Controlled hydrothermal synthesis of tri-wing tellurium nanoribbons and their template reaction†
Hangtian
Zhu
ab,
Jun
Luo
*b,
He
Zhang
ab,
Jingkui
Liang
bc,
Guanghui
Rao
b,
Jingbo
Li
b,
Guangyao
Liu
b and
Zhenmin
Du
a
aDepartment of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, P. R. China
bBeijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: jluo@aphy.iphy.ac.cn
cInternational Center for Materials Physics, Chinese Academy of Sciences, Shenyang 110016, P. R. China
First published on 18th October 2011
Abstract
Single crystalline tri-wing tellurium nanoribbons with well-defined outer surfaces and geometrical shapes are synthesized by a facile hydrothermal method in the presence of poly(vinyl alcohol) as the surfactant. The tri-wing Te nanoribbons with a length of tens of micrometres have a very thin wing structure with an average thickness about 20 nm. A two-step growth mechanism is proposed for the formation of tri-wing Te nanoribbons based on time dependent morphology evolution. By adjusting the pH value of the reaction solution, varied Te nanostructures including nanofeathers, nanobelts, tri-wing nanoribbons and nanorods are obtained, indicating that the reduction rate plays a key role in fabricating tri-wing Te nanoribbons since the pH value has a dramatic influence on the reduction reaction rate of TeO32−. With tri-wing Te nanoribbons as the precursor and template, Ag2Te nanoribbons in the same shape and similar size are synthesized through the direct reaction of Te with AgNO3 at room temperature, demonstrating that the Te nanoribbons with a very thin tri-wing structure are ideal templates to synthesize other Te related nanocompounds.
Introduction
As a valuable p-type narrow-bandgap semiconducting material and a promising template to fabricate various novel nanostructures of tellurides, Te nanostructures have been a subject of intensive research.1–12 Trigonal tellurium (t-Te) has an anisotropic crystal structure which is composed of insulating parallel helical chains held by van der Waals cohesion.13,14 As a result, the growth rate along the c axis is relatively high and a one-dimensional (1D) Te nanostructure is facilely obtained, which makes the 1D Te nanostructure an important and ideal candidate to investigate the morphology and shape control of 1D nanostructures.15–25In solution synthesis, the morphology and shape of nanostructures mainly depend on the control of nucleation and growth process. The growth process is closely related to the inherent anisotropy of the crystal structure and the reactivity of the crystal surface, leading to the anisotropic growth of nanostructures. Generally, a crystal face with a significantly different growth rate results in 1D or 2D nanostructures, depending on the growth rate of the crystal face being higher or lower. However, nanobelts or nanoribbons are formed if different crystal faces have three distinguished growth rates. Moreover, the morphology and shape of nanostructures could be highly modulated by separating the nucleation and growth process and/or by the elaborated growth control of different crystal planes.
Several simple 1D Te nanostructures such as rods24–26 and tubes17–19 were prepared by a simple reduction reaction. With the presence of surfactant, Te nanowires with high aspect ratio were synthesized in the solution method, where the surfactant played an important role in tailoring the morphology and shape of the Te nanostructures.12,22–24,27 Furthermore, Te nanobelts or nanoribbons were also obtained by adding appropriate surfactant,22–24 indicating that the growth rate of crystal faces could be adjusted by selective absorption of the surfactant on different crystal planes. Moreover, the supersaturation of Te in the reaction media is tunable due to the strong pH-dependence of reduction reaction rate.23,26,28 As a consequence, we believe that more complex quasi-1D Te nanostructures could be fabricated by carefully manipulating the nucleation and growth rate with suitable surfactant.
In this contribution, we present a controlled hydrothermal route to synthesize unique 1D Te nanostructures. By the reduction of Na2TeO3 in NaH2PO2·H2O solution with poly(vinyl alcohol) (PVA) as the surfactant, tri-wing Te nanoribbons are obtained, which manifest clearly the three-fold symmetry of their crystal structures. This quasi-1D nanostructure with a tri-wing shape grows along the c axis and the surfaces of three wings are mainly enclosed by the {100} planes with a three-fold rotational symmetry relationship. A two-step formation mechanism of the tri-wing nanostructure is proposed based on time dependent morphology evolution. The influence of pH value on the morphology and shape of Te nanostructures is investigated, and the synthesis of varied Te nanostructures is attributed to the different reduction reaction rates under different pH conditions. The tri-wing Te nanostructure is demonstrated to be an ideal template to prepare other telluride nanocompounds through the successful synthesis of tri-wing Ag2Te nanoribbons.
Experimental
Synthesis of Te tri-wing nanoribbons
All the reagents used in the experiment were of analytical purity purchased and used without further purification. In a typical experiment, 102 mg (0.4 mmol) of K2TeO3 was dissolved in 40 mL of deionized water with 5.3 g (50 mmol) of NaH2PO2·H2O as the reducing agent and 123 mg of poly(vinyl alcohol) (PVA) (98–99% hydrolyzed) as the surfactant by continually stirring. Then the pH value of the reaction solution was adjusted to 9–10 by the addition of NaOH. The formed clear reactant solution was transferred to a 60 mL Teflon-lined stainless-steel autoclave, which was sealed and put into an oven of 140 °C. After a reaction time of 12 h at 140 °C, the container was cooled to room temperature naturally. The precipitates were collected by centrifuging and washed several times by deionized water and absolute ethanol, and then dried at 80 °C for 4 h.Synthesis of Ag2Te tri-wing nanoribbons
The obtained Te tri-wing nanoribbons were washed and re-dispersed ultrasonically in 200 mL of deionized water, and then 50 mL of 30 mM AgNO3 solution was added dropwise into the solution at room temperature. The mixture was stirred for 30 min and the precipitates were collected from the solution by centrifuging and washed several times by deionized water and absolute ethanol, and then dried at 80 °C for 4 h.Characterization
The obtained Te nanostructures and Ag2Te tri-wing nanoribbons were characterized by X-ray powder diffraction (XRD), field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). Phase identification and structure analysis of the sample were carried out by XRD using a Rigaku D/max 2500 diffractometer with Cu-Kα radiation (50 kV × 250 mA) and a graphitic monochromator. XRD data were collected by the step-scan mode with a step width of 2θ = 0.02° and a sampling time of 1 s. The chemical composition of the sample was identified by energy-dispersive X-ray spectroscopy (EDS). The overview morphologies and sizes of the samples were obtained by FESEM performed on a FEI-Sirion scanning electron microanalyzer at 10 kV. TEM images, high-resolution TEM (HRTEM) images, and selected area electron diffraction (SAED) patterns were recorded on a JEM-2010 transmission electron microscope using an accelerating voltage of 200 kV.Results and discussion
Te tri-wing nanoribbons
Fig. 1a shows a typical SEM image of tri-wing Te nanoribbons with a length of tens of micrometres and a width of hundreds of nanometres. As shown in Fig. 1b, an individual Tri-wing nanoribbon is composed of three wings with a thickness of about 20 nm. A TEM image of the tri-wing nanoribbon is displayed in Fig. 2a. The HRTEM images and SAED patterns are taken from two different wings of this nanoribbon, as shown in Fig. 2b and 2c. Both of the HRTEM images show lattice fringes perpendicular to the wings with a spacing of 5.93 Å, corresponding to the (001) plane of t-Te, indicating that the wings grow along the [001] direction (c-axis). The SAED patterns have been obtained by focusing the electron beam along the [110] direction as shown in Fig. 2d, and the SAED patterns recorded from different wings are virtually identical. This implies that the three wings belong to one single crystal and the wing surfaces are mainly enclosed by {100} facets. The tri-wing nanoribbon shows a three-fold symmetry and the three wings are separated from each other by an angle of 120° between them. | ||
| Fig. 1 SEM images of Te tri-wing nanoribbons. (a) A general overview of the product and (b) a high magnification SEM image showing clearly the tri-wing structure. | ||
 | ||
| Fig. 2 (a) A TEM image of the Te tri-wing nanoribbon. (b, c) HRTEM images and the corresponding SAED patterns recorded from the marked areas of (b) and (c). (d) A schematic illustration of the cross-section of the tri-wing nanoribbon with the direction of TEM electron beams denoted by the arrows. | ||
Fig. 3 shows the XRD pattern of tri-wing Te nanoribbons. All the peaks can be readily indexed to a pure trigonal phase of tellurium (space group: P3121), which is in good agreement with the standard XRD data (JCPDS 36-1452). For the random powder sample pressed and fixed in a conventional sample holder, no obvious preferred orientation is observed (bottom panel of Fig. 3). However, as shown in the middle panel of Fig. 3, the XRD pattern of the sample dispersed on a glass slide shows remarkable preferred orientation. A similar phenomenon, as displayed on the top panel of Fig. 3, is also observed for the 2D nanobelts synthesized by adjusting the pH condition (detailed discussion is presented below). The preferential orientation presented on the XRD pattern can be explained based on the shape and symmetry of the nanostructure. For 1D and 2D nanostructures dispersed on glass slides, they show a tendency to lie down on the substrate. The quasi-1D tri-wing nanoribbons, as shown in Fig. 1b and Fig. 2a, prefer to lie on the substrate by their two side wings, leading to the intensified (110) and (100) peaks of the XRD pattern (middle panel of Fig. 3). For the single-crystal Te nanobelts, they grow in the ac plane and lie on the substrate with the ac plane parallel to the substrate, resulting in the obviously intensified (100) peak of the XRD pattern (top panel of Fig. 3).
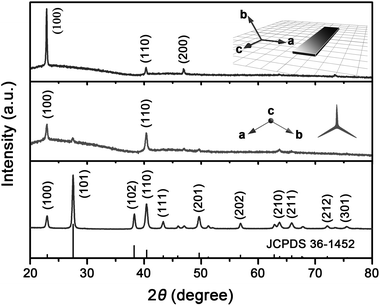 | ||
| Fig. 3 XRD patterns of Te nanostructures. Low panel shows the XRD pattern of the random powder sample of Te tri-wing nanoribbons pressed and fixed in a conventional sample holder. The middle and top panels show the XRD patterns of quasi-1D tri-wing Te nanoribbons and 2D Te nanobelts dispersed on glass slides, respectively. | ||
Time dependent morphology evolution has been investigated to understand the formation process of this unique tri-wing nanostructure. As shown in Fig. 4a, nanorods with a length of several hundred nanometres are formed at the stage of 30 min. After a hydrothermal treatment of 1 h, nanobelts, nanoplates and tri-wing nanoribbons with a length of several micrometres are obtained (Fig. 4b). The control experiment with a reaction time of 12 h at 120 °C shows Te nanostructures similar to those of Fig. 4b (Fig. S1†). The tri-wing structure is observed after a reaction time of 1.5 h. The above experiments indicate that the tri-wing Te nanoribbons are synthesized by a two-step process. Te nanorods are first formed probably because of the low reaction rate at low temperature, and then the tri-wing structure with a three-fold symmetry is obtained due to higher reaction rate at higher temperature.
 | ||
| Fig. 4 SEM images of the Te nanostructures with a reaction time of (a) 30 min, (b) 1 h, and (c) 1.5 h. (d) SEM image of the product obtained in the absence of PVA. | ||
According to the previous report, a single-crystal Te nanobelt has a tendency to enclose its top surface with {100} planes,18,22 which is attributed to the lower surface free energy of {100} planes. Similarly to the 2D Te nanobelts, the three wings of the Te nanoribbon also grow from the stem along the {100} planes in the presence of PVA as the surfactant. In the solution synthesis of Te nanobelts, surfactants play an important role in tailoring the morphology and shape of the nanostructures by selectively absorbing on the surfaces and reducing the free energy of the {100} planes.22–24 According to our experiments, the addition of PVA is also essential for the formation of tri-wing Te nanoribbons. As shown in Fig. 4d, only nanorods are obtained in the absence of PVA.
It is well known that the reduction reaction rate of TeO32− shows strong pH value dependence, which is decreased with increasing pH value.23,26,28 In our experiment, the morphology and shape of the Te nanostructures are controlled by the content of NaOH, and the pH value dependent morphology evolution is shown in Fig. 5. Due to the higher growth rate in lower pH condition, 2D Te nanofeathers are synthesized in the absence of NaOH (Fig. 5a and 5b). The quick growth in both lateral and longitudinal dimensions leads to the formation of dendrites with a featherlike morphology. With the addition of 5 mg of NaOH, imperfect tri-wing nanostructures are obtained (Fig. 5c). By increasing NaOH to 10 mg in the reaction solution, the tri-wing Te nanoribbons with smooth and intact wings, as shown in Fig. 5d, are obtained due to further decreased growth rate. With the addition of even more NaOH, degeneration of the tri-wing nanoribbons is observed (Fig. 5e), and only normal 2D nanobelts can be observed when 20 mg of NaOH is put into the solution (Fig. 5f). Furthermore, nanorods are obtained with the addition of 60 mg of NaOH (Fig. 5g), and they grow bigger in diameter in the presence of 1.6 g of NaOH (Fig. 5h). The sharp ends of nanorods displayed in Fig. 5g and 5h should be ascribed to the effect of the surfactant PVA. This indicates that the Te nanostructures are very sensitive to the pH value, which is closely related to the reaction rate, and the elaborated control of reaction rate is critical to the synthesis of tri-wing Te nanobelts.
 | ||
| Fig. 5 SEM images of Te nanostructures prepared in different pH conditions. (a) Te nanofeathers synthesized in the absence of NaOH, (b) an individual Te nanofeather, (c), (d), and (e) the tri-wing Te nanoribbons obtained with 5, 10, and 15 mg of NaOH, respectively, (f) Te nanobelts synthesized with 20 mg of NaOH, (g) and (h) Te nanorods obtained with 60 mg and 1.6 g of NaOH, respectively. | ||
Normally, a Te crystal grows preferentially along the [001] direction, and various 1D Te nanostructures have been synthesized. The successfully fabrication of tri-wing Te nanoribbons indicates that more complex quasi-1D Te nanostructures could be obtained by a combination of appropriate surfactant and careful reaction rate control.
Tri-wing Ag2Te nanobelts
Due to its high chemical activity, Te nanostructures can react with many other elements to synthesize Te-based nanocompounds.3–8 Therefore, Te nanostructures serve as ideal templates to synthesize novel nanostructures of tellurides with various applications. Herein, we demonstrate that tri-wing Ag2Te nanoribbons can be facilely prepared by the direct reaction of tri-wing Te nanoribbons with AgNO3 at room temperature. Similarly to the synthesis of ultrathin Ag2Te nanowires,29 the reaction takes place fast at room temperature, and the colour of the solution changes from blue to black immediately after the addition of AgNO3.The obtained product is a pure phase of Ag2Te, as indicated by the XRD pattern shown in Fig. 6a. All of the diffraction peaks can be perfectly indexed to the monoclinic phase of Ag2Te (space group: P21/c), which is consistent with the reported XRD data (JCPDS 81-1820). Energy-dispersive spectrometry (EDS) analysis (inset in Fig. 6a) indicates the sample is composed of Ag and Te, and the atomic ratio of Ag to Te is about 2.09, which is very close to 2. Fig. 6b shows the overview SEM image of tri-wing Ag2Te nanoribbons. The wing of the nanoribbon has a thickness of 20–30 nm, which is almost the same as that of the tri-wing Te nanoribbon. A large crystal boundary across the Ag2Te nanoribbon is clearly observed in the high-magnification image (Fig. 6c), and the orientations of the two crystals are determined by the HRTEM image and SAED patterns, as shown in Fig. 6d. This indicates the polycrystalline nature of the tri-wing Ag2Te nanoribbon, which leads to the curvature of the nanoribbons as shown in Fig. 6b.
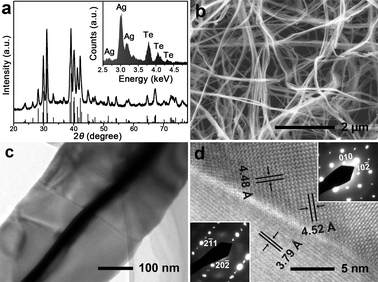 | ||
Fig. 6 (a) XRD pattern of tri-wing Ag2Te nanoribbons, and the inset shows the EDS pattern. (b) An overview SEM image of the tri-wing Ag2Te nanoribbons. (c) TEM image of a single tri-wing Ag2Te nanoribbon, and the crystal boundary is observed clearly. (d) HRTEM image of the crystal boundary. The lattice fringes with spacings of 4.48 Å, 4.52 Å and 3.79 Å corresponding to the (010), (10![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ) and (20 ) and (20![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ) lattice planes of monoclinic Ag2Te, respectively. The SAED patterns inserted in the image's left and right sides correspond to the left and right areas of the crystal boundary, respectively. ) lattice planes of monoclinic Ag2Te, respectively. The SAED patterns inserted in the image's left and right sides correspond to the left and right areas of the crystal boundary, respectively. | ||
Conclusion
In summary, unique tri-wing Te nanoribbons have been successfully synthesized through a simple surfactant assisted hydrothermal method. Based on the time dependent morphology evolution, a two-step formation mechanism is proposed for the Te tri-wing nanostructures. According to our experiments, the reduction reaction rate, which is pH value dependent, plays an important role in the controlled synthesis of tri-wing Te nanoribbons. By adjusting only the alkalinity of the reaction solution, the morphology of Te nanostructures can be successfully modulated from nanofeathers to tri-wing nanoribbons, normal 2D nanobelts and nanorods, which is attributed to the different reaction rate in different pH condition. The Te nanoribbons with very thin wings are demonstrated to be ideal templates to prepare novel telluride nanostructures through the successful synthesis of tri-wing Ag2Te nanoribbons.Acknowledgements
The work was financially supported by the National Natural Science Foundation of China (grant no. 11144002) and the National Basic Research Program of China (grant no. 2007CB925003).Notes and references
- Z. H. Lin, Z. S. Yang and H. T. Chang, Cryst. Growth Des., 2008, 8, 351–357 CAS.
- W. H. Xu, J. M. Song, L. Sun, J. L. Yang, W. P. Hu, Z. Y. Ji and S. H. Yu, Small, 2008, 4, 888–893 CrossRef CAS.
- H. W. Liang, S. Liu and S. H. Yu, Adv. Mater., 2010, 22, 3925–3937 CrossRef CAS.
- H. W. Liang, S. Liu, J. Y. Gong, S. B. Wang, L. Wang and S. H. Yu, Adv. Mater., 2009, 21, 1850–1854 CrossRef CAS.
- G. D. Moon, S. Ko, Y. Xia and U. Jeong, ACS Nano, 2010, 4, 2307–2319 CrossRef CAS.
- A. K. Samal and T. Pradeep, J. Phys. Chem. C, 2009, 113, 13539–13544 CAS.
- G. A. Tai, W. L. Guo and Z. H. Zhang, Cryst. Growth Des., 2008, 8, 2906–2911 CAS.
- G. A. Tai, B. Zhou and W. L. Guo, J. Phys. Chem. C, 2008, 112, 11314–11318 CAS.
- T. S. Sreeprasad, A. K. Samal and T. Pradeep, J. Phys. Chem. C, 2009, 113, 1727–1737 CAS.
- H. S. Qian, S. H. Yu, L. B. Luo, J. Y. Gong, L. F. Fei and X. M. Liu, Chem. Mater., 2006, 18, 2102–2108 CrossRef CAS.
- G. C. Xi, C. Wang, X. Wang, Y. T. Qian and H. Q. Xiao, J. Phys. Chem. C, 2008, 112, 965–971 CAS.
- G. C. Xi, Y. K. Liu, X. Q. Wang, X. Y. Liu, Y. Y. Peng and Y. T. Qian, Cryst. Growth Des., 2006, 6, 2567–2570 CAS.
- A. von Hippel, J. Chem. Phys., 1948, 16, 372–380 CrossRef CAS.
- S. Sen, U. M. Bhatta, V. Kumar, K. P. Muthe, S. Bhattacharya, S. K. Gupta and J. V. Yakhmi, Cryst. Growth Des., 2008, 8, 238–242 CAS.
- Y. Rheem, C. H. Chang, C. M. Hangarter, D. Y. Park, K. H. Lee, Y. S. Jeong and N. V. Myung, Electrochim. Acta, 2010, 55, 2472–2476 CrossRef CAS.
- Y. J. Zhu, W. W. Wang, R. J. Qi and X. L. Hu, Angew. Chem., Int. Ed., 2004, 43, 1410–1414 CrossRef CAS.
- B. Mayers and Y. N. Xia, Adv. Mater., 2002, 14, 279–282 CrossRef CAS.
- M. S. Mo, J. H. Zeng, X. M. Liu, W. C. Yu, S. Y. Zhang and Y. T. Qian, Adv. Mater., 2002, 14, 1658–1662 CrossRef CAS.
- Z. B. He, S. H. Yu and J. P. Zhu, Chem. Mater., 2005, 17, 2785–2788 CrossRef CAS.
- Z. Y. Tang, Y. Wang, K. Sun and N. A. Kotov, Adv. Mater., 2005, 17, 358–363 CrossRef CAS.
- J. M. Song, Y. Z. Lin, Y. J. Zhan, Y. C. Tian, G. Liu and S. H. Yu, Cryst. Growth Des., 2008, 8, 1902–1908 CAS.
- H. S. Qian, S. H. Yu, J. Y. Gong, L. B. Luo and L. F. Fei, Langmuir, 2006, 22, 3830–3835 CrossRef CAS.
- B. Zhang, W. Y. Hou, X. C. Ye, S. Q. Fu and Y. Xie, Adv. Funct. Mater., 2007, 17, 486–492 CrossRef CAS.
- U. K. Gautam and C. N. R. Rao, J. Mater. Chem., 2004, 14, 2530–2535 RSC.
- B. Mayers and Y. N. Xia, J. Mater. Chem., 2002, 12, 1875–1881 RSC.
- Z. P. Liu, S. Li, Y. Yang, Z. K. Hu, S. Peng, J. B. Liang and Y. T. Qian, New J. Chem., 2003, 27, 1748–1752 RSC.
- W. J. Lan, S. H. Yu, H. S. Qian and Y. Wan, Langmuir, 2007, 23, 3409–3417 CrossRef CAS.
- G. C. Xi, Y. Y. Peng, W. C. Yu and Y. T. Qian, Cryst. Growth Des., 2005, 5, 325–328 CAS.
- H. T. Zhu, H. Zhang, J. K. Liang, G. H. Rao, J. B. Li, G. Y. Liu, Z. M. Du, H. M. Fan and J. Luo, J. Phys. Chem. C, 2011, 115, 6375–6380 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Chemical reaction mechanism and SEM image of the product synthesized at 120 °C. See DOI: 10.1039/c1ce05734k |
| This journal is © The Royal Society of Chemistry 2012 |
