Green chemistry: solvent- and metal-free Prins cyclization. Application to sequential reactions†
Damien
Clarisse
,
Béatrice
Pelotier
,
Olivier
Piva
and
Fabienne
Fache
*
Université de Lyon, Université Lyon 1, ICBMS, équipe SURCOOF, CNRS, UMR 5246, Bât. Raulin, 43 Bd du 11 Nov. 1918, 69622 Villeurbanne cedex, France. E-mail: fache@univ-lyon1.fr; Fax: +33 472448136; Tel: +33472448521
First published on 21st October 2011
Abstract
Prins cyclization between a homoallylic alcohol and an aldehyde, promoted by trimethylsilyl halide, afforded 4-halo-tetrahydropyrans with good to excellent yields. Thanks to the absence of the solvent and metal, the THP thus obtained have been implicated without purification in several other reactions, in a sequential way, affording in particular new indole derivatives.
The tetrahydropyran ring (THP) is present in numerous natural products and therefore considerable efforts have been made to develop methods which give access to this structure,1 such as oxa-Michael reactions2 or radical cyclizations.3 Among them, the Prins cyclization has a major impact4 as confirmed by the continuous flow of articles which were published, and are still published in the literature on the subject.5–7 In the course of our study on the synthesis of THP-containing natural products,8,9 we examined the possibility of using the Prins approach. Several types of metals have been successfully applied as catalysts such as Fe,10 In,11Sc,12 salts. Ionic liquids derived from Lewis13 or Brönsted acids14 have also been developed. A proper choice of the metal salts or the presence of an additive such as a trimethylsilyl halide allowed the introduction of a halogen at the 4-position of the THP ring,15,16 leading to the formation of one C–C bond, one heteroatom–carbon bond and one halogen–carbon bond starting from homoallylic alcohols and aldehydes. Interested by all the concepts which allow the development of green organic chemistry, we especially turn our attention to sequential reactions which avoid intermediate purifications.17,18 One of the major problems to overcome is the choice of the solvent, which has to be efficient in different reactions, solventless conditions being the ideal case. There are only a few examples of solvent-free Prins reaction in the literature: Borah et al. used the SiO2p-TSA catalyst under solventless microwave irradiation conditions for the synthesis of 1,3-dioxanes19 and Dos Santos et al. reported the use of the same catalyst for the synthesis of THP odorants.20Prins reaction of styrenes with formalin was also described using HPA salts leading also to 1,3-dioxane derivatives,21 but to our knowledge there is no report of carbon–halogen bond formation under solvent free conditions. We thus examined the possibility of working under neat conditions and our first results showed that neither the solvent (usually CH2Cl2) nor the catalyst were necessary (Fig. 1).
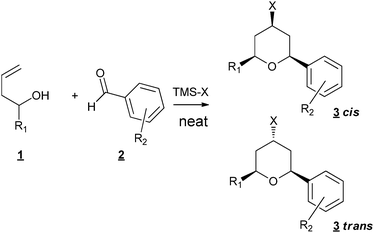 | ||
| Fig. 1 Solvent- and metal-free Prins cyclisation. | ||
In a typical procedure, 1 mmol of homoallylic alcohol, 1 mmol of aldehyde and 1.5 mmol of trimethylsilyl halide were stirred at room temperature for the required time (20 min–2 h). Column chromatography on silica allowed the isolation of the pure product with good isolated yields (Table 1).
| Entry | R1 | R2 | X | Product: isolated yield (%) | Cis/trans ratio |
|---|---|---|---|---|---|
| a 20 min reaction. b In CH2Cl2 (10 mL). c In this case, R2 = 3,4-methylenedioxy-6-nitrophenyl. | |||||
| 1 | H | i-Bu | Cl | 3a: 93 | 100/0 |
| 2 | H | i-Bu | Br | 3b: 50a | 100/0 |
| 3 | H | n-C5H11 | Cl | 3c: 76 | 100/0 |
| 4 | H | c-C6H11 | Cl | 3d: 90 | 100/0 |
| 5 | H | Ph | Cl | 3e: 0b | — |
| 6 | H | Ph | Cl | 3e: 83 | 100/0 |
| 7 | H | Ph | Br | 3f: 85a | 85/15 |
| 8 | H | Ph | I | 3g: 70a | 70/30 |
| 9 | H | p-NO2Ph | Cl | 3h: 84 | 76/24 |
| 10 | H | o-NO2Ph | Cl | 3i: 80 | 75/25 |
| 11 | H | p-MeOPh | Cl | 3j: 70 | 99/1 |
| 12 | H | R2c | Cl | 3k: 85 | 75/25 |
| 13 | H | 3-NO2,4-MePh | Cl | 3l: 78 | 91/9 |
| 14 | H | 2-NO2,6-ClPh | Cl | 3m: 88 | 55/45 |
| 15 | Ph | Ph | Cl | 3n: 81 | 89/11 |
| 16 | c-C6H11 | c-C6H11 | Cl | 3o: 87 | 80/20 |
3-Buten-1-ol combined with different aldehydes, aliphatic (Table 1, entries 1–4) or aromatic (Table 1, entries 6–14), gave good to excellent isolated yields of the corresponding THP derivatives. It is to be noticed that working under classical solvent conditions led only to recovered starting materials (Table 1, entry 5). In the case of aliphatic aldehydes, only the cis adducts were observed. With aromatic aldehydes, mixtures of stereoisomers were obtained, with a cis/trans ratio in favour of the cis isomers. Most of the time, the two isomers can be separated by column chromatography. The formation of the trans isomer is not usually reported under classical diluted conditions (most of the time CH2Cl2 as a solvent). Nevertheless, Rychnovsky et al.22 observed axial-selective Prins cyclization in the case of homoallylic ethers by solvolysis of bromoether intermediates when TMSBr was used, invoking the formation of an intimate ion pair followed by a possible proximal addition of the bromide, leading to the axial adduct. Our particularly concentrated conditions could be in favour of the formation of this intimate ion pair and thus explain this unusual formation.
Under our conditions, trimethylsilyl chloride, bromide or iodide can be indifferently used. Nevertheless, in the case of aliphatic aldehydes, polymerization of the starting aldehyde occurred when using TMSBr, explaining the relatively low yield of cyclization (Table 1, entry 2). Substituted homoallylic alcohols also led to good isolated yields of THP products (Table 1, entries 15 and 16). When R1 is different from R2, a mixture of products is obtained, due to an oxonia-Cope rearrangement prior to Prins cyclization.
Taking advantage of the absence of both the solvent and the catalyst, we engaged our halogeno-THP obtained via the Prins-cyclization in different other reactions, in a sequential way, without purification of the Prins intermediate. We first tested the Bartoli reaction, which allowed the formation of indoles from nitro aromatic compounds (Fig. 2).23
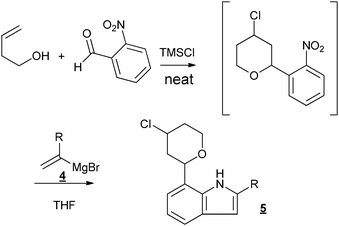 | ||
| Fig. 2 Sequential Prins cyclization–Bartoli reaction. | ||
Different new indole derivatives bearing a chloro-THP substituent were obtained with moderate yields (20–35% over the two steps) (Table 2). Considering the good isolated yields (around 80%) measured for the first step, the Prins cyclization, the yields for the Bartoli reaction can be estimated around 40%, which are usual yields for this reaction.24
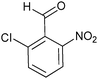
| Entry | Aldehyde 2 | R3 | Compound 5 | Isolated yield (%) (cis/trans) |
|---|---|---|---|---|
| 1 |
|
H |
|
30 (80/20) |
| 2 |
|
H |
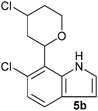
|
35 (26/74) |
| 3 |
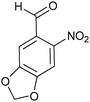
|
H |
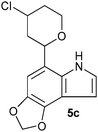
|
22 (60/40) |
| 4 |
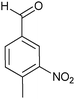
|
H |
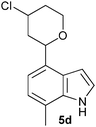
|
30 (80/20) |
| 5 |
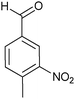
|
CH3 |
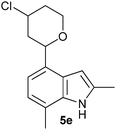
|
24 (70/30) |
Radical reduction and elimination reaction under basic conditions were also tested, leading to saturated or unsaturated THP, depending on the reaction conditions with good overall isolated yields (Fig. 3).
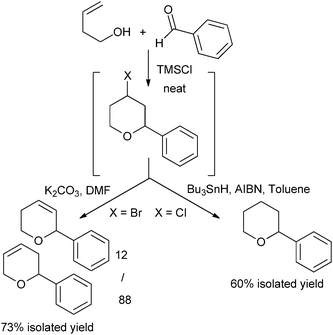 | ||
| Fig. 3 Sequential reactions. | ||
In conclusion, we proposed an efficient and mild green method to access 4-halo THP with good to excellent isolated yields. Both the cis and trans adducts can be obtained even if the reaction is mainly in favour of the cis isomer. Thanks to the absence of both the solvent and catalyst, the Prins cyclization product can be engaged in a further reaction without purification, as illustrated by three sequential examples described in this article: Prins cyclization followed by Bartoli reaction, by elimination reaction or by radical reduction. But the field of possibilities is open and we are currently working on other sequential reactions.
Acknowledgment is made to the Ministère Français de l'Education Supérieure et de la Recherche (D. Clarisse) and Université Lyon 1 for financial support.
Notes and references
- P. A. Clarke and S. Santos, Eur. J. Org. Chem., 2006, 2045 CrossRef CAS
; P. A. Clarke, Tetrahedron, 2011, 67, 4959 CrossRef
and references cited therein.
- C. F. Nising and S. Bräse, Chem. Soc. Rev., 2008, 37, 1218 RSC
; C. F. Nising and S. Bräse, Chem. Soc. Rev., 2012 10.1039/C1CS15167C
.
- E. Lee, Pure Appl. Chem., 2005, 77, 2073 CrossRef CAS
.
- C. Olier, M. Kaafarani, S. Gastaldi and M. P. Bertrand, Tetrahedron, 2010, 66, 413 CrossRef CAS
.
- J. S. Yadav, M. R. Pattanayak, P. P. Das and D. K. Mohapatra, Org. Lett., 2011, 13, 1710 CrossRef CAS
.
- E. A. Crane, T. P. Zabawa, R. L. Farmer and K. A. Scheidt, Angew. Chem., Int. Ed., 2011, 50, 9112 CrossRef CAS
; J. Wang, E. A. Crane and K. A. Scheidt, Org. Lett., 2011, 13, 3086 CrossRef
.
- S. N. Chavre, H. Choo, J. K. Lee, A. N. Pae, Y. Kim and Y. S. Cho, J. Org. Chem., 2008, 73, 7467 CrossRef CAS
; K. Tadpetch and S. D. Rychnovsky, Org. Lett., 2008, 10, 4839 CrossRef
.
- E. Bourcet, F. Fache and O. Piva, Tetrahedron, 2010, 66, 1319 CrossRef CAS
; E. Bourcet, F. Fache and O. Piva, Eur. J. Org. Chem., 2010, 4075 CrossRef
.
- M. A. Hiebel, B. Pelotier and O. Piva, Tetrahedron, 2007, 63, 7874 CrossRef CAS
.
- P. O. Miranda, D. D. Diaz, J. I. Padron, J. Bermejo and V. S. Martin, Org. Lett., 2003, 5, 1979 CrossRef CAS
.
- X. F. Yang, J. T. Mague and C. J. Li, J. Org. Chem., 2001, 66, 739 CrossRef CAS
; F. Liu and T.-P. Loh, Org. Lett., 2007, 9, 2063 CrossRef
.
- J. S. Yadav, B. V. S. Reddy, A. V. Ganesh and G. G. K. S. Narayana Kumar, Tetrahedron Lett., 2010, 51, 2963 CrossRef CAS
.
- J. S. Yadav, B. V. S. Reddy, M. D. Reddy, N. Niranjan and A. R. Prasad, Eur. J. Org. Chem., 2003, 1779 CrossRef CAS
.
- W. Wang, L. Shao, W. Cheng, J. Yang and M. He, Catal. Commun., 2008, 9, 337 CrossRef CAS
.
- P. O. Miranda, R. M. Carballo, V. S. Martin and J. I. Padron, Org. Lett., 2009, 11, 357 CrossRef CAS
.
- G. Sabitha, K. B. Reddy, M. Bhikshapathi and J. S. Yadav, Tetrahedron Lett., 2006, 47, 2807 CrossRef CAS
.
- M. A. Hiebel, B. Pelotier, P. Goekjian and O. Piva, Eur. J. Org. Chem., 2008, 713 CrossRef CAS
.
- F. Batt, C. Gozzi and F. Fache, Chem. Commun., 2008, 5830 RSC
.
- K. J. Borah, M. Phukan and R. Borah, Synth. Commun., 2008, 38, 3082 CrossRef CAS
.
- A. Macedo, E. P. Wendler, A. A. Dos Santos, J. Zukerman-Schpector and E. R. T. Tiekink, J. Braz. Chem. Soc., 2010, 21, 1563 CrossRef CAS
.
- W. Zhang, Y. Leng, P. Zhao, J. Wang, D. Zhu and J. Huang, Green Chem., 2011, 13, 832 RSC
.
- R. Jasti, J. Vitale and S. D. Rychnovsky, J. Am. Chem. Soc., 2004, 126, 9904 CrossRef CAS
.
- M. Bosco, R. Dalpozzo, G. Bartoli, G. Palmieri and M. Petrini, J. Chem. Soc., Perkin Trans. 2, 1991, 657 RSC
.
- L. F. Silva, Jr and M. V. Craveiro, Org. Lett., 2008, 10, 5417 CrossRef CAS
; A. Dobbs, J. Org. Chem., 2001, 66, 638 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available: Complete synthetic procedures, characterization data, 1H and 13C NMR spectra of all new compounds. See DOI: 10.1039/c1cc16501a |
| This journal is © The Royal Society of Chemistry 2012 |
