one-dimensional lanthanide-radical chain complexes bridged by the NITPhOEt radical, and the other two are mononuclear tri-spin compounds with the NITPhOCH2Ph radical, due to the steric hindrance effect. In addition, the temperature dependence of the magnetic susceptibilities for the four complexes are also studied, and the results suggested that there are antiferromagnetic interactions between Ln(III) (Ce(III) or Pr(III)) ions and the radicals in all four Ln(III)-radical complexes.
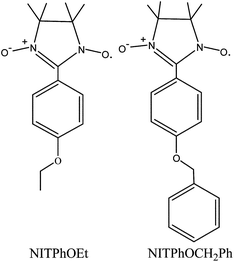 |
| | Scheme 1 Structures of the two nitronyl nitroxide radicals with different steric hindrance. | |
Experimental
Materials
All of the reagents used in the syntheses were of analytical grade, the hexafluoroacetylacetone, 4-(ethoxy)benzaldehyde and 4-(benzyloxy)benzaldehyde were purchased from Alfa Chemical Company. Syntheses of the starting radicals23 and Ce(hfac)3·2H2O and Pr(hfac)3·2H2O,24 have been performed according to the literature methods.
Syntheses of [Ce(hfac)3(NITPhOEt)]n (1), [Pr(hfac)3(NITPhOEt)]n (2), [Ce(hfac)3(NITPhOCH2Ph)2] (3) and [Pr(hfac)3(NITPhOCH2Ph)2] (4)
All of the four complexes were synthesized by the same method. Therefore, the synthesis of compound 1 is detailed herein. Ce(hfac)3·2H2O (0.1 mmol) was dissolved in boiling n-heptane (20 mL). After stirring for 1 h, the solution was cooled to 65 °C, to which NITPhOEt (0.1 mmol) in CH2Cl2 (5 mL) was added with refluxing for 30 min. Then the solution was cooled to room temperature, filtrated and the filtrate was stored in a refrigerator at 4 °C for several days to give blue-violet crystals, which were suitable for X-ray analysis.25 The compound of 2 was obtained in a similar manner using Pr(hfac)3·2H2O instead of Ce(hfac)3·2H2O; compound 3 was obtained using the NITPhOCH2Ph radical instead of the NITPhOEt radical; and compound of 4 was obtained in a similar manner using NITPhOCH2Ph and Pr(hfac)3·2H2O.
Anal. Calcd (1) for C30H24F18CeN2O9 (Yield: 0.0099 g, 9.5%): C, 34.69; H, 2.33; N, 2.70%. Found: C, 34.81; H, 2.38; N, 2.81%. IR (KBr cm−1): 1651(vs), 1608(w), 1558(w), 1394(w), 1379(w), 1255(vs), 1198(vs), 1093(w); Anal. Calcd (2) for C30H24F18PrN2O9 (Yield: 0.0284 g, 27.3%): C, 34.67; H, 2.33; N, 2.70%. Found: C, 35.35; H, 2.42; N, 2.89%. IR (KBr cm−1): 1651(vs), 1607(w), 1554(w), 1395(w), 1375(w), 1254(vs), 1202(vs), 1099(w); Anal. Calcd (3) for C55H49F18CeN4O12 (Yield: 0.0171 g, 11.9%): C, 45.87; H, 3.43; N, 3.89%. Found: C, 44.76; H, 3.34; N, 3.79%. IR (KBr cm−1): 1655(vs), 1605(w), 1556(w), 1397(w), 1376(w), 1256(vs), 1208(vs), 1093(w); Anal. Calcd (4) for C55H49F18PrN4O12 (Yield: 0.0289 g, 20.1%): C, 45.85; H, 3.43; N, 3.89%. Found: C, 44.67; H, 3.32; N, 3.72%. IR (KBr cm−1): 1652(vs), 1607(w), 1550(w), 1398(w), 1373(w), 1255(vs), 1205(vs), 1096(w).
Crystal structure determination
Determination of the unit cell and data collection for the four complexes were performed with Mo-Kα radiation (λ = 0.71073 Å) on a Bruker Smart 1000 diffractometer and equipped with a CCD camera. Both of the structures were solved primarily by direct methods and refined by the full-matrix least squares method. The computations were performed with the SHELXL-97 program. All non-hydrogen atoms were refined with anisotropic thermal parameters. The hydrogen atoms were set in calculated positions and refined as riding atoms with a common fixed isotropic thermal parameter.26,27Crystal data and details of structural determination refinement are summarized in Table 1 (the selected bond lengths and angles have been placed in the Electronic Supplementary Information†).
Table 1
Crystal data and structure refinements for complexes 1–4
| |
1
|
2
|
3
|
4
|
| Empirical formula |
C30H24F18CeN2O9 |
C30H24F18PrN2O9 |
C55H49F18CeN4O12 |
C55H49F18PrN4O12 |
| Formula weight |
1038.63 |
1039.42 |
1440.10 |
1440.89 |
|
T/K |
113(2) |
293(2) |
113(2) |
293(2) |
| Crystal system |
Monoclinic |
Monoclinic |
Triclinic |
Triclinic |
| Space group |
P21/c |
P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
|
|
a/Å |
10.953(5) |
11.259(2) |
12.056(2) |
12.421(3) |
|
b/Å |
17.082(8) |
17.207(3) |
15.763(3) |
15.856(3) |
|
c/Å |
23.293(9) |
23.884(6) |
17.490(4) |
18.210(4) |
|
α (°) |
90 |
90 |
73.04(3) |
69.13(3) |
|
β (°) |
116.58(17) |
117.99(2) |
74.49(3) |
73.10(3) |
|
γ (°) |
90 |
90 |
78.36(3) |
89.59(3) |
| Volume/Å3 |
3897(3) |
4085.9(14) |
3030.5(10) |
3187.2(12) |
|
Z
|
4 |
4 |
2 |
2 |
|
ρ
c/g cm−3 |
1.770 |
1.690 |
1.578 |
1.501 |
|
μ/mm−1 |
1.305 |
1.323 |
0.868 |
0.876 |
|
F(000) |
2040 |
2044 |
1446 |
1448 |
| Reflections collected |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 785 785 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 606 606 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 709 709 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 632 632 |
| Unique/parameters |
6852/546 |
7291/658 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 626/819 626/819 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 197/879 197/879 |
|
R(int) |
0.0481 |
0.0796 |
0.0312 |
0.0549 |
| Completeness to θ = 27.48 |
100.0% |
99.8% |
99.2% |
99.7% |
| Max./min. transmission |
1.000/0.745 |
0.899/0.852 |
0.8881/0.8319 |
0.858/0.804 |
| Goodness-of-fit on F2 |
1.045 |
1.128 |
1.060 |
1.102 |
| Final R indices [I > 2σ(I)] |
R
1 = 0.0344, wR2 = 0.0815 |
R
1 = 0.0666, wR2 = 0.1244 |
R
1 = 0.0325, wR2 = 0.0702 |
R
1 = 0.0796, wR2 = 0.2258 |
|
R indices (all data) |
R
1 = 0.0366, wR2 = 0.0830 |
R
1 = 0.1000, wR2 = 0.1369 |
R
1 = 0.0381, wR2 = 0.0726 |
R
1 = 0.1007, wR2 = 0.2400 |
Materials and physical techniques
Elemental analysis for C, H, and N were obtained on a Perkin-Elmer elemental analyzer model 240. Variable-temperature magnetic susceptibilities were measured on a SQUID MPMS XL-7 magnetometer in the range of 2–300 K. Diamagnetic corrections were made with Pascal's constants for all of the constituent atoms.
Results and discussion
Description of the crystal structure
The molecular structures of 1 and 2 are similar, which crystallize in the monoclinic P21/c space group, and both consist of linear chains built by Ce(hfac)3 or Pr(hfac)3 units bridged by NITPhOEt radicals through their N–O groups. The structure of complex 1 is shown in Fig. 1. Each Ce(III) atom is eight-coordinated by three bidentate hfac ligands and two nitroxide groups from the radicals. The Ce–O bond lengths are in the range of 2.417(10)–2.472(8) Å. The intrachain Ce⋯Ce distance is 8.607 Å, and the nearest interchain Ce⋯Ce contact is 12.493 Å. For complex 2, [Pr(hfac)3(NITPhOEt)]n is isomorphic to complex 1 except for the substitution of Ce(III) with the Pr(III) ion, which makes the bond distances and angles vary a little.
![Crystal structure of [Ce(hfac)3(NITPhOEt)]n (1). All hydrogen and fluorine atoms are omitted for clarity.](/image/article/2012/CE/c1ce06016c/c1ce06016c-f1.gif) |
| | Fig. 1 Crystal structure of [Ce(hfac)3(NITPhOEt)]n (1). All hydrogen and fluorine atoms are omitted for clarity. | |
Both complexes 3 and 4 crystallize in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, and consist of isolated molecules where the nitronyl nitroxide radical acts as a monodentate ligand towards Ce(III) or Pr(III) through the oxygen atom of the N–O group to form the monometallic Radical–Ln(III)–Radical complexes. The structure of complex 3 is shown in Fig. 2, where the Ce(III) ion is in the center of a distorted dodecahedron with triangular faces28,29 and coordinated by six oxygen atoms of three hfac molecules with the Ce–O bond lengths in the range of 2.4421(18)–2.4875(19) Å, and two oxygen atoms from two NITPhOCH2Ph radicals with the Ce–O bond lengths of 2.4060(19) and 2.4504(19) Å, respectively.
space group, and consist of isolated molecules where the nitronyl nitroxide radical acts as a monodentate ligand towards Ce(III) or Pr(III) through the oxygen atom of the N–O group to form the monometallic Radical–Ln(III)–Radical complexes. The structure of complex 3 is shown in Fig. 2, where the Ce(III) ion is in the center of a distorted dodecahedron with triangular faces28,29 and coordinated by six oxygen atoms of three hfac molecules with the Ce–O bond lengths in the range of 2.4421(18)–2.4875(19) Å, and two oxygen atoms from two NITPhOCH2Ph radicals with the Ce–O bond lengths of 2.4060(19) and 2.4504(19) Å, respectively.
Comparing the structure of the two systems of complexes, here 1 and 3 for example, they are both cerium(III) complexes consisting of nitronyl nitroxide radicals and hfac ligands. The only difference between them is the substituent group of the nitronyl nitroxide radical ligand, which makes their structures significantly different. In complex 1, the radical of NITPhOEt with the small steric hindrance makes a chain structure with Ce(hfac)3, whereas when the steric hindrance is enlarged as big as NITPhOCH2Ph, the mononuclear tri-spin structure was obtained for complexe 3. The larger steric hindrance effect of NITPhOCH2Ph in the mononuclear tri-spin complex 3 induces the nearest Ce⋯Ce distance to be as large as 14.341 Å, which is much larger than the nearest intrachain Ce⋯Ce distance (8.607 Å) and the interchain Ce⋯Ce contact (12.493 Å) in chain complex 1 (with the smaller steric hindrance effect of NITPhOEt). It can be concluded that the chains are to be assigned to a dynamical regime dominated by steric hindrance effects.3,31 The smaller steric hindrance benefits forming the chain complex, while the larger steric hindrance enlarges the Ce⋯Ce distance and destroys the stability of the possible formation of the chain complex, and eventually forms the mononuclear complex.
![Perspective view of complex [Ce(hfac)3(NITPhOCH2Ph)2] (3). All hydrogen and fluorine atoms are omitted for clarity.](/image/article/2012/CE/c1ce06016c/c1ce06016c-f2.gif) |
| | Fig. 2 Perspective view of complex [Ce(hfac)3(NITPhOCH2Ph)2] (3). All hydrogen and fluorine atoms are omitted for clarity. | |
Magnetic properties
The temperature dependence of magnetic susceptibilities for complexes 1 and 2 with chain structure are studied and shown in Fig. 3 and 4, and the χMT values at room temperature are 1.34 and 1.98 cm3 K mol−1 for Ce(III) and Pr(III) complexes, respectively. Both the values are close to the expected values of 1.18 and 1.97 cm3 K mol−1 for an uncoupled system of one Ln(III) ion (2F5/2, g = 6/7, for Ce(III) and 3H4, g = 4/5 for Pr(III)) plus one radical (S = 1/2, 0.375 cm3 K mol−1). Upon cooling, the χMT values of both 1 and 2 decrease gradually and reach a minimum of 0.2 and 0.1 cm3 K mol−1 at 2.0 K, respectively, which could arise from a selective depopulation of the excited crystal field state and antiferromagnetic interaction between Ln(III) ion and radical. There is no available expression to determine the magnetic susceptibility of such a 1D system with large anisotropy. However, to obtain a rough quantitative estimation of the magnetic interactions between paramagnetic species (Ce(III) [or Pr(III)] ion and radical), the magnetic susceptibility χtotal of the complex can be assumed as the sum of χLn of the isolated Ce(III) or Pr(III) ion and χRad of one radical (eqn (1)). The Ce(III) [or Pr(III)] ion may be assumed to exhibit a splitting of the mj energy levels (Ĥ = ΔĴz2) in an axial crystal field.14e,32 Thus χCe and χPr can be described as eqn (3) and (4), respectively. In the expression, Δ is the zero-field-splitting parameter, g is the Lande factor, k is the Boltzmann constant, β is the Bohr magneton constant, and N is Avogadro's number. The Weiss constant, θ, is introduced (eqn (5)) to roughly simulate the magnetic interactions between all the paramagnetic species in the system.32a,33 Thus the magnetic data can be analyzed by the following approximate treatment of eqn (1)–(5).| | 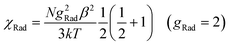 | (2) |
| | 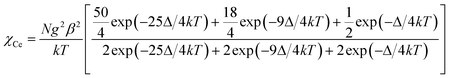 | (3) |
| | 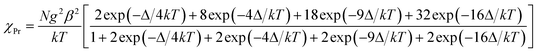 | (4) |
| | 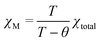 | (5) |
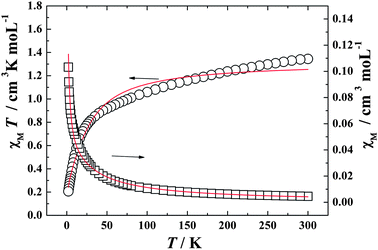 |
| | Fig. 3
χ
M
T (○) versus T and χM (□) versus T plots for complex 1 in the range 2–300 K in the field of 2000 Oe. The solid lines represent the theoretical. | |
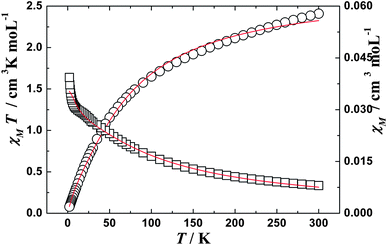 |
| | Fig. 4
χ
M
T (○) versus T and χM (□) versus T plots for complex 2 in the range 2–300 K in the field of 1000 Oe. The solid lines represent the theoretical. | |
The observed χMT data were well reproduced (Fig. 3 and 4) by using the above approximate eqn (1)–(5), giving the best fitting parameters of g = 0.93, Δ = 6.99, θ = −0.79 K for Ce(III) complex 1 and g = 0.84, Δ = 20.22, θ = −11.72 K for Pr(III) complex 2, which indicates that there are antiferromagnetic interactions between the paramagenetic ions (Ln(III) [Ce(III) or Pr(III)] and radical) in the 1D chain system.
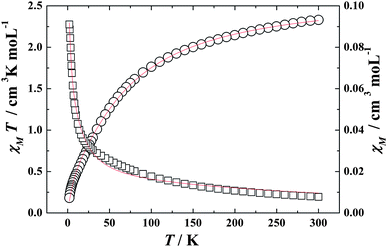
Variable-temperature magnetic susceptibilities for mononuclear complexes of 3 and 4 were also studied and shown in Fig. 5 and 6. The χMT values at 300 K are 1.54 and 2.18 cm3 K mol−1 for Ce(III) and Pr(III) mononuclear complexes, respectively. Both the values are very close to the expected values of 1.55 and 2.35 cm3 K mol−1 for an uncoupled system of one Ln(III) ion (Ce(III) or Pr(III)) plus two radicals. On decreasing the temperature, the χMT values of both 3 and 4 decrease gradually and reach a minimum of 0.18 cm3 K mol−1 at 2.0 K for both the compounds. To obtain a rough quantitative estimate of the magnetic interaction parameters between paramagnetic species (Ln(III) and radical) in the mononuclear tri-spin system, we assumed that the total magnetic susceptibility χtotal is given by the sum of the isolated Ln(III) ion and two radicals (eqn (6)), and the Weiss constant, θ, is also introduced to roughly simulate the magnetic interactions between all the paramagnetic species in the system (eqn (5)).32a,33
| | | χ(total) = χLn + 2χRad | (6) |
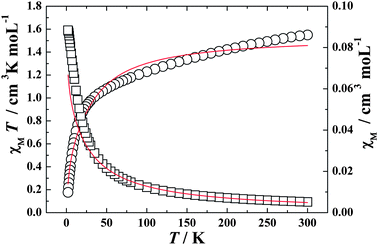 |
| | Fig. 5
χ
M
T (○) versus T and χM (□) versus T plots for complex 3 in the range 2–300 K in the field of 1000 Oe. The solid lines represent the theoretical. | |
 |
| | Fig. 6
χ
M
T (○) versus T and χM (□) versus T plots for complex 4 in the range 2–300 K in the field of 1000 Oe. The solid lines represent the theoretical. | |
Giving the best fitting parameters of g = 0.87, Δ = 13.82, θ = −0.89 K for Ce(III) complex 3 and g = 0.83, Δ = 22.96, θ = −9.13 K for Pr(III) complex 4, which indicates the antiferromagnetic interaction between the paramagenetic ions (Ln(III) and radicals) in both of the two mononuclear tri-spin systems.
Conclusions
In conclusion, by modifying the nitroxide radicals with different steric hindrance effects, we have successfully prepared two novel one-dimensional lanthanide-radical chain complexes bridged by NITPhOEt radicals and two mononuclear tri-spin compounds with NITPhOCH2Ph, which indicate that the small steric hindrance of the radical will benefit the formation of the chain structure of lanthanide-radical complexes. The rough magnetic studies show that there are antiferromagnetic interactions between the paramagenetic ions (Ln(III) and radical) in all the four Ln(III)-radical complexes.
Acknowledgements
This work was supported by the National Natural Science Foundation of China 21101096, 90922032, 21071085, 20971072, National Basic Research Program of China (973 Program, 2007CB815305), NSF of Tianjin 09JCYBJC05500, Research Fund for the Doctoral Program of Higher Education 20100031120013, and Fundamental Research Funds for the Central Universities, National Undergraduates Innovating Experimentation Project 101005533.
Notes and references
-
(a) K. E. Vostrikova, Coord. Chem. Rev., 2008, 252, 1409 CrossRef CAS;
(b) A. Caneschi, D. Gatteschi and R. Sessoli, Acc. Chem. Res., 1989, 22, 392 CrossRef CAS;
(c) A. Caneschi and D. Gatteschi, Prog. Inorg. Chem., 1991, 39, 331 CrossRef.
-
(a)
O. Kahn, Molecular Magnetism, VCH Publishers, Inc., New York, 1993 Search PubMed;
(b) A. Cogne, J. Laugier, D. Luneau and P. Rey, Inorg. Chem., 2000, 39, 5510 CrossRef CAS;
(c) R. Sessoli and A. K. Powell, Coord. Chem. Rev., 2009, 253, 2328 CrossRef CAS.
- K. Bernot, L. Bogani, A. Caneschi, D. Gatteschi and R. Sessoli, J. Am. Chem. Soc., 2006, 128, 7947 CrossRef CAS.
- L. Bogani, C. Sangregorio, R. Sessoli and D. Gatteschi, Angew. Chem., Int. Ed., 2005, 44, 5817 CrossRef CAS.
- G. Poneti, K. Bernot, L. Bogani, A. Caneschi, R. Sessoli, W. Wernsdorferc and D. Gatteschia, Chem. Commun., 2007, 1807 RSC.
- D. Luneau and P. Rey, Coord. Chem. Rev., 2005, 249, 2591 CrossRef CAS.
- T. Tsukuda, T. Suzuki and S. Kaizaki, J. Chem. Soc., Dalton Trans., 2002, 1721 RSC.
- C. Train, L. Norel and M. Baumgarten, Coord. Chem. Rev., 2009, 253, 2342 CrossRef CAS.
-
(a) L. Y. Wang, L. F. Ma, Z. H. Jiang, D. Z. Liao and S. P. Yan, Inorg. Chim. Acta, 2005, 358, 820 CrossRef CAS;
(b) K. Hayakawa, D. Shiomi, T. Ise, K. Sato and T. Takui, J. Mater. Chem., 2006, 16, 4146 RSC;
(c) J. Y. Zhang, C. M. Liu, D. Q. Zhang, S. Gao and D. B. Zhu, Inorg. Chim. Acta, 2007, 360, 3553 CrossRef CAS;
(d) R. N. Liu, L. C. Li, X. Y. Xing and D. Z. Liao, Inorg. Chim. Acta, 2009, 362, 2253 CrossRef CAS.
-
(a) C. Lescopa, G. Bussiereb, R. Beaulacb, H. Belisleb, E. Belorizkyc, P. Rey, C. Reberb and D. Luneau, J. Phys. Chem. Solids, 2004, 65, 773 CrossRef;
(b) T. Tsukuda, T. Suzuki and S. Kaizaki, Polyhedron, 2007, 26, 3175 CrossRef CAS;
(c) C. Benelli, Inorg. Chim. Acta, 2008, 361, 4157 CrossRef CAS;
(d) K. Bernot, L. Bogani, R. Sessoli and D. Gatteschi, Inorg. Chim. Acta, 2007, 360, 3807 CrossRef CAS.
- C. Benelli, A. Caneschi, D. Gatteschi and R. Sessoli, Inorg. Chem., 1993, 32, 4797 CrossRef CAS.
- M. L. Kahn, J. P. Sutter, S. Golhen, P. Guionneau, L. Ouahab, O. Kahn and D. Chasseau, J. Am. Chem. Soc., 2000, 122, 3413 CrossRef CAS.
- C. Benelli, A. Caneschi, D. Gatteschi, L. Pardi and P. Rey, Inorg. Chem., 1990, 29, 4223 CrossRef CAS.
-
(a) R. N. Liu, L. C. Li, X. L. Wang, P. P. Yang, C. Wang, D. Z. Liao and J. P. Sutter, Chem. Commun., 2010, 46, 2566 RSC;
(b) C. Lescop, E. Belorizky, D. Luneau and P. Rey, Inorg. Chem., 2002, 41, 3375 CrossRef CAS;
(c) N. Zhou, Y. Ma, C. Wang, G. F. Xu, J. K. Tang, J. X. Xu, S. P. Yan, P. Cheng, L. C. Li and D. Z. Liao, Dalton Trans., 2009, 8489 RSC;
(d) R. N. Liu, Y. Ma, P. P. Yang, X. Y. Song, G. F. Xu, J. K. Tang, L. C. Li, D. Z. Liao and S. P. Yan, Dalton Trans., 2010, 39, 3321 RSC;
(e) J. X. Xu, Y. Ma, D. Z. Liao, G. F. Xu, J. K. Tang, C. Wang, N. Zhou, S. P. Yan, P. Cheng and L. C. Li, Inorg. Chem., 2009, 48, 8890 CrossRef CAS.
-
(a) L. Bogani, C. Sangregorio, R. Sessoli and D. Gatteschi, Angew. Chem., Int. Ed., 2005, 44, 5817 CrossRef CAS;
(b) G. Poneti, K. Bernot, L. Bogani, A. Caneschi, R. Sessoli, W. Wernsdorferc and D. Gatteschi, Chem. Commun., 2007, 43, 1807 RSC.
- C. Benelli, A. Caneschi, D. Gatteschi, L. Pardi, P. Rey, D. P. Shum and R. L. Carlin, Inorg. Chem., 1989, 28, 272 CrossRef.
- C. Lescop, E. Belorizky, D. Luneau and P. Rey, Inorg. Chem., 2002, 41, 3375 CrossRef CAS.
-
(a) C. Benelli, A. Caneschi, D. Gatteschi and L. Pardi, Inorg. Chem., 1992, 31, 741 CrossRef CAS;
(b) G. Poneti, K. Bernot, L. Bogani, A. Caneschi, R. Sessoli, W. Wernsdorfer and D. Gatteschi, Chem. Commun., 2007, 1807 RSC.
-
(a) J. P. Sutter, M. L. Kahn, S. Golhen, L. Ouahab and O. Kahn, Chem.–Eur. J., 1998, 4, 571 CrossRef CAS;
(b) M. L. Kahn, J. P. Sutter, S. Golhen, P. Guionneau, L. Ouahab, O. Kahn and D. Chasseau, J. Am. Chem. Soc., 2000, 122, 3413 CrossRef CAS;
(c) T. Tsukuda, T. Suzuki and S. Kaizaki, Polyhedron, 2007, 26, 3175 CrossRef CAS.
- K. Bernot, L. Bogani, R. Sessoli and D. Gatteschi, Inorg. Chim. Acta, 2007, 360, 3807 CrossRef CAS.
- C. Benelli, A. Caneschi, D. Gatteschi and R. Sessoli, J. Appl. Phys., 1993, 73, 5333 CrossRef CAS.
- A. Caneschi, D. Gatteschi, N. Lalioti, C. Sangregorio and R. Sessoli, J. Chem. Soc., Dalton Trans., 2000, 3907 RSC.
- E. F. Ullman, J. H. Osiecki, D. G. B. Boocock and R. Darcy, J. Am. Chem. Soc., 1972, 94, 7049 CrossRef CAS.
- M. F. Richardson, W. F. Wagner and D. E. Sands, J. Inorg. Nucl. Chem., 1968, 30, 1275 CrossRef CAS.
- Q. H. Zhao, Y. P. Ma, L. Du and R. B. Fang, Transition Met. Chem., 2006, 31, 593 CrossRef CAS.
- T. Shiga, M. Ohba and H. Okawa, Inorg. Chem. Commun., 2003, 6, 15 CrossRef CAS.
- S. Akine, T. Matsumoto, T. Taniguchi and T. Nabeshima, Inorg. Chem., 2005, 44, 3270 CrossRef CAS.
- C. Benelli, A. Caneschi, D. Gatteschi, J. Laugier and P. Rey, Angew. Chem., Int. Ed. Engl., 1987, 26, 913 CrossRef.
- K. Bernot, J. Luzon, L. Bogani, M. Etienne, C. Sangregorio, M. Shanmugam, A. Caneschi, R. Sessoli and D. Gatteschi, J. Am. Chem. Soc., 2009, 131, 5573 CrossRef CAS.
- N. Zhou, Y. Ma, C. Wang, G. F. Xu, J. K. Tang, S. P. Yan and D. Z. Liao, J. Solid State Chem., 2010, 183, 927 CrossRef CAS.
- H. Miyasaka, R. Clerac, K. Mizushima, K. Sugiura, M. Yamashita, W. Wernsdorfer and C. Coulon, Inorg. Chem., 2003, 42, 8203 CrossRef CAS.
-
(a) I. A. Kahwa, J. Selbin, C. J. O'Connor, J. W. Foise and G. L. McPherson, Inorg. Chim. Acta, 1988, 148, 265 CrossRef CAS;
(b) J. K. Tang, Q. L. Wang, S. F. Si, D. Z. Liao, Z. H. Jiang, S. P. Yan and P. Cheng, Inorg. Chim. Acta, 2005, 358, 325 CrossRef CAS;
(c) B. Li, W. Gu, L. Z. Zhang, J. Qu, Z. P. Ma, X. Liu and D. Z. Liao, Inorg. Chem., 2006, 45, 10425 CrossRef CAS;
(d) Y. Ouyang, W. Zhang, N. Xu, G. F. Xu, D. Z. Liao, K. Yoshimura, S. P. Yan and P. Cheng, Inorg. Chem., 2007, 46, 8454 CrossRef CAS;
(e) N. Xu, W. Shi, D. Z. Liao, S. P. Yan and P. Cheng, Inorg. Chem., 2008, 47, 8748 CrossRef CAS;
(f) N. Xu, C. Wang, W. Shi, S. P. Yan, P. Cheng and D. Z. Liao, Eur. J. Inorg. Chem., 2011, 15, 2387 CrossRef.
- Y. Liao, W. W. Shum and J. S. Miller, J. Am. Chem. Soc., 2002, 124, 9336 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 785
785![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 606
606![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 709
709![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 632
632![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 626/819
626/819![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 197/879
197/879![Crystal structure of [Ce(hfac)3(NITPhOEt)]n (1). All hydrogen and fluorine atoms are omitted for clarity.](/image/article/2012/CE/c1ce06016c/c1ce06016c-f1.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, and consist of isolated molecules where the nitronyl nitroxide radical acts as a monodentate ligand towards Ce(III) or Pr(III) through the oxygen atom of the N–O group to form the monometallic Radical–Ln(III)–Radical complexes. The structure of complex 3 is shown in Fig. 2, where the Ce(III) ion is in the center of a distorted dodecahedron with triangular faces28,29 and coordinated by six oxygen atoms of three hfac molecules with the Ce–O bond lengths in the range of 2.4421(18)–2.4875(19) Å, and two oxygen atoms from two NITPhOCH2Ph radicals with the Ce–O bond lengths of 2.4060(19) and 2.4504(19) Å, respectively.
space group, and consist of isolated molecules where the nitronyl nitroxide radical acts as a monodentate ligand towards Ce(III) or Pr(III) through the oxygen atom of the N–O group to form the monometallic Radical–Ln(III)–Radical complexes. The structure of complex 3 is shown in Fig. 2, where the Ce(III) ion is in the center of a distorted dodecahedron with triangular faces28,29 and coordinated by six oxygen atoms of three hfac molecules with the Ce–O bond lengths in the range of 2.4421(18)–2.4875(19) Å, and two oxygen atoms from two NITPhOCH2Ph radicals with the Ce–O bond lengths of 2.4060(19) and 2.4504(19) Å, respectively.![Perspective view of complex [Ce(hfac)3(NITPhOCH2Ph)2] (3). All hydrogen and fluorine atoms are omitted for clarity.](/image/article/2012/CE/c1ce06016c/c1ce06016c-f2.gif)