Synthetic and crystallographic studies of bicyclo[3.3.1]nonane derivatives: from strong to weak hydrogen bonds and the stereochemistry of network formation†
Carl-Johan
Wallentin
a,
Edvinas
Orentas
b,
Magnus T.
Johnson
a,
Nikoletta B.
Báthori
c,
Eugenijus
Butkus
*b,
Ola F.
Wendt
*a,
Kenneth
Wärnmark
*a and
Lars
Öhrström
*d
aCentre for Analysis and Synthesis, Department of Chemistry, Lund University, P. O. Box 124, SE-221 00, Lund, Sweden. E-mail: ola.wendt@organic.lu.se; Fax: +46 46 2228209; Tel: +46 46 222 0000Carl-Johan.Wallentin@organic.lu.se; Magnus.Johnson@organic.lu.se; Kenneth.Warnmark@organic.lu.se
bDepartment Organic Chemistry, University of Vilnius, Naugarduko 24, Vilnius, 03225, Lithuania. E-mail: edvinas.orentas@chf.vu.lt; Fax: +370-5-2330987; Tel: +370-682-63092Eugenijus.Butkus@chf.vu.lt
cDepartment of Chemistry, Faculty of Applied Sciences, Cape Peninsula University of Technology, South Africa. E-mail: bathorin@cput.ac.za
dDepartment of Chemical and Biological Engineering, Chalmers University of Technology, SE-412 96, Göteborg, Sweden. E-mail: ohrstrom@chalmers.se; Fax: +46 31 772 3858
First published on 19th October 2011
Abstract
The syntheses and crystal structures of four unsaturated bicyclo[3.3.1]nonane derivatives containing various functionalities are presented and their intermolecular interactions examined. The impact of unsaturation on crystal structures and intermolecular networks of the six membered rings was found to be significant compared to the saturated analogues. Thus, unsaturated diolrac-1, in striking contrast to its saturated analogue rac-6, does not crystallise with spontaneous resolution. The hydrogen bonds in the starting bicyclononane diene diol rac-1, and the weaker hydrogen bonds in the dienone rac-2, and the bromo and nitrile derivatives, rac-3, rac-4 and (+)-4, respectively, were found significant for the overall structure. The two bromine atoms in rac-3 have significant halogen–halogen interactions. In several structures 2D nets can be identified and overall structures can be interpreted as close packing of these layers. The crystal structures were also subject to independent analysis by Hirshfeld surfaces, and this method provided additional insights, especially for the structural role of the unsaturation. The possible relation between chiral networks and conglomerate formation is discussed.
Introduction
Synthetic and structural studies of the bicyclo[3.3.1]nonanes (BCNs) (structure A, Fig. 1) have attracted much interest for several reasons. First, this framework is a common motif in many natural and biologically active compounds.1 This includes potential therapeutics for Alzheimer's disease such as garsubellin A (structure B, Fig. 1)2 and huperzine A (structure C, Fig. 1).3![The bicyclo[3.3.1]nonane framework (A), natural products containing this frame work: garsubellin A (B), and huperzine A (C).](/image/article/2012/CE/c1ce05673e/c1ce05673e-f1.gif) | ||
| Fig. 1 The bicyclo[3.3.1]nonane framework (A), natural products containing this frame work: garsubellin A (B), and huperzine A (C). | ||
Second, the stereochemical multiplicity of this semi-rigid framework allows functional groups to be placed in defined spatial positions. An important feature of this ring system is that it consists of two cyclohexane rings which both can adopt either a chair or a boat conformation.1 The framework may also be chiral by virtue of the molecular topology only. The unique molecular shape of the BCN framework, when substituted with suitable hydrogen-bond donors and acceptors or other groups capable of generating supramolecular synthons, has been successfully employed in the self-assembly of a wide range of supramolecular structures and inclusion complexes with various guest molecules.4
Some hydroxyl derivatives of this ring skeleton, the so-called “tubuland diols”, give controllable crystal structures with a variety of inclusion guests, illustrating the potential of BCNs in crystal engineering.5 We recently showed how an analysis of the network topology of BCN diols can provide a general way of both understanding and comparing these structures.6a Also in the case of less strongly interacting entities, a network analysis approach7 can be profitable, although more caution is recommended when the structure directing power of, for example, weak hydrogen bonds is discussed. Nevertheless, we have recently prepared organometallic systems where such interactions consistently give rise to 3D-nets and large, solvent filled channels.8
An additional aspect that renders the BCN interesting from a stereochemical point of view is that some of these aforementioned diols crystallise as conglomerates, i.e. they form homochiral crystals from a racemic mixture in solution.
In this work we report on our continued exploration of the synthetic and structural chemistry of the BCN framework, compounds 1–4, see Fig. 2. We are particularly interested in the effect that unsaturation in the BCN framework may have on crystal structures and networks, since this renders the six membered rings significantly flattened and the BCN skeleton more conformationally restrained.
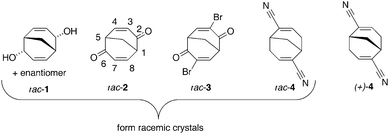 | ||
| Fig. 2 The compounds 1–4 discussed in this investigation. For each racemic structure, one enantiomer is shown. | ||
We furthermore explore what effect the potential, weak, hydrogen bonds introduced by the unsaturation will have on the solid state structure of the so formed compounds rac-1, rac-2, rac-3, rac-4, and (+)-4. It is worth noting that the introduction of a bromine atom in the unsaturated BCN skeleton, rac-3, also introduces another intermolecular force, the halogen–halogen interaction (not to be confused with the halogen bond). In addition to the network analysis of the crystal structures of compounds 1–4, a traditional analysis, by inspection of intermolecular atom–atom geometries, is compared to an independent approach based on Hirshfeld surfaces.9
For discussion purposes the structures of 1–4 will be compared to the crystal structures of the saturated compounds rac-2,6-dimethylbicyclo[3.3.1]nonane-exo-2,exo-6-diol chloroacetic acid clathrate,4brac-5 and rac-bicyclo[3.3.1]nonane-endo-2,endo-6-diol,6rac-6, both of which crystallise with spontaneous resolution, thus forming conglomerates. Finally, the solid state structure of rac-bicyclo[3.3.1]nonane-2,6-dione, rac-7,10 forming racemic crystals, and the solid state structure of the corresponding enantiopure compound (+)-(1S,5S)-7,6a will also be used as reference substances (Fig. 3).
![The bicyclo[3.3.1]nonane diolsrac-5, rac-6 and diones rac-7 and (+)-7 used as reference compounds for the discussions in this work.](/image/article/2012/CE/c1ce05673e/c1ce05673e-f3.gif) | ||
| Fig. 3 The bicyclo[3.3.1]nonane diolsrac-5, rac-6 and diones rac-7 and (+)-7 used as reference compounds for the discussions in this work. | ||
Results and discussion
Synthesis
The synthetic procedures are presented in Scheme 1. Both rac-bicyclo[3.3.1]nona-3,7-diene-endo-2,endo-6-diol, rac-1, and rac-3,7-dibromobicyclo[3.3.1]nona-3,7-diene-2,6-dione, rac-3, were synthesised using rac-bicyclo[3.3.1]nona-3,7-diene-2,6-dione, rac-2,11 as starting compound. Hence, reducing rac-2 under Luche conditions afforded rac-1 in 95% yield. The previous synthesis of the bromodienone rac-3 was based on a bromination–elimination sequence of the saturated dienone.12 However, we found that the synthesis of bromodienone based on a bromination–elimination protocol developed for α,β-unsaturated ketones works better. Thus, in this way rac-3 was synthesized from dienone rac-2 in 65% yield.13 Dicyanodiene rac-4 was synthesised in accordance with the literature procedure.14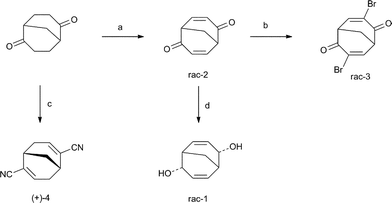 | ||
| Scheme 1 Reagents and conditions: (a) (i) PhSOOMe, NaH, THF and (ii) Na2CO3, PhMe, 80%; (b) Br2, CCl4, Et3N, 65%; (c) (i) TMSCl, ZnI2, NaCN, DCM and (ii) POCl3, pyridine, 76%; (d) NaBH4, CeCl3, MeOH, 95%. | ||
Crystal structure analysis
rac-Bicyclo[3.3.1]nona-3,7-diene-endo-2,endo-6-diol, rac-1. A displacement ellipsoid plot of the molecular unit and the hydrogen-bond pattern of the crystal structure of the dienediol rac-1 are displayed in Fig. 4. No unusual features are present at the molecular level.
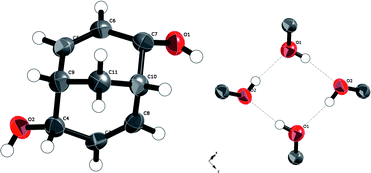 | ||
| Fig. 4 Left: a displacement ellipsoid (50%) plot of the crystal structure of dienediol rac-1. Right: hydrogen bond squares. | ||
In the crystal structure, the hydrogen bonds involving the hydroxyl groups are the only hydrogen bonds present. Both hydroxyl groups in each molecule are involved and each hydroxyl group acts both as a hydrogen-bond acceptor and donor, forming a cyclic hydrogen-bond network involving eight atoms and four molecules of rac-1. The hydrogen-bonded squares shown in Fig. 4 connect these dienediols into a sheet structure, cf.Fig. 5, distinctively different from the 3D hydrogen bond nets of the saturated diolrac-5, containing two exo-hydroxyl groups, (83)-eta, and rac-6, containing two endo-hydroxyl groups (82.12)-utg.6a The resulting puckered sheets are close packed as displayed in Fig. 6. The topological representation of the 3-connected 2D net in the crystal structure of rac-1 is shown in Fig. 7.
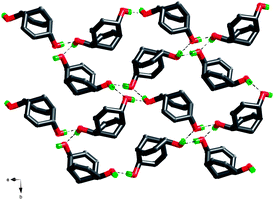 | ||
| Fig. 5 The hydrogen-bonded network in the crystal structure of dienediol rac-1. Note that no C(sp2)–H⋯O hydrogen bonds are detected. | ||
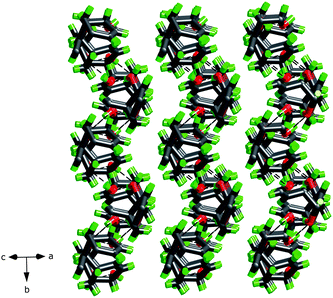 | ||
| Fig. 6 A side view of the packing of the hydrogen bonded sheets of dienediol rac-1. | ||
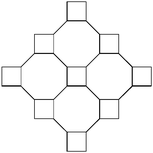 | ||
| Fig. 7 The most symmetric form of the three-connected net (topology symbol fes) in rac-1. | ||
For further discussion it will be important to note that in this case a racemic solution of rac-1 crystallises as a racemate, whereas rac-5 and rac-6 both crystallise as conglomerates.
rac-Bicyclo[3.3.1]nona-3,7-diene-2,6-dione, rac-2. A displacement-ellipsoid plot of the molecular unit and the hydrogen bonds of the crystal structure of rac-2 are displayed in Fig. 8 (left). No unusual features are present at the molecular level.
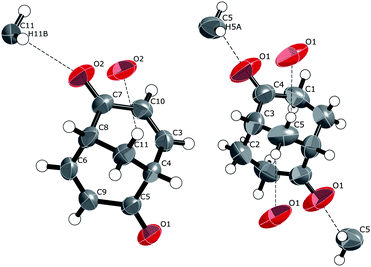 | ||
| Fig. 8 Left: displacement ellipsoid (50%) plot of the crystal structure of rac-2 showing also the weak hydrogen bond interactions. Right: the corresponding plot for the crystal structure of enantiopure saturated dione (+)-7. The hydrogen bonding between the methine hydrogen (C9–H) and carbonyl oxygen (O1) on an adjacent rac-2 is not displayed for clarity. See Fig. 9 for the complete view. | ||
The shortest intermolecular O⋯H interaction is between H11B residing on the bridgehead carbon C11 and O2: 2.53 Å, 3.373 Å and the OCH angle being 140° (Fig. 8, left). This is very similar to what was observed for the enantiomerically pure crystals of the corresponding saturated dione (+)-7,6a (2.52 Å, 3.401(2) Å, 150°). As can be seen by comparing the displacement ellipsoid plots of the two compounds (Fig. 8), the orientations of the interactions are almost identical, except that for rac-2 this connection uses only one bridgehead hydrogen atom instead of two as in (+)-7. Completing the analogy between the crystal structure of rac-2 and (+)-7, we would expect this intermolecular bond to occur between the enantiomers of the same absolute configuration in the crystal structure of rac-2, and this is indeed the case, giving single-enantiomer chains, or flat helices, along the b-axis of the crystal, 50% of the chains having enantiomers of one absolute configuration of molecule rac-2 and 50% having the opposite. This indeed results in racemic crystals.
The overall crystal structure of rac-2 is displayed in Fig. 9. The α-hydrogen atom (connected to C(sp2)) and the adjacent carbonyl oxygen atom in one of the enone systems of each molecule of rac-2 are engaged in hydrogen-bonding with the same enone system in an adjacent molecule of rac-2, forming a cyclic hydrogen-bonding system involving eight atoms and two molecules, with the graph set notation R(2,2)8 of the first level. Furthermore, the carbonyl oxygen atom of the other enone system in each rac-2 is, together with one of the bridgehead hydrogen atoms, engaged in a propagating helical hydrogen-bonding system with two other next neighbours (different from the one involved in the first type of hydrogen-bonding network). Overall this results in a herringbone (6,3)-2D net displayed in Fig. 9.
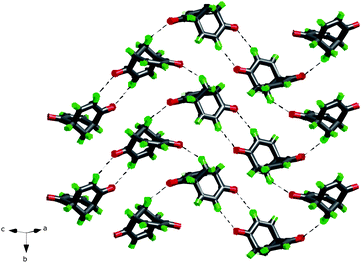 | ||
| Fig. 9 Herringbone (6,3)-2D net formed by weak O⋯H–C interactions in rac-2. All hydrogen-bonding interactions are displayed. | ||
Comparing the structure of unsaturated rac-2 to the saturated analogue rac-7,10 we see that the latter contains a similar (2.621 Å, 3.493 Å, 144°) but double O⋯H–C bridgehead interaction, giving chains perpendicular to the y-axis. However, if every second of the R(2,2)8 O⋯H–C interactions in rac-2 is ignored, something akin to the close packing of rods emerges, just as for rac-7 (Fig. 10). This illustrates the difficulty in drawing conclusions on the structure directing power of individual weak interactions.
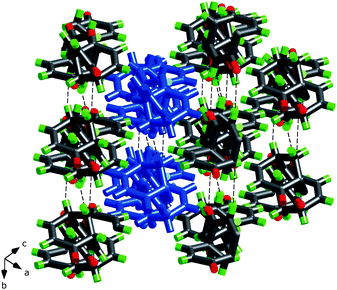 | ||
| Fig. 10 The packing of the sheets in rac-2 (Fig. 9). One sheet has been highlighted in blue. | ||
rac-Dibromobicyclo[3.3.1]nona-3,7-diene-2,6-dione, rac-3. Substituting each of the two hydrogen atoms in the α-positions in ketonerac-2 for two bromine atoms gives compound rac-3. In rac-3, the carbonyl oxygen at carbon 2 (O20) and the hydrogen atom at carbon 8 (hydrogen atom attached to C4) form a cyclic R(2,2)10 hydrogen-bonding network with the carbonyl oxygen atom at carbon 6 (O21) and the hydrogen atom at carbon 4 (hydrogen atom attached to C15) in an adjacent molecule of rac-3 (Fig. 11). This hydrogen-bonded system contains 10 atoms and each molecule of rac-3 forms two such interactions. Concerning the bromine atoms, each bromine atom is engaged in halogen–halogen bonding15 to another neighbouring molecule (not the same as is engaged in hydrogen bonding (Fig. 11)). Thus, the weak self-complementary R(2,2)8 bonding pattern involving the carbonyl oxygen atom and the α hydrogen atom attached to a C(sp2) that was seen between two adjacent molecules in the crystal structure of rac-2 is now disabled being replaced by another weak interaction—the halogen–halogen interaction.
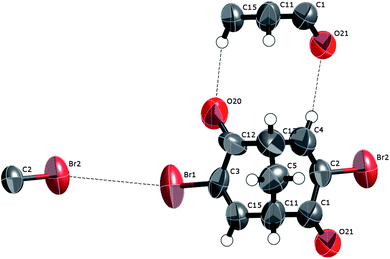 | ||
| Fig. 11 A displacement ellipsoid (50%) plot of the crystal structure of rac-3 also indicating the major intermolecular interactions. | ||
This interaction has been described based on the angles θ1 and θ2 (θ1 = C1–Br1⋯Br2, θ2 = Br1⋯Br2–C2) as either “type-I” with θ1 = θ2 and usually >110°, or “type-II” with θ1 = 180° and θ2 = 90°. In the general case, both types arise because of the polarisation (anisotropy) of the bromine electron density16 and the basic geometry of this interaction can be readily explained by a simple circular 2D model of the Br atom with a positive segment traversing the centre from side to side and smaller negatively polarised segments on the top and at the bottom.15b (Typically for this interaction, the 3D-electrostatic potential energy iso-surface looks much like a stuffed olive.)
The Br⋯Br interactions in this structure (3.901 Å, θ1 = 145°, θ2 = 140°) are rather typical examples of a type I interaction as these have also somewhat longer Br⋯Br distances than type II.15b Possibly, the self-complementary O⋯H–C weak hydrogen bonds are reinforced by the inductive effect of the bromine atom as they are not observed in any of the other compounds in the series and because the interaction seems somewhat stronger (2.436 Å, 3.282 Å, 143°). Finally, these (4,4) 2D nets, shown in Fig. 12, are interconnected by Br⋯H–C intermolecular forces in the expected geometrical range 3.2–3.3 Å and 130–134°. (A plot of Br⋯H–C geometry data from the CSD17 is included in the ESI as Fig. S1†.)
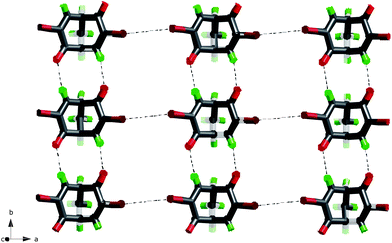 | ||
| Fig. 12 The 2D net in the crystal structure of rac-3. | ||
At the molecular level, the introduction of the bromine atoms means that the molecules take on a much more specific “cleft” shape as the distance from the “peak” (C5 in Fig. 11) to the base is increased from around 2.8 Å (C11⋯C10 distance in rac-2) to 4.6 Å (C5⋯Br distance in rac-3). Based on this one might argue that the crystal structure of rac-3 could better be described as a close packing of these “cleft” molecules in the solid state than being a result of a few particular intermolecular interactions. The space filling plot in Fig. 13 indicates that this may be the case.
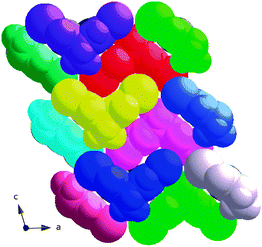 | ||
| Fig. 13 Space filling plot of the crystal structure of rac-3 in the ac-plane. | ||
(+)-Bicyclo[3.3.1]nona-2,6-diene-2,6-dicarbonitrile, (+)-4. The molecular unit of enantiomerically pure (+)-4 with the shortest C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interactions indicated in the crystal structure is plotted in Fig. 14. The nitrile nitrogen atom is a hydrogen-bond acceptor to one α-hydrogen atom (attached to C(sp2)) in an adjacent molecule (+)-4 and one endo-β-hydrogen atom (attached to C(sp3)) in another adjacent molecule (+)-4, creating again an R(2,2)10 ten-atom cyclic hydrogen-bonding system involving four molecules of (+)-4 (Fig. 15).
N⋯H interactions indicated in the crystal structure is plotted in Fig. 14. The nitrile nitrogen atom is a hydrogen-bond acceptor to one α-hydrogen atom (attached to C(sp2)) in an adjacent molecule (+)-4 and one endo-β-hydrogen atom (attached to C(sp3)) in another adjacent molecule (+)-4, creating again an R(2,2)10 ten-atom cyclic hydrogen-bonding system involving four molecules of (+)-4 (Fig. 15).
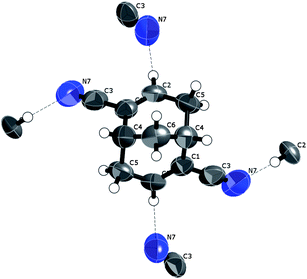 | ||
| Fig. 14 A displacement ellipsoid (50%) plot of the crystal structure of (+)-4 indicating also the shortest intermolecular contacts. | ||
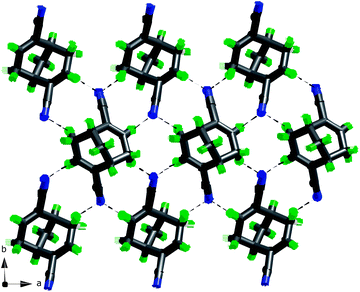 | ||
Fig. 15 The 2D net in the crystal structure of (+)-4 defined by the shortest C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H contacts (two types). N⋯H contacts (two types). | ||
Again, these shortest interactions (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H–C(sp2)) (2.561 Å, 3.487 Å 141°) are within the expected intermolecular attractive region (see plot in Fig. S2†) and define a 2D network in which also the second shortest (C
N⋯H–C(sp2)) (2.561 Å, 3.487 Å 141°) are within the expected intermolecular attractive region (see plot in Fig. S2†) and define a 2D network in which also the second shortest (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H–C(sp3)) contact takes part (N⋯H: 2.94 Å), see Fig. 15. For a space filling packing diagram of these 2D-nets, see Fig. 16.
N⋯H–C(sp3)) contact takes part (N⋯H: 2.94 Å), see Fig. 15. For a space filling packing diagram of these 2D-nets, see Fig. 16.
The crystal structure of the racemate, rac-4,10 displays the same kind of weak hydrogen bonds, giving the same 2D pattern, and consequently cell parameters that are very close. Possibly, this is due to the fact that the hydrogen bond acceptor (CN) is protruding far away (2.6 Å) from the chiral core of the molecule, making the same kind of hydrogen bonds possible in the two cases.
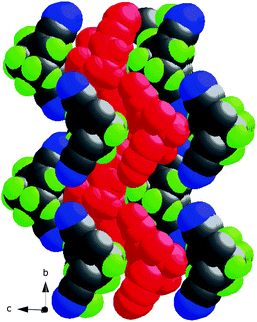 | ||
| Fig. 16 Packing of (+)-4 with one 2D sheet in red. | ||
Hirshfeld surface analysis
The analysis of a crystal structure using only a few selected geometric data is at most approximate and at worst misleading. A remedy has been proposed based on the analysis of so-called Hirshfeld surfaces,9 a boundary surface of a molecule in a structure. At each point of this surface one can measure the distances to the closest atom on the inside (di) and outside (de) of the surface. The so-generated 2D plots provide not only a unique fingerprint of the environment surrounding each type of molecule in a structure, but also hint at various types of intermolecular interactions. For example, hydrogen bonds D–H⋯A tend to generate a number of points where the H and A atoms are the closest. If the molecule hydrogen bonds to itself, two distinct traces will be seen in the plot, one with the H inside the surface and A outside, giving short de values and long di values starting at de + di equal to the shortest H⋯A distances, and vice versa.It is important to note that there is no new information generated by the Hirshfeld surfaces and the various variables that can be mapped on it, but they do have the advantage of providing a single picture of all the intermolecular atom–atom interactions in a structure.
The following examination was made more or less independently of the classical analysis in the preceding section and we can therefore briefly comment on the added value of the Hirshfeld approach.
We have compared the crystal structure of the racemic dienediol, rac-1, with that of the rac-bicyclo[3.3.1]nonane-endo-2,endo-6-diol, rac-6, earlier prepared and analysed by us,7 that forms a hydrogen-bonded 3D-net upon crystallisation. In Fig. 17 we see that the most prominent features of the crystal structures of diolrac-6 and dienediol rac-1 are the same, namely the symmetric spikes for the H⋯O interaction extending towards the lower left of the diagrams, accounting for 18% (rac-6) and 20% (rac-1) of the points on the Hirshfeld surface. However, in the crystal structure of rac-1 there is clearly an additional feature manifested in the “wings” symmetrically extending down to (de = 1.6, di = 1.1). As CrystalExplorer™ also allows the mapping of individual atom–atom contacts it is easy to identify this as short C–H⋯C contacts (Fig. 17, right), and subsequent inspection of the surface and the crystal structure identifies this as the specific interaction between the hydrogen on C4 and the sp2 hybridised C3 (9.5% of the surface points), reinforcing the network interaction in the b-direction. Thus a distinguishing feature of the crystal structure of rac-1 compared to the one of rac-6 is that the sp2 hybridised carbon atoms open up the hydrogen covered bicyclononane framework to render the C⋯H interactions accessible.
![Hirshfeld fingerprint plot of rac-bicyclo[3.3.1]nonane-endo-2,endo-6-diol, rac-6 (left), rac-bicyclo[3.3.1]nona-3,7-diene-endo-2,endo-6-diol, rac-1 (middle) and highlighted CH interactions in rac-1 (right, blue, other interactions faded to gray). Note that these plots display all points on the Hirshfeld surface and the colour coding (red, many points; blue, few points, at each x, y pair) is identical in all plots.](/image/article/2012/CE/c1ce05673e/c1ce05673e-f17.gif) | ||
| Fig. 17 Hirshfeld fingerprint plot of rac-bicyclo[3.3.1]nonane-endo-2,endo-6-diol, rac-6 (left), rac-bicyclo[3.3.1]nona-3,7-diene-endo-2,endo-6-diol, rac-1 (middle) and highlighted CH interactions in rac-1 (right, blue, other interactions faded to gray). Note that these plots display all points on the Hirshfeld surface and the colour coding (red, many points; blue, few points, at each x, y pair) is identical in all plots. | ||
![Hirshfeld fingerprint plots of unsaturated rac-bicyclo[3.3.1]nona-3,7-diene-2,6-dione, rac-2 (left), compared to the plots of the corresponding saturated rac-bicyclo[3.3.1]nonane-2,6-dione, rac-7 (middle), and the structure of the enantiopure saturated dione (+)-7 (right). See text for further explanation.](/image/article/2012/CE/c1ce05673e/c1ce05673e-f18.gif) | ||
| Fig. 18 Hirshfeld fingerprint plots of unsaturated rac-bicyclo[3.3.1]nona-3,7-diene-2,6-dione, rac-2 (left), compared to the plots of the corresponding saturated rac-bicyclo[3.3.1]nonane-2,6-dione, rac-7 (middle), and the structure of the enantiopure saturated dione (+)-7 (right). See text for further explanation. | ||
Immediately we can notice the more diffuse character of the diene based framework and again, this corresponds to C⋯H (10.4%), but also to C⋯O (1.6%) and C⋯C (2%) interactions made possible by the sp2 carbons. The spikes at the flanks indicate weak C–H⋯O hydrogen bonds and are somewhat more pronounced in the enantiopure crystals of (+)-7, Fig. 18, right, as is the (repulsive) H⋯H contact demonstrated by the diagonal spikes. The larger area of (+)-7 spreading up to higher values of de indicates a less dense structure compared to rac-7.
As observed in the “manual” analysis, we can see both similarities and differences in these plots, but perhaps the more diffuse points in the plots for rac-2 and rac-7 compared to (+)-7 indicate that the close packing motif is important for these compounds.
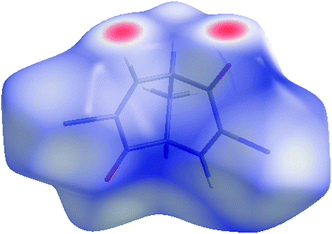 | ||
| Fig. 19 Hirshfeld surface mapped with the normalised contact distance dnorm for dibromodienedione rac-3. | ||
The self-complementary weak hydrogen bonds found in the manual analysis are clearly seen as two bright red spots, and this interaction covers 24.8% of the Hirshfeld surface. The Br⋯Br interactions also discussed in the preceding section are not found in this plot, and although present in the fingerprint plot in Fig. 20, they cover only 6% of the surface, so in this respect both Br⋯O (8.5%) and, especially, Br⋯H (34.0%) seem to be more important.
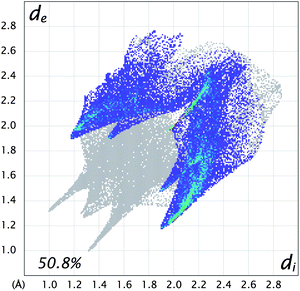 | ||
| Fig. 20 Hirshfeld fingerprint plot with bromide interactions to other atoms highlighted for dibromodienedione rac-3. | ||
In this case, however, it is important to understand that the Hirshfeld analysis does not take angular information into account, and as especially the larger halogen atoms Br and I have a pronounced anisotropy, responsible for the weak halogen–halogen interactions found in rac-3 the use of only Hirshfeld plots might actually be misleading in such cases.
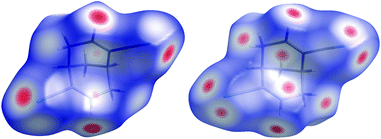 | ||
| Fig. 21 Hirshfeld surfaces mapped with the normalised contact distance dnorm for enantiopure dicyanodiene, (+)-4 (left) and racemic dicyanodiene rac-4 (right). Red colour indicates short contacts, blue colour no contacts. | ||
Network formation and chirality
In our earlier work on BCN network compounds6a we noted that spontaneous resolution (conglomerate formation) was concomitant with chiral nets in the crystals. We summarise these results and the work presented here in Table 1.Prior to formulating a tentative statement based on these data, we note two problems that possibly limit the value of our discussion. Firstly, this analysis is limited to BCN structures, and a more comprehensive search of the literature will be necessary to reach more general conclusions about conglomerate formation. Secondly, we have not explored the possibility of polymorphism of these compounds; perhaps a broader search of crystal growth conditions will lead to other polymorphs suggesting other conclusions.
We would like to suggest the following hypothesis that obviously needs further experimental data to be verified or falsified: conglomerate formation is advantageous when the strongest intermolecular interactions present have the geometric requirements to form a chiral net. Note that there seems to be nothing forbidding an achiral network, such as the diamond (dia) net, to adopt a chiral conformation (embedding).
This agrees with the data in Table 1, as the 2D net of rac-1 is achiral and the crystals racemic, whereas for rac-5 and rac-6 the networks are chiral and they crystallize as conglomerates. The enantiopure saturated dione, (+)-7, gives a chiral 3D net. However the more dense structure of rac-7 gives crystals having a higher melting point and thus a thermodynamically more stable product, in this case a racemic crystal structure. This observation further suggests that strong hydrogen-bonding motifs, such as hydroxyl groups, are a prerequisite for spontaneous resolution for this particular compound class.
Other chiral motifs formed by strong hydrogen bonds, like helices and their stacking, have also been implicated in conglomerate formation,18a and a related discussion of chiral coordination networks appeared in 2005.18b Thus the idea that an intrinsic chiral motif in the overall crystal packing is important in obtaining spontaneous resolution is not entirely new.
Summary and conclusions
Upon introducing double unsaturation in the skeleton of BCN derivatives new hydrogen-bond donors are introduced. The basic unsaturated BCN skeleton was modified by the introduction of different functional groups, leading to compounds 1–4. Each of these four unsaturated molecules has a different hydrogen-bonding pattern. Their solid-state structures were analysed in detail and when possible a network descriptor was assigned. More specifically, upon introducing double unsaturation, the resulting crystal structures display weak hydrogen bonds to the hydrogen atoms attached to C(sp2). This is clearly seen in the difference of the Hirshfeld plots of dienediol rac-1 compared to diolrac-6. Similar effects can be seen in dienedione rac-2 compared to dione rac-7. By introducing bromine atoms into rac-2 to give the dibromodienone, rac-3, another, less known intermolecular force, the halogen–halogen interaction could be observed. The crystal structures have been subjected to independent analysis by inspection of intermolecular atom–atom geometries and by Hirshfeld surfaces, proving the utility of this latter methodology. For example, in the Hirschfeld analysis we could show that the intermolecular forces in (+)-4 are dominated by a few strong C–H⋯N interactions, whereas the corresponding racemic structure, rac-4, displays a larger number of weaker hydrogen bonds.The unsaturated diol derivative displays 2D sheet structure, where the corresponding saturated compound displays a chiral 3D network and conglomerate formation. This prompted us to call attention to the possible connection between conglomerate formation and chiral nets, but we are reluctant to draw any firm conclusions based on our limited study. However, formation of chiral 3D nets seems to be dependent on the presence of strongly hydrogen-bonding motifs, such as hydroxyl groups, but a wider set of compounds from the BCN family are needed to confirm this hypothesis which is based solely on the present observations.
Experimental
General procedures and materials
Compounds rac-2 and (+)-4 were prepared according to the literature.10,14 All commercially available reagents were purchased from Sigma Aldrich and used as received. 1H and 13C NMR spectra were recorded in CDCl3 on a Bruker Avance 400 or a Bruker DR400 spectrometer working at 400 MHz. Chemical shifts are given in ppm downfield from TMS using residual solvent peaks as reference. Elemental analyses were performed by H. Kolbe, Mülheim an der Ruhr, Germany.1H-NMR: δ 7.70 (d, J = 6.85, 2H), 3.68 (m, 2H), 5.26 (t, J = 2.90, 2H); 13C-NMR: δ 185.1, 145.7, 122.0, 48.2, 34.2; Anal. calcd for C9H6Br2O2: C 35.33; H 1.98. Found: C 35.63; H 2.14%.
X-Ray crystallography
Crystals suitable for X-ray diffraction analysis of rac-1 were grown from a saturated chloroform solution at 4 °C overnight. Crystals of rac-3 were obtained by slowly letting a layer of heptane diffuse into a saturated solution of rac-3 in chloroform and crystals of rac-2 were obtained by slow evaporation of a petroleum ether (40–60 °C) solution. Intensity data were collected at 293 K with an Oxford Diffraction Xcalibur 3 system using ω-scans and Mo-Kα (λ = 0.71073 Å).19CCD data were extracted and integrated using Crysalis RED.20 The structures were solved using direct methods and refined by full-matrix least-squares calculations on F2 using SHELXTL 5.1.1.21 Non-H atoms were refined with anisotropic displacement parameters. Hydrogen atoms were constrained to parent sites, using a riding model. Crystal data and details about data collection are given in Table 2. Three letter codes for the network topologies were taken from O'Keeffe et al.,22 and graph set symbols given according to Etter et al.23| rac - 1 | rac - 2 | rac - 3 | (+)-4 | |
|---|---|---|---|---|
| Chemical formula | C9H12O2 | C9H6Br2O2 | C9H8O2 | C11H10N2 |
| Formula weight | 152.19 | 305.96 | 148.16 | 170.21 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Orthorhombic |
| Space group | P21/n | P21/c | P21/c | P21212 |
| a/Å | 9.2834(7) | 10.3618(7) | 6.8088(3) | 7.3655(5) |
| b/Å | 8.5265(5) | 6.6849(5) | 7.4891(4) | 11.7786(9) |
| c/Å | 9.8621(7) | 14.6052(11) | 14.8396(8) | 5.5546(4) |
| β/° | 93.062(6) | 107.353(7) | 100.854(5) | 90 |
| Volume/Å3 | 779.52(9) | 965.62(12) | 743.16(7) | 481.89(6) |
| Z | 4 | 4 | 4 | 2 |
| ρ calc/Mg m−3 | 1.288 | 2.105 | 1.324 | 1.173 |
| μ/mm−1 | 0.090 | 8.356 | 0.093 | 0.071 |
| No. of reflections collected | 4831 | 5869 | 7381 | 3059 |
| No. of independent reflections | 1373 | 1678 | 2627 | 519 |
| θ range/° | 2.94–25.02 | 2.92–25.02 | 2.80–33.09 | 3.26–25.02 |
| R(int) | 0.0692 | 0.0502 | 0.0175 | 0.0238 |
| No. of parameters | 102 | 118 | 100 | 60 |
| Goodness-of-fit | 0.890 | 0.873 | 1.029 | 0.918 |
| R1 (I > 2σ(I)) | 0.0394 | 0.0710 | 0.0412 | 0.0310 |
| wR2 (all data) | 0.0633 | 0.2735 | 0.1337 | 0.0727 |
Acknowledgements
This work was supported by Nordforsk via the Nordic-Baltic Network in Crystal Engineering and Supramolecular Materials and the Swedish Research Council. We thank the Knut and Alice Wallenberg Foundation for funding the diffractometer and the Swedish Research Links program for a travel grant to LÖ.Notes and references
- N. S. Zefirov, Russ. Chem. Rev., 1975, 44, 196–211 Search PubMed; Russ. Chem. Rev., 1975, 44, 413 Search PubMed.
- (a) Y. Fukuyama, A. Kuwayama and H. Minami, Chem. Pharm. Bull., 1997, 45, 947–949 CAS; (b) Y. Fukuyama, A. Kuwayama and H. Minami, Phytochemistry, 1998, 49, 853–857 CrossRef CAS.
- R. Wang, H. Yan and H. C. Tang, Acta Pharmacol. Sin., 2006, 27, 1 Search PubMed.
- (a) A. T. Ung, R. Bishop, D. C. Craig, I. G. Dance and M. L. Scudder, Chem. Mater., 1994, 6, 1269 CrossRef CAS; (b) A. T. Ung, D. Gizachew, R. Bishop, M. L. Scudder, I. G. Dance and D. C. Craig, J. Am. Chem. Soc., 1995, 117, 8745 CrossRef CAS; (c) S. Stoncius, E. Butkus, A. Zilinskas, K. Larsson, L. Öhrström, U. Berg and K. Wärnmark, J. Org. Chem., 2004, 69, 5196 CrossRef CAS; (d) S. Stoncius, E. Orentas, E. Butkus, L. Öhrström, O. F. Wendt and K. Wärnmark, J. Am. Chem. Soc., 2006, 128, 8272 CrossRef CAS; (e) V. T. Nguyen, R. Bishop, D. C. Craig and M. L. Scudder, Supramol. Chem., 2001, 13, 103 CrossRef CAS.
- R. Bishop, Acc. Chem. Res., 2009, 42, 67 CrossRef CAS.
- (a) C.-J. Wallentin, E. Orentas, M. T. Johnson, E. Butkus, O. F. Wendt, L. Öhrström and K. Wärnmark, CrystEngComm, 2009, 11, 1837 RSC; (b) V. T. Nguyen, I. Y. H. Chan, R. Bishop, D. C. Craig and M. L. Scudder, New J. Chem., 2009, 33, 1736–1741 RSC.
- L. Öhrström and K. Larsson, Molecule-Based Materials: the structural Network Approach, Elsevier, Amsterdam, 2005 Search PubMed.
- R. Johansson, L. Öhrström and O. F. Wendt, Cryst. Growth Des., 2007, 7, 1974 CrossRef CAS.
- (a) M. A. Spackman and J. J. McKinnon, CrystEngComm, 2002, 378–392 Search PubMed; (b) J. J. McKinnon, M. A. Spackman and A. S. Mitchell, Acta Crystallogr., Sect. B: Struct. Sci., 2004, 60, 627–668 CrossRef; (c) M. A. Spackman and D. Jayatilaka, CrystEngComm, 2009, 11, 19 RSC.
- H. Quast, C. Becker, E. Geissler, K. Knoll, E. M. Peters, K. Peters and H. G. von Schnering, Liebigs Ann., 1994, 109 Search PubMed.
- E. Orentas, G. Bagdžiūnas, U. Berg, A. Zilinskas and E. Butkus, Eur. J. Org. Chem., 2007, 4251 CrossRef CAS.
- T. A. Klimova, M. M. Krayushkin, V. V. Sevostyanova, S. S. Novikov and N. F. Karpenko, Izv. Akad. Nauk SSSR, Ser. Khim., 1975, 1565–1969 CAS; Chem. Abstr., 1975, 83, 147411g Search PubMed.
- M. Ogasawara, A. Okada, K. Nakajima and T. Takahashi, Org. Lett., 2009, 11, 177 CrossRef CAS.
- H. Quast, M. Witzel,, E.-M. Peters,, K. Peters, and H. G. von Schnering,, Liebigs Ann., 1995, 725 Search PubMed.
- (a) F. F. Awwadi, R. D. Willett, K. A. Peterson and B. Twamley, Chem.–Eur. J., 2006, 12, 8952 CrossRef CAS; (b) M. Ghazzali, V. Langer, C. Lopes, A. Eriksson and L. Öhrström, New J. Chem., 2007, 31, 1777 RSC.
- (a) C. M. Reddy, M. T. Kirchner, R. C. Gundakaram, K. A. Padmanabhan and G. R. Desiraju, Chem.–Eur. J., 2006, 12, 2222 CrossRef CAS; (b) G. R. Desiraju and R. Parthasarathy, J. Am. Chem. Soc., 1989, 111, 8725 CrossRef CAS; (c) V. R. Pedireddi, D. S. Reddy, B. S. Goud, D. C. Craig, A. D. Rae, and G. R. Desiraju,, J. Chem. Soc., Perkin Trans. 2, 1994, 2353 RSC.
- F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380 CrossRef.
- (a) K. Kinbara, Y. Hashimoto, M. Sukegawa, H. Nohira and K. Saigo, J. Am. Chem. Soc., 1996, 118, 3441–3449 CrossRef CAS; (b) A. Johansson, M. Håkansson and S. Jagner, Chem.–Eur. J., 2005, 11, 5311–5318 CrossRef CAS.
- Crysalis CCD, Oxford Diffraction Ltd., Abingdon, Oxfordshire, UK, 2005 Search PubMed.
- Crysalis RED, Oxford Diffraction Ltd., Abingdon, Oxfordshire, UK, 2005 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112.
- (a) M. O'Keeffe, O. M. Yaghi and S. Ramsden, Reticular Chemistry Structure Resource, Australian National University Supercomputer Facility, 2011, http://rcsr.anu.edu.au/ Search PubMed; (b) M. O'Keeffe, M. A. Peskov, S. Ramsden and O. M. Yaghi, Acc. Chem. Res., 2008, 41, 1782–1789 CrossRef CAS.
- M. C. Etter, J. C. MacDonald and J. Bernstein, Acta Crystallogr., 1990, B46, 256–262 CAS.
Footnote |
| † CCDC reference numbers 768528–768531. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c1ce05673e |
| This journal is © The Royal Society of Chemistry 2012 |
