Layer-by-layer evolution and a hysteretic single-crystal to single-crystal transformation cycle of a flexible pillared-layer open framework†
Xiao-Feng
Wang
ab,
Yu
Wang
a,
Yue-Biao
Zhang
a,
Wei
Xue
a,
Jie-Peng
Zhang
*a and
Xiao-Ming
Chen
a
aMOE Key Laboratory of Bioinorganic and Synthetic Chemistry, State Key Laboratory of Optoelectronic Materials and Technologies, School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou 510275, China. E-mail: zhangjp7@mail.sysu.edu.cn
bSchool of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China
First published on 9th November 2011
Abstract
Two hypothetical crystal-growth intermediates of a new pillared-layer framework have been isolated. The seemingly rigid pillared-layer framework also undergoes temperature-induced, time-dependent, hysteretic framework distortion.
Porous coordination polymers (PCPs) have received mushrooming attention owing to their intriguing properties,1 especially the framework flexibility, where the host framework structure can change in response to an external stimulus.2 Many types of reversible and irreversible structural changes, such as shrinkage and expansion,3 distortion,4 sliding,5 ligand exchange,6 and even catenation rearrangement,7 have been reported. However, the exploration is generally based only on measurements of the crystal structures of the initial and final states. The kinetics or detailed pathway of the structural change is still rarely investigated.8
Unlike the controllable molecule-level step-by-step organic syntheses, supramolecular PCPs were usually obtained by one-pot reactions, in which the assembly processes or mechanisms are extremely difficult to be experimentally observed and clarified due to the current technological limitation. A few types of PCPs have been proposed to be assembled in a step-by-step approach.9 For example, the secondary building unit (SBU) strategy adopts organic ligands to link the preformed discrete clusters to specific topological nets (net-based approach). However, the formation of discrete SBUs has been scarcely monitored during the crystal-growth process.10 Pillared-layer PCPs (Scheme 1) are regarded as another type of structure constructed by multi-step assembly, in which the two-dimensional (2D) layers are first built from 0D molecular building blocks, which are further pillared by exo-bidentate ligands into the final 3D architecture. Although the pillared-layer approach has been successfully used for the construction of PCPs,11 the initially formed (or non-pillared) 2D layer and a subsequent layer-by-layer growth process have not been directly observed so far. Herein, we report the interesting self-assembly and properties of a flexible pillared-layer PCP, including the identification of two 2D intermediate non-pillared and pillared structures and an intriguing single-crystal to single-crystal (SCSC) structural transformation cycle with a temperature hysteresis.
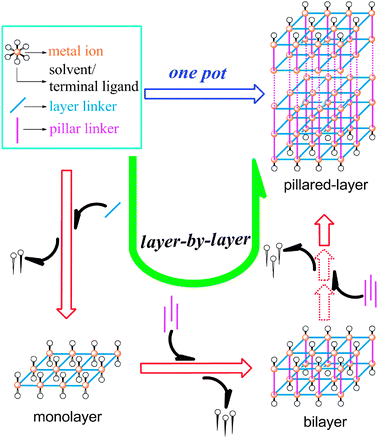 | ||
| Scheme 1 Principles of the self-assembly of a pillared-layer structure. | ||
In the reaction of Cd(NO3)2, 2-nitro-1,4-benzene-dicarboxylic acid (H2nbdc), and pyrazine (pyz) in N,N-dimethylformamide (DMF) at room temperature, crystals of [Cd2(nbdc)2(pyz)2]·4DMF (3, MCF-33) started to deposit after ca. 3 weeks. Interestingly, in the initial period a few crystals of [Cd2(nbdc)2(DMF)4]·2DMF (1, ca. 5–7 days) and [Cd2(nbdc)2(pyz)(DMF)2]·3DMF (2, ca. 2 weeks) were isolated and have been characterized by single-crystal diffraction. Parallel experiments showed that 1 and 2 only occasionally appeared in a few cases among many trials, indicating that their crystallization may be affected by some imperceptible perturbations. Powder X-ray diffraction (PXRD) patterns of the final products did not exhibit observable signals of 1 or 2. When higher reactant concentrations were used, 3 could be obtained in a shorter time without the observation of 1 or 2. These facts imply that both 1 and 2 should be unstable intermediates and can be easily transformed into 3, or should have extremely low yields and can be hardly detected from large amounts of 3.
Crystal structure of 1 exhibits a 2D framework containing Cd2(RCOO)4(DMF)4 clusters as SBUs (Fig. 1).12 Each Cd atom adopts a distorted pentagonal-bipyramidal coordination environment, in which the equatorial and apical positions are occupied by five oxygen atoms from three carboxylate groups and two oxygen atoms from two terminal DMF ligands, respectively. The two adjacent Cd atoms are bridged by two μ-O atoms from two carboxylates into a SBU. These SBUs are extended through the nbdc2− ligands as linkers into a grid-like (sql topology) Cd(nbdc) layer with DMF molecules anchored on both sides.
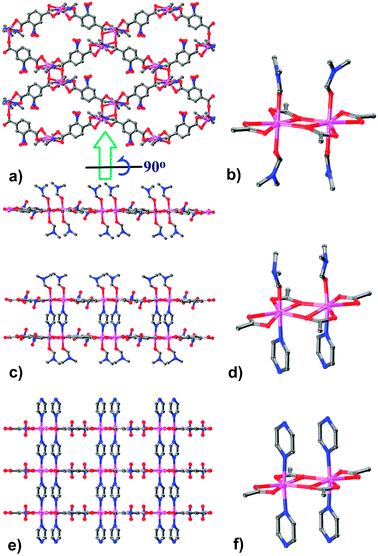 | ||
| Fig. 1 Framework and local coordination structures of 1–3. (a) Top and side views of the monolayer 1, (b) the Cd2(RCOO)4(DMF)4 SBU in 1, (c) side view of the bilayer 2, (d) the Cd2(RCOO)4(pyz)(DMF)2 SBU in 2, (e) side view of the pillared-layer open framework 3, (f) the Cd2(RCOO)4(pyz)2 SBU in 3. | ||
Crystal structure of 2 reveals dinuclear SBUs and 2D layers similar to those in 1. However, two of the four terminal DMF molecules, coordinated on the same side of the SBU, are replaced by two pyz ligands. Consequently, a pair of adjacent Cd(nbdc) layers are connected into a bilayer with the pyz ligands as pillars. The remaining terminal DMF molecules are anchored on the two outer sides of this bilayer.
Although 3 also contains similar dinuclear SBUs and 2D layers found in 1 and 2, all the terminal DMF molecules are now replaced by bridging pyz ligands, resulting in a formal pillared-layer 3D framework with pcu topology. Consequently, 3 features 3D intersecting channels with 46% void, which are filled by solvated DMF molecules.
The structures of 1–3 may represent the stepwise evolution of 3. It can be rationally speculated that, the Cd(II) ions in solution are originally coordinated by monodentate solvent molecules (DMF), which are rather labile compared with the bridging ligands, especially the anionic nbdc2−. Therefore, the DMF molecules are first substituted by the dicarboxylates, which linked the Cd(II) ions to a neutral Cd(nbdc) monolayer as observed in 1. Nevertheless, the monolayer should be still unstable when a strong ligand pyz is present. The terminal DMF molecules are then replaced by pyz ligands, which can serve as a template for the growth of the second Cd(nbdc) layer, leading to the formation of 2. Repeating this procedure would sequentially increase the number of layers and finally lead to the macroscopic crystals of 3. It should be noted that, when the number of layers (n) is small (e.g., n = 1, 2, 3, etc.), the corresponding species are too thin to be observed, and they can transform into the final 3D structure very fast in the presence of sufficient pyz ligands.
Interestingly, although 3 is constructed by rigid organic ligands, temperature-induced framework flexibility was observed by SCSC transformations (Fig. 2). When 3 was cooled from 293 to 150 K, the lattice changed from orthorhombic (3) to monoclinic (3′, β = 99.132(4)°) with a significant change of an axial angle (rather than in the axial lengths), which reveals a drastic framework distortion. Actually, the Cd(nbdc) layers are not distorted, while the pillars become slanted in 3′. More amazingly, when the crystal was warmed up back to 293 K, another monoclinic phase (3′′, β = 92.650(5)°) with a less slanted orientation of the pillars than for 3′, rather than the original orthorhombic one, was captured. Therefore, 3′′ can be regarded as an intermediate between 3′ and 3. Actually, 3′′ is unstable, being implied by the poorer diffraction data, and it can be completely transformed back into 3 after more than 3 hours without any further treatment. The temperature-induced (3 to 3′ to 3′′) and time-dependent (3′′ to 3) SCSC transformation cycle represents an unprecedented structural dynamic phenomenon.13 The hysteretic behavior indicates that 3 has multiple metastable structural conformations and the transformation is slow. Attempt to further monitor the temperature- and time-dependent unit-cell parameters of 3 was not successful so far, because of the easy cracking behavior of single crystals of flexible PCPs during the heating/cooling process.14
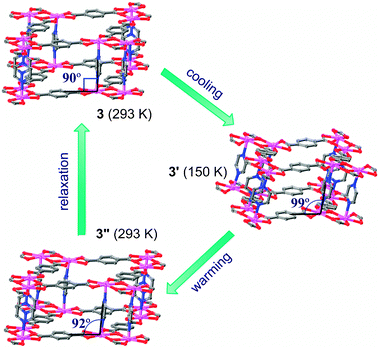 | ||
| Fig. 2 The temperature-induced, hysteretic SCSC structural transformation of 3. | ||
The framework thermal stability and porosity of 3 have been characterized by thermogravimetry analysis (TGA), PXRD, and N2 sorption isotherm measurements. TGA of the as-synthesized sample showed gradual loss of 26.5 wt% below 220 °C which matches the expected value of 26.7 wt% for the removal of four DMF molecules per Cd2 formula unit. The PXRD pattern of the degassed sample is basically identical to that of the as-synthesized one, which is quite unusual because lowering temperature can induce a significant framework distortion. The N2 sorption isotherm at 77 K (Fig. 3) is type I, with apparent Langmuir surface area of 590 m2 g−1.
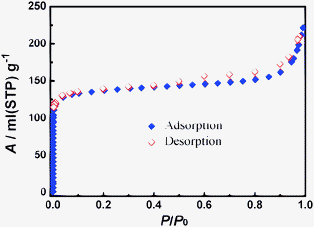 | ||
| Fig. 3 The N2 gas sorption isotherms of 3 at 77 K. | ||
In summary, the layer-by-layer self-assembly process of a new pillared-layer PCP has been demonstrated by capturing of two intermediates. In contrast with hydro(solvo)thermal syntheses, the mild conditions in conventional solution syntheses with lower temperature and concentration may substantially slow down the kinetics of assembly of PCPs and provide a possibility to seize the intermediates in the crystal growth course. Moreover, a unique temperature-induced, hysteretic framework dynamic behavior has been also observed. These findings should be instructive for understanding the self-assembly and properties of PCPs.
This work was supported by the ‘973 Project’ (2012CB821706) and the NSFC (20821001 & 21001120).
Notes and references
- (a) D. Farrusseng, S. Aguado and C. Pinel, Angew. Chem., Int. Ed., 2009, 48, 7502 CrossRef CAS; (b) B. Chen, X. Zhao, A. Putkham, K. Hong, E. B. Lobkovsky, E. J. Hurtad, A. J. Fletcher and K. M. Thomas, J. Am. Chem. Soc., 2008, 130, 6411 CrossRef CAS; (c) Y.-B. Zhang, W.-X. Zhang, F.-Y. Feng, J.-P. Zhang and X.-M. Chen, Angew. Chem., Int. Ed., 2009, 48, 5287 CrossRef CAS; (d) S. K. Henninger, H. A. Habib and C. Janiak, J. Am. Chem. Soc., 2009, 131, 2776 CrossRef CAS; (e) K. C. Stylianou, R. Heck, S. Y. Chong, J. Bacsa, J. T. A. Jones, Y. Z. Khimyak, D. Bradshaw and M. J. Rosseinsky, J. Am. Chem. Soc., 2010, 132, 4119 CrossRef CAS; (f) D. Yuan, D. Zhao, D. J. Timmons and H.-C. Zhou, Chem. Sci., 2011, 2, 103 RSC.
- (a) J. J. Vittal, Coord. Chem. Rev., 2007, 251, 1781 CrossRef CAS; (b) J.-R. Li and H.-C. Zhou, Nat. Chem., 2010, 2, 893 CrossRef CAS; (c) R. Medishetty, L. L. Koh, G. K. Kole and J. J. Vittal, Angew. Chem., Int. Ed., 2011, 50 DOI:10.1002/anie.201104106.
- (a) K. Barthelet, J. Marrot, D. Riou and G. Férey, Angew. Chem., Int. Ed., 2002, 41, 281 CrossRef CAS; (b) D. N. Dybtsev, H. Chun and K. Kim, Angew. Chem., Int. Ed., 2004, 43, 5033 CrossRef CAS; (c) T. K. Maji, K. Uemura, H.-C. Chang, R. Matsuda and S. Kitagawa, Angew. Chem., Int. Ed., 2004, 43, 3269 CrossRef CAS.
- G. J. Halder, C. J. Kepert, B. Moubaraki, K. S. Murray and J. D. Cashion, Science, 2002, 298, 1762 CrossRef CAS.
- (a) K. Biradha, Y. Hongo and M. Fujita, Angew. Chem., Int. Ed., 2002, 41, 3395 CrossRef CAS; (b) E.-Y. Choi, K. Park, C.-M. Yang, H. Kim, J.-H. Son, S. W. Lee, Y. H. Lee, D. Min and Y.-U. Kwon, Chem.–Eur. J., 2004, 10, 5535 CrossRef CAS; (c) M.-H. Zeng, X.-L. Feng and X.-M. Chen, Dalton Trans., 2004, 2217 RSC.
- (a) A. Galet, M. C. Munoza and J. A. Real, Chem. Commun., 2006, 4321 RSC; (b) T. K. Maji, R. Matsuda and S. Kitagawa, Nat. Mater., 2007, 6, 142 CrossRef CAS.
- J.-P. Zhang, Y.-Y. Lin, W.-X. Zhang and X.-M. Chen, J. Am. Chem. Soc., 2005, 127, 14162 CrossRef CAS.
- (a) G. J. Halder and C. J. Kepert, J. Am. Chem. Soc., 2005, 127, 7891 CrossRef CAS; (b) Y. Liu, J.-H. Her, A. Dailly, A. J. Ramirez-Cuesta, D. A. Neumann and C. M. Brown, J. Am. Chem. Soc., 2008, 130, 11813 CrossRef CAS.
- (a) A. C. Sudik, A. P. Côté, A. G. Wong-Foy, M. O'Keeffe and O. M. Yaghi, Angew. Chem., Int. Ed., 2006, 45, 2528 CrossRef CAS; (b) F. Nouar, J. F. Eubank, T. Bousquet, L. Wojtas, M. J. Zaworotko and M. Eddaoudi, J. Am. Chem. Soc., 2008, 130, 1833 CrossRef CAS; (c) D. Zacher, R. Schmid, C. Wöll and R. A. Fischer, Angew. Chem., Int. Ed., 2011, 50, 176 CrossRef CAS; (d) B. J. Burnett, P. M. Barron, C. Hu and W. Choe, J. Am. Chem. Soc., 2011, 133, 9984 CrossRef CAS.
- (a) S. Surblé, F. Millange, C. Serre, G. Férey and R. I. Walton, Chem. Commun., 2006, 1518 RSC; (b) J. A. Rood, W. C. Boggess, B. C. Noll and K. W. Henderson, J. Am. Chem. Soc., 2007, 129, 13675 CrossRef CAS; (c) D. Zacher, K. Yusenko, A. Bétard, S. Henke, M. Molon, T. Ladnorg, O. Shekhah, B. Schüpbach, T. Arcos, M. Krasnopolski, M. Meilikhov, J. Winter, A. Terfort, C. Wöll and R. A. Fischer, Chem.–Eur. J., 2011, 17, 1448 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS.
- (a) S. M. Hawxwell, G. M. Espallargas, D. Bradshaw, M. J. Rosseinsky, T. J. Prior, A. J. Florence, J. van de Streek and L. Brammer, Chem. Commun., 2007, 1532 RSC; (b) M. H. Mir, L. L. Koh, G. K. Tan and J. J. Vittal, Angew. Chem., Int. Ed., 2010, 49, 390 CAS.
- (a) M. P. Suh and Y. E. Cheon, Aust. J. Chem., 2006, 59, 605 CrossRef CAS; (b) S. Kitagawa and K. Uemura, Chem. Soc. Rev., 2005, 34, 109 RSC.
- (a) J.-P. Zhang and S. Kitagawa, J. Am. Chem. Soc., 2008, 130, 907 CrossRef CAS; (b) G.-C. Xu, X.-M. Ma, L. Zhang, Z.-M. Wang and S. Gao, J. Am. Chem. Soc., 2010, 132, 9588 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, crystallographic details, additional structural plots, TGA, DSC, PXRD curves, as well as X-ray crystallographic files. CCDC 840366–840371. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1cc15891k |
| This journal is © The Royal Society of Chemistry 2012 |
