Metal-assisted salphen organic frameworks (MaSOFs) with high surface areas and narrow pore-size distribution†
Michael
Mastalerz
*a,
Hans-Jochen S.
Hauswald
b and
Raphael
Stoll
b
aInstitute of Organic Chemistry II & Advanced Materials, Ulm University, 89081 Ulm, Germany. E-mail: michael.mastalerz@uni-ulm.de; Fax: +49 731-50-22840; Tel: +49 731-50-22855
bRUBiospek, Ruhr-University of Bochum, 44780 Bochum, Germany
First published on 3rd November 2011
Abstract
The one-pot three component synthesis of metal containing microporous organic polymers with high BET surface areas is presented. The metal salphen units were built during the formation of the porous polymers. Selective gas adsorption depending on the metal ions is discussed.
Since the introduction of covalent organic frameworks (COFs),1 porous organic polymers2 are recognized in a broader sense as a new alternative class of porous materials besides metal–organic frameworks (MOFs)3 or zeolites.4 These porous organic compounds are usually synthesized from one or two components either in reversible1,5,6 or irreversible reactions.7–11 High surface areas were achieved with reversible as well as irreversible methods.1,8
Till date, metal complexes of those porous organic polymers are rare and are either created by the introduction of metal ions via post functionalization,12 or by the use of predefined metal phthalocyanine or porphyrine building blocks as reactants.13 Nevertheless, “metal-doped” porous organic frameworks become more and more into the focus of interest, because free metal sites evidently influence gas adsorption in a positive manner.14 Furthermore, those metal sites can act as catalytic centers.15
To the best of our knowledge, there is only one type of porous covalent organic framework described, where the metal ions are incorporated into the network through the polymerization of the bis(phthalodinitriles), having a template as well as a catalytic effect on the formation of the covalent bonded network.16 These porous networks have BET surface areas between 489 m2 g−1 and 895 m2 g−1. It was also demonstrated that those networks had catalytic properties.17
Salphens and salens are versatile tetradentate ligands and their corresponding metal complexes are well-known for their excellent catalytic activities for a number of transformations.18 There are a few examples of porous materials containing salphen metal units within the pores. For instance, salens are introduced as linkers for the construction of zinc-MOFs,19 which are catalytically active.
We envisioned the introduction of metal salphen units into the porous material by constructing the frameworks and the metal salphen in the same synthetic step. Therefore, we exploited the well-known Schiff base reaction of salicylaldehydes20 (e.g.1) with o-phenylene diamine 2 in the presence of metal acetate to give the corresponding metal salphens 3a or 3b (Scheme 1), which can be seen as sectional parts of our polymeric porous metal-assisted salphen organic frameworks (MaSOFs, Scheme 2).
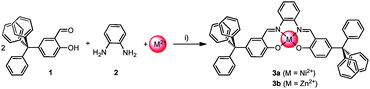 | ||
| Scheme 1 One-pot three component reaction of salicylaldehyde 1, o-phenylene diamine 2 and metal(II) ions to metal salphen complexes 3a and 3b, respectively. (i) Ethanol, 95 °C, 1d; yields: 82% (3a); 69% (3b). | ||
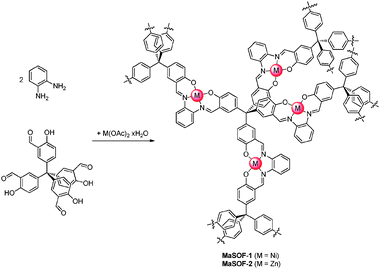 | ||
| Scheme 2 Synthesis of metal-assisted salphen covalent organic frameworks (MaSOFs). | ||
We started to investigate the reaction of rigid tetrahedral tetrakis salicylaldehyde 420 with o-phenylene diamine 2 and nickel acetate tetrahydrate, to give MaSOF-1. First we tested various solvents for the reaction such as ethanol, acetonitrile, N,N-dimethylformamide (DMF) and toluene at elevated temperatures. Exclusively in DMF at 95 °C a voluminous reddish brown homogeneous precipitate was formed in 98% yield. Thermogravimetric analysis (TGA) of the freshly prepared, air-dried compound in a nitrogen atmosphere shows three major steps of mass loss: at low temperatures (<80 °C) the compound loses 10% of the mass, suggesting that this is loosely bound solvent from the outer surface of the particles; second step is assigned to the loss of solvent bound inside the pores (15% at 270 °C); and third step between 270 and 400 °C (4% mass loss), which corresponds probably to the removal of solvent molecules bound on the microporous surface through weak interactions with the generated nickel salphen sites. A TGA measurement of the same material in an oxygen atmosphere showed a very sharp mass reduction at 300 °C, remaining 16.8% of mass, even after further heating to 1000 °C. Assuming that in an oxygen atmosphere exclusively nickel(II)oxide remains, a nickel content of 13.2% of the as-synthesized material can be calculated. In comparison, the activated material (see below) remains 20.4% of mass, which corresponds to 15.8% of nickel content. This result fits the expected value of 15.6%, calculated from the ideal molecular formula of MaSOF-1. In the FT-IR of the as-synthesized, air-dried material a sharp peak at 1612 cm−1 is observed, which can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-stretching in nickel salphen compounds. No band corresponding to a C
N-stretching in nickel salphen compounds. No band corresponding to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the precursor salicylaldehyde at 1658 cm−1 was detected any more, revealing a full conversion of the salicylaldehyde functional groups (see Fig. 1). Additionally, the stretching band of the DMF carbonyl bond can clearly be assigned at 1666 cm−1. We exchanged the DMF by washing the material extensively with ethanol and “activated” the material at 200 °C in vacuum for several hours. Besides the results of FT-IR spectroscopy (the band from DMF is not detected any more, see Fig. 1c), the 13C MAS NMR spectrum revealed that the material consists certain expected characteristic signals, referred to the 13C NMR spectrum of model compound 3a. For instance, a peak at δ = 61 ppm can be assigned to the central quarternary carbon nuclei of the tetraphenylmethane nodes. Furthermore, a peak of the imine carbon resonates at δ = 166 ppm, which is very similar to the signals (172 ppm) of the model compound 3a in DMSO-d6 solution. Additionally, no peaks at approx. 200 pm are recognized, which is again an evidence for the full conversion of all aldehyde moieties.
O stretching of the precursor salicylaldehyde at 1658 cm−1 was detected any more, revealing a full conversion of the salicylaldehyde functional groups (see Fig. 1). Additionally, the stretching band of the DMF carbonyl bond can clearly be assigned at 1666 cm−1. We exchanged the DMF by washing the material extensively with ethanol and “activated” the material at 200 °C in vacuum for several hours. Besides the results of FT-IR spectroscopy (the band from DMF is not detected any more, see Fig. 1c), the 13C MAS NMR spectrum revealed that the material consists certain expected characteristic signals, referred to the 13C NMR spectrum of model compound 3a. For instance, a peak at δ = 61 ppm can be assigned to the central quarternary carbon nuclei of the tetraphenylmethane nodes. Furthermore, a peak of the imine carbon resonates at δ = 166 ppm, which is very similar to the signals (172 ppm) of the model compound 3a in DMSO-d6 solution. Additionally, no peaks at approx. 200 pm are recognized, which is again an evidence for the full conversion of all aldehyde moieties.
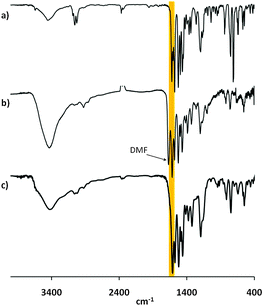 | ||
Fig. 1 Comparison of FT-IR spectra of (a) model compound 3a, (b) MaSOF-1, as-synthesized and (c) MaSOF-1, “activated”. The orange bar marks the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching bonds. N stretching bonds. | ||
To investigate the porosity of the material, we measured the nitrogen sorption of a sample, dried in vacuum at 200 °C for 5 h (Fig. 2). The isotherm can be classified as a type I isotherm, which is typical for a microporous material.21 The isotherm shows no hysteresis between adsorption and desorption, providing the information that the network is rather rigid than flexible. A total amount of 398 cm3 g−1nitrogen was adsorbed at P/P0 = 0.95. The measured surface areas are 647 m2 g−1 (BET-model) and 741 m2 g−1 (Langmuir-model), respectively. Although the powder-X-ray diffraction (PXRD) shows no defined signals—suggesting that the material is amorphous—the compound shows a narrow pore-size distribution with maxima at 5.0 Å (Horvath–Kawazoe-model) or 4.2 Å (NLDFT-model), which were calculated from adsorption at low P/P0 (see Fig. 2, inset).
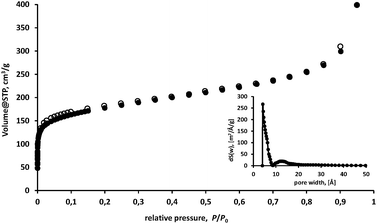 | ||
| Fig. 2 Nitrogen sorption measurement of MaSOF-1 at 77.35 K. Filled circles (adsorption), open circles (desorption). The inset frame depicts the NL-DFT derived pore-size distribution. | ||
The metal ion seems to play a crucial role in the formation of the porous solid: in the absence of nickel acetate no precipitate is formed at all under similar conditions (same concentration of reactants, 95 °C reaction temperature). The solution stays clear even after several days. Also the addition of trifluoroacetic acid (TFA) does not induce any precipitation, again suggesting that nickel(II) ions probably do not act as Lewis acidic centers to accelerate the imine bond formation, but rather as a template. We assume that the formation of the metal salphen is irreversible (or less reversible), which would further explain the amorphous nature of the material.
We also tested to incorporate zinc(II) instead of nickel(II) ions during the framework formation by using zinc(II) acetate dihydrate as reagent. After completion of the reaction a bright yellow powder (MaSOF-2) was formed. Similar to MaSOF-1, the FT-IR spectrum of the as-synthesized material shows a characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching band at 1615 cm−1, which is comparable to those of typical zinc-salphen complexes.20 Again, remaining DMF can be detected as a peak at ca. 1660 cm−1. This peak disappears when the compound was washed with ethanol and dried in vacuum at 150 °C for 18 hours. The measured BET surface area was 630 m2 g−1, which is similar to the surface area of MaSOF-1. The compound shows a narrow pore-size distribution with maxima at 5.5 Å (HK-model) or 4.2 Å (NL-DFT-model). Again, FT-IR and 13C MAS NMR spectroscopy revealed a full conversion of all aldehyde moieties.
N stretching band at 1615 cm−1, which is comparable to those of typical zinc-salphen complexes.20 Again, remaining DMF can be detected as a peak at ca. 1660 cm−1. This peak disappears when the compound was washed with ethanol and dried in vacuum at 150 °C for 18 hours. The measured BET surface area was 630 m2 g−1, which is similar to the surface area of MaSOF-1. The compound shows a narrow pore-size distribution with maxima at 5.5 Å (HK-model) or 4.2 Å (NL-DFT-model). Again, FT-IR and 13C MAS NMR spectroscopy revealed a full conversion of all aldehyde moieties.
The relative small pore-sizes of 4.2 to 5.5 Å and the presence of charged metal centers, which, in the ideal case, should contain accessible, free coordination sites, forced us to test gas adsorption of various analytes (H2, CO2, CH4) that perhaps interact with the metal centers. MaSOF-1, with nickel ions, adsorbs at 77 K and 1 bar 4.46 mmol g−1 H2 and 3.49 mmol g−1 at 87 K, which is equal to 0.90 wt% and 0.70 wt%, respectively (Fig. 3). The heat of adsorption via the Clausius–Clapeyron equation is estimated to be −6.8 kJ mol−1.22MaSOF-2 with zinc ions adsorbs similar amounts under the same conditions (1 bar, 77 K: 4.14 mmol g−1, 0.84 wt%; at 87 K: 3.27 mmol g−1, 0.66 wt%). Here, the calculated heat of adsorption is −7.8 kJ mol−1, about 1 kJ mol−1 lower than for MaSOF-1. This suggests that the nature of the metal ion influences the adsorption properties. It is worth mentioning that the heat of adsorption for both materials decreases with increasing surface coverage (see Fig. S25 in the ESI†), which, again, can be seen as a hint that first the metal interacts with the adsorbent. Methane is adsorbed at 273 K and 1 bar by both MaSCOFs in lower amounts: 0.43 mmol g−1 (0.69 wt%) for MaSOF-1 and 0.61 mmol g−1 (0.98 wt%) for MaSOF-2. For the adsorption of CO2 at 273 K and 1 bar, a bigger difference can be detected: MaSOF-1 adsorbs 1.83 mmol g−1 (8.1 wt%), whereas MaSOF-2 takes up more CO2 (2.23 mmol g−1, 9.8 wt%). This is a hint that the more Lewis acidic zinc centers stronger interact with CO2 than the weaker Lewis acidic nickel centers.22,23
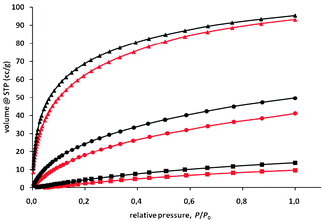 | ||
| Fig. 3 Adsorption of H2 at 77 K and P0 = 1 bar (triangles), CO2 (circles) and methane (squares) at 273 K and P0 = 1 bar of MaSOF-1 (red) and MaSOF-2 (black). | ||
In conclusion, we described a one-pot three component reaction of metal salphen containing porous organic polymers, with high BET surface areas, narrow pore-size distribution and selective gas adsorption properties. To the best of our knowledge, this is the first three-component synthesis of a metal-functionalized porous organic framework. Several MaSOFs, with a broad variety of incorporated metal ions, will be synthesized to study the influence of charged, free metal sites in similar porous networks.24 Furthermore, homochiral MaSOFs will be synthesized, to combine the extraordinary catalytic properties of metal salens18 with porous materials in heterogeneous catalysis.15,25
The authors like to thank the “Deutsche Forschungsgemeinschaft” and the “Fond der Chemischen Industrie” for financial support. M. M. likes to thank C. Egger for nitrogen sorption, S. Blessing for PXRD, and G. Weber (all Ulm University) for TGA measurements.
Notes and references
- (a) A. P. Côté, A. I. Benin, N. W. Ockig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166 CrossRef; (b) H. M. El-Kaderi, J. R. Hunt, J. L. Mendoza-Cortés, A. P. Côté, R. E. Taylor, M. O'Keeffe and O. M. Yaghi, Science, 2007, 316, 268 CrossRef CAS; (c) R. W. Tilford, R. Gemmil, H.-C. zur Loye and J. J. Lavigne, Chem. Mater., 2006, 18, 5296 CrossRef CAS.
- (a) N. B. McKeown and P. M. Budd, Macromolecules, 2010, 43, 5163 CrossRef CAS; (b) J.-X. Xiang and A. I. Cooper, Top. Curr. Chem., 2010, 293, 1 Search PubMed; (c) A. Thomas, Angew. Chem., Int. Ed., 2010, 49, 8328 CrossRef CAS; (d) M. Mastalerz, Angew. Chem., Int. Ed., 2008, 47, 445 CrossRef CAS.
- (a) S. Kitagawa, R. Kitaura and S.-i. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS; (b) G. Férey, Chem. Soc. Rev., 2008, 37, 191 RSC.
- Introduction to Zeolite Science and Practice, ed. H. Ceijka, H. van Bekkum, A. Corma and F. Schüth, Elsevier, Amsterdam, Netherlands, 3rd edn, 2007 Search PubMed.
- (a) F. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klöck, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 4570 CrossRef CAS; (b) M. G. Schwab, B. Fassbender, H. W. Spiess, A. Thomas, X. Feng and K. Müllen, J. Am. Chem. Soc., 2009, 131, 7216 CrossRef CAS; (c) P. Pandey, A. P. Katsoulidis, I. Eryazici, Y. Wu, M. G. Kanatzidis and S. T. Nguyen, Chem. Mater., 2010, 22, 4974 CrossRef CAS.
- (a) P. Kuhn, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2008, 47, 3450 CrossRef CAS; (b) P. Kuhn, A. Forget, D. Su, A. Thomas and M. Antonietti, J. Am. Chem. Soc., 2008, 130, 13333 CrossRef CAS; (c) P. Kuhn, A. Thomas and M. Antonietti, Macromolecules, 2009, 42, 4426 CrossRef; (d) H. Ren, T. Ben, E. S. Wang, X. F. Jing, M. Xue, B. B. Liu, Y. Cui, S. L. Qiu and G. S. Zhu, Chem. Commun., 2009, 291 Search PubMed.
- (a) J.-X. Jiang, F. Su, A. Trewin, C. D. Wood, N. L. Campbell, H. Niu, C. Dickinson, A. Y. Ganin, M. J. Rosseinsky, Y. Z. Khimyak and A. I. Cooper, Angew. Chem., Int. Ed., 2007, 46, 8574 CrossRef CAS; (b) J.-X. Jiang, F. Su, A. Trewin, C. D. Wood, H. Niu, J. T. A. Jones, Y. Z. Khimyak and A. I. Cooper, J. Am. Chem. Soc., 2008, 130, 7710 CrossRef CAS; (c) R. Dawson, A. Laybourn, R. Clowes, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Macromolecules, 2009, 42, 8809 CrossRef CAS.
- (a) T. Ben, H. Rao, S. Ma, D. Cao, J. Lan, X. Jing, W. Wang, J. Xu, F. Deng, J. M. Simmons, S. Qui and G. Zhu, Angew. Chem., Int. Ed., 2009, 48, 9457 CrossRef CAS; (b) J. Schmidt, M. Werner and A. Thomas, Macromolecules, 2009, 42, 4426 CrossRef CAS.
- N. B. McKeown, B. Gahnem, K. J. Msayib, P. M. Budd, C. E. Tattershall, K. Mahmood, S. Tan, D. Book, H. W. Langmi and A. Walton, Angew. Chem., Int. Ed., 2006, 45, 1804 CrossRef CAS.
- P. Pandey, O. K. Farha, A. M. Spokoyny, C. A. Mirkin, M. G. Kanatzidis, J. T. Hupp and S. T. Nguyen, J. Mater. Chem., 2011, 21, 1700 RSC.
- B. Li, R. Gong, W. Wang, X. Huang, W. Zhang, H. Li, C. Hu and B. Tan, Macromolecules, 2011, 44 Search PubMed , dx.doi.org/10.1021/ma200630s.
- (a) R. Palkovits, M. Antonietti, P. Kuhn, A. Thomas and F. Schüth, Angew. Chem., Int. Ed., 2009, 48, 6913 CrossRef; (b) P. M. Budd, B. Ghanem, K. Msayib, N. B. McKeown and C. Tattershall, J. Mater. Chem., 2003, 13, 2721 RSC; (c) L. Ma, M. Wanderley and W. Lin, ACS Catal., 2011, 1, 691 CrossRef CAS.
- L. Chen, Y. Yang and D. Jiang, J. Am. Chem. Soc., 2010, 132, 9138 CrossRef CAS.
- For a recent example, see: K. Gedrich, I. Senkovska, N. Klein, U. Stoeck, A. Henschel, M. R. Lohe, I. A. Baburin, U. Mueller and S. Kaskel, Angew. Chem., Int. Ed., 2010, 49, 8489 CrossRef CAS.
- P. Kaur, J. T. Hupp and S. T. Nguyen, ACS Catal., 2011, 1, 819 CrossRef CAS.
- N. B. McKeown, S. Makhseed and P. M. Budd, Chem. Commun., 2002, 2780 RSC.
- H. J. Mackintosh, P. M. Budd and N. B. McKeown, J. Mater. Chem., 2008, 18, 573 RSC.
- P. G. Cozzi, Chem. Soc. Rev., 2004, 33, 410 RSC.
- C. Wang, M. Zheng and W. Lin, J. Phys. Chem. Lett., 2011, 2, 1701 CrossRef CAS.
- M. Mastalerz and I. M. Oppel, Eur. J. Org. Chem., 2011, 5971 CrossRef CAS.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquèrol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- B. Panella, M. Hirscher, H. Pütter and U. Müller, Adv. Funct. Mater., 2006, 16, 520 CrossRef CAS.
- M. M. Belmonte, S. J. Wezenberg, R. M. Haak, D. Anselmo, E. C. Escudero-Adán, J. Benet-Buchholz and A. W. Kleij, Dalton Trans., 2010, 39, 4541 RSC.
- B. Schmitz, U. Müller, N. Trukhan, M. Schubert, G. Férey and M. Hirscher, ChemPhysChem, 2008, 9, 2181 CrossRef CAS.
- A. Zulauf, M. Mellah, X. Hong and E. Schulz, Dalton Trans., 2010, 39, 6911 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details and analytical data of 3a, 3b, MaSOF-1 and MaSOF-2. See DOI: 10.1039/c1cc14805b |
| This journal is © The Royal Society of Chemistry 2012 |
