Phosphopeptide enrichment and fractionation by using Click OEG-CD matrix
Yanyan
Zhao
ab,
Xiuling
Li
*a,
Jingyu
Yan
a,
Zhimou
Guo
a and
Xinmiao
Liang
*a
aKey Lab of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China. E-mail: lixiuling@dicp.ac.cn; liangxm@dicp.ac.cn; Fax: +86-411-84379539; Tel: +86-411-84379523
bPharmacy College, Dalian Medical University, Dalian 116044, China
First published on 2nd April 2012
Abstract
Reversible protein phosphorylation regulates many significant cellular processes. Identification of phosphorylation sites is vital to elucidate their biofunctions. To aid in phosphoproteome characterization, several selective enrichment methods have been developed, including immobilized metal-ion affinity chromatography (IMAC) and titanium dioxide (TiO2). However, the high pH elution step applied in these two methods, which tends to degrade phosphopeptides. In order to improve phosphopeptide enrichment efficiency, a hydrophilic interaction liquid chromatography (HILIC) based material, cyclodextrin (CD) bonded silica (Click OEG-CD), was synthesized in our group and applied to phosphopeptide enrichment and fractionation. Taking tryptic digest of standard protein α-casein as model sample, the performance of Click OEG-CD in phosphopeptide isolation was investigated. It was found that both the acetonitrile and salt concentrations in mobile phase influence the phosphopeptide enrichment selectivity of the matrix. Under optimized enrichment condition, Click OEG-CD has a similar phosphopeptide enrichment selectivity as commercial TiO2. Meanwhile, phosphopeptides with same charges could be fractionated according to hydrophilicity difference on Click OEG-CD under acetonitrile gradient. Different selectivity is proved with Click OEG-CD from conventional SAX. We demonstrate that Click OEG-CD is an efficient matrix in phosphopeptide enrichment and fractionation.
1. Introduction
Protein phosphorylation as one of the most important post-translational modifications plays a vital role in cellular events. Comprehensive analysis of phosphoproteome is challenging, primarily originating from their low abundance and ion suppression effect of non-phosphopeptides. It is necessary to separate phosphopeptides from complex mixtures prior to mass spectrometry (MS) analysis.1–4 Enrichment by solid phase extraction (SPE) and fractionation by HPLC are the two primary ways for phosphopeptide separation. The approach to be applied depends on the complexity of samples.Phosphopeptide enrichment is usually performed with convenient SPE mode. Immobilized metal-ion affinity chromatography (IMAC) (with Fe3+, Ga3+, Ni2+, Zr4+)5–7 is the commonly used method for phosphopeptide enrichment. However, acidic non-phosphopeptides bind to IMAC materials. Metal oxide affinity chromatography representative with TiO2 attracts many interests in recent years.7–10 Compared with IMAC, TiO2 shows higher enrichment selectivity for phosphopeptide. However, the elution steps of both IMAC and MOAC are performed under high pH, which tends to degrade phosphopeptides.11 More phosphopeptide enrichment methods should be developed, which feature with complementary enrichment selectivity to IMAC or TiO2 and acidic elution condition.
For large-scale phosphoproteome analysis, fractionation by HPLC could reduce the complexity of samples and enhance the selectivity of subsequent SPE enrichment. Ion exchange chromatography (IXC) and hydrophilic interaction liquid chromatography (HILIC) are frequently applied in phosphopeptide fractionation. Strong cation-exchange chromatography (SCX) in peptide isolation was primarily based on peptides' solution charge states. Phosphopeptides with one or more negative phosphate groups are eluted in early fractions and separated from non-phosphopeptides. However, acidic non-phosphopeptides coelute with phosphopeptides and decrease the enrichment selectivity for phosphopeptides.12 Although the enrichment selectivity was enhanced when IMAC was applied to purify phosphopeptides from the early eluted SCX fractions,13,14 some multi-phosphopeptides are lost in the flowthrough of SCX. Phosphopeptides can be enriched on strong anion-exchange (SAX) material, based on the electrostatic interaction between negatively charged phosphate groups in phosphopeptides and the surface charge of the ion exchange matrix. Fractionation can be performed using salt or pH stepwise gradient. High coverage of phosphopeptide was achieved when SAX was coupled with IMAC.15,16 Nevertheless, IXC suffers from low resolution and contamination of non-phosphopeptides with same charges. HILIC is a variant of normal phase chromatography and is used primarily for separation of very polar compounds. On HILIC, phosphopeptides could be separated from their counterparts because of the high hydrophilicity.17,18 Compared to SCX, HILIC shows better chromatographic resolution and absence of clustering of prevalent +2 and +3 charged peptides.19 Electrostatic repulsion-hydrophilic interaction chromatography (ERLIC) as a derivative of HILIC was used to enrich phosphopeptides, based on both electrostatic interaction and hydrophilic interaction on weak anion exchange chromatography (WAX).20,21 Efficient as the HILIC and ERLIC approaches are, limitation arises from the scarcity of commercial available HILIC or WAX matrices. In further studies, materials providing different interactions from that of commercial columns should be applied in the field.
The series of “Click CD” materials have been synthesized in our group and were used to separate polar compounds.22,23 When CD group bonded on the surface of silica through oligo (ethylene glycol) (OEG) spacer, high polar selectivity also could be obtained with Click OEG-CD matrix.24,25 CD group together with OEG spacer and triazole group on Click OEG-CD provide hydrogen bonding and anion-exchange interactions, which will contribute to the high phosphopeptide enrichment selectivity. Meanwhile, the elution step is performed under low pH, avoiding phosphopeptide degradation. Based on the above consideration, we speculate that Click OEG-CD matrix might perform well in phosphopeptide separation and be complementary to the conventional method. In this work, phosphopeptide enrichment and fractionation was carried out with Click OEG-CD matrix under HILIC mode. Tryptic digest of standard protein α-casein was selected as a model sample. The effect of acetonitrile and salt concentrations on the phosphopeptide enrichment selectivity was studied. Comparison was carried out between Click OEG-CD and commercial TiO2 in phosphopeptide enrichment. Phosphopeptide fractionation was carried out under appropriate chromatography conditions.
2. Experimental
2.1 Material and reagents
α-Casein, human serum albumin (HSA) and ammonium bicarbonate were obtained from Sigma-Aldrich (St. Louis, MO). Trypsin was from Promega (Madison, WI). Ammonium hydroxide was purchased from Fluka (St. Louis, MO). Water was prepared with Millipore (Millipore, Bedford, MA). Acetonitrile (HPLC grade) for analysis was purchased from Fisher (New Jersey, USA). Formic acid (HPLC grade) was purchased from Acros (New Jersey, USA). Ammonium formate was purchased from Tedia (Fairfield, USA). GELoader tips were obtained from Eppendorf (Hamburg, Germany).Click OEG-CD matrix, which was synthesized in house, was applied in phosphopeptide enrichment and fractionation. The synthesis processes were described in our previous article.24 The chemistry of Click OEG-CD is shown in Fig. 1. Click OEG-CD column (2.1 mm × 100 mm, 5 μm, homemade) for fractionation was packed in house. TiO2 particle used for comparison were purchased from GL Science (Tokyo, Japan). ZipTip C18 was obtained from Millipore (Bedford, MA, USA).
 | ||
| Fig. 1 The structure of Click OEG-CD matrix. | ||
2.2 Apparatus
The HPLC system (Agilent 1100, Agilent Technologies, Waldbronn, Germany) consisted of a degasser, an automatic thermostatic column compartment, a quaternary pump, and an autosampler and diode array detection (DAD) system.MS analysis was performed on a X′Treme Simple nano-LC system (Micro-Tech Scientific, Vista, CA) coupled to a quadrupole time-of-flight (Q-TOF) mass spectrometer (Waters MS Technologies, Manchester, UK) in positive ion electrospray ionization (ESI) mode.
2.3 Desalting with C18 material
ZipTip C18 microcolumn was conditioned with 40 μL of 50% ACN/0.1% FA and equilibrated with 80 μL of 0.1% FA aqueous solution before use. Then, tryptic digest was loaded onto the column. After washing with 80 μL of 0.1% FA aqueous solution, peptides were eluted with 40 μL of 50% ACN/0.1% FA. The resulting fraction was dried and redissolved in 20 μL of 50% ACN/0.1% FA before MS analysis.2.4 Phosphopeptide enrichment and fractionation
α-Casein (1.0 mg) was dissolved with 1 mL of ammonium bicarbonate (50 mM, pH = 8.0) and digested in trypsin for 18 h at 37 °C with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (w/w) enzyme-to-protein ratio. The digested solution was lyophilized by a vacuum concentration. The dried digest was redissolved in loading solution (90% ACN/0.1% FA for Click OEG-CD isolation, 300 mg mL−1 lactic acid in 0.1% TFA/90% ACN for TiO2 enrichment).
40 (w/w) enzyme-to-protein ratio. The digested solution was lyophilized by a vacuum concentration. The dried digest was redissolved in loading solution (90% ACN/0.1% FA for Click OEG-CD isolation, 300 mg mL−1 lactic acid in 0.1% TFA/90% ACN for TiO2 enrichment).
Phosphopeptide enrichment was carried out with GELoader tips packed with 1.5 mg Click OEG-CD matrix. α-Casein digest (86 pmol) was loaded onto the GELoader tips. After washing with 120 μL 80% ACN/0.1% FA, the captured phosphopeptides were eluted with 30 μL 10 mM NH4FA/70% ACN (pH 4.0). The phosphopeptide fraction was dried and redissolved in 20 μL 0.1% FA aqueous solution, then was desalted before MS analysis.
For comparison, α-casein digest was loaded onto GELoader tips packed with 1.5 mg TiO2. After successive washing with 60 μL 300 mg mL−1 lactic acid/0.1% TFA/80% ACN and 30 μL 80% ACN/0.1% TFA, the captured peptides were eluted with 30 μL 2% ammonium hydroxide. The eluted solution was acidified with 10% FA aqueous solution and desalted with ZipTip C18 before MS analysis.
For enrichment of tryptic digest mixture of α-casein and HSA (the molar ratio of α-casein to HSA is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), the chromatography condition of Click OEG-CD was the same to that with digest of α-casein.
5), the chromatography condition of Click OEG-CD was the same to that with digest of α-casein.
Phosphopeptide fractionation was carried out in a HPLC system. Solvent A (ACN/0.1% FA), solvent B (water/0.1% FA), and solvent C (50 mM NH4FA aqueous, pH = 4.0) were used to develop a gradient. Flow rate: 0.2 mL min−1; column temperature: 25 °C. Chromatography conditions: 80% A/20% B/10% C – 30 min – 80% A/20% B/10% C – 30 min – 50% A/40% B/10% C. A total of 20 fractions were collected manually from 1.5 min to 60.0 min at 3.0 min intervals and they were denoted as fraction 1 to fraction 20 orderly. All fractions were desalted offline before MS analysis.
3. Results and discussion
3.1 The rationale of phosphopeptide isolation by using Click OEG-CD matrix
To selectively separate phosphopeptides from a complex biological mixture, a chromatography method needs to be established. Compared to their counterparts, phosphopeptides are usually more hydrophilic and acidic. Therefore, phosphopeptides are stronger retained on HILIC or anion-exchange (AX) materials than their counterparts. The commercial available HILIC materials are primarily bonded with amine, cyano or diol groups, and the commercial available AX materials are mostly bonded with amine. However, reducing saccharides irreversibly adsorb on amine bonded materials, leading to the low recovery of these kinds of compounds. It is desirable to develop more novel materials featuring with high separation selectivity.Click OEG-CD was applied to separate polar compounds under HILIC mode.25 Hydrogen bonding and weak anion exchange interactions are two primary interactions provided by Click OEG-CD under HILIC mode. Click OEG-CD under HILIC are expected to separate phosphopeptides from non-phosphopeptides and be complementary to commercial phosphopeptide enrichment methods due to the following reasons:
(1) Compared to their counterparts, phosphopeptides bind strongly onto Click OEG-CD because of their relative high hydrophilicity. Non-phosphopeptides can be removed with high content of acetonitrile, phosphopeptides can then be eluted with high content of water;
(2) Phosphate groups on phosphopeptides are negatively charged when pH is above their pKa values. At low pH, phosphopeptides with one or more negative phosphate groups bind more tightly onto the Click OEG-CD than their counterparts due to the weak anion exchange interaction, and can be eluted from the matrix with higher concentration of salt;
(3) The separation mechanisms of Click OEG-CD in HILIC mode are different from that of conventional TiO2 and SAX. Click OEG-CD has the potential to be complementary material to TiO2 and SAX in phosphopeptide isolation.
(4) The whole isolation process is carried out under low pH condition, low pH loading and elution conditions can avoid the degradation of phosphopeptides.
Phosphopeptide isolation with Click OEG-CD matrix is dependent on the complexity of the requirement of analysis. For phosphopeptide enrichment, convenient SPE is the suitable method. For large scale phosphoproteome analysis, fractionation by using HPLC is appropriate.
3.2 Optimization of phosphopeptide enrichment
Condition optimization for phosphopeptide enrichment was carried out in microcolumns packed with Click OEG-CD matrix. To evaluate the ability of Click OEG-CD for phosphopeptide isolation, tryptic digest of phosphoprotein α-casein was selected as the model sample, which contains mono-phosphopeptides, multi-phosphopeptides and acidic peptides. The phosphopeptides detected in this study are shown in Table 1.| No. | Molecular weighta (calcd) | m/zb (found) | Number of phosphorylation sites | Amino acid sequence |
|---|---|---|---|---|
| a Peptide mass. b Inside the bracket is the number of charge. | ||||
| 1 | 1466.63 | 733.64(2+) | 1 | TVDMEpSTEVFTK |
| 2 | 1660.81 | 830.69(2+) | 1 | VPQLEIVPNpSAEER |
| 3 | 1832.86 | 916.95(2+) | 1 | YLGEYLIVPNpSAEER |
| 4 | 1927.72 | 964.37(2+) | 2 | DIGpSEpSTEDQAMEDIK |
| 5 | 1951.97 | 976.25(2+)/651.17(3+) | 1 | YKVPQLEIVPNpSAEER |
Under optimized conditions, the selectivity of Click OEG-CD matrix for phosphopeptide enrichment could be modulated to meet the different isolation requirement. The two basic interactions provided by Click OEG-CD under HILIC mode are hydrogen bonding and weak anion exchange interactions. Therefore, the most influencing factors in loading and elution steps should be the acetonitrile and salt concentrations.
To evaluate the influence of acetonitrile concentration for phosphopeptide retention, peptide mixture in 90% ACN/0.1% FA was loaded onto Click OEG-CD microcolumn, the captured peptides were eluted with 90% ACN/0.1% FA aqueous solution (40 μL), 80% ACN/0.1% FA aqueous solution (40 μL), 70% ACN/0.1% FA aqueous solution (40 μL), and 50% ACN/0.1% FA aqueous solution (40 μL), sequentially. Mass spectrum of each desalted fraction before and after enrichment is shown for comparison in Fig. 2. Before enrichment, signals of phosphopeptides were suppressed by large amount of non-phosphopeptides (Fig. 2A). After enrichment, peptides with different polarity were concentrated in different eluting solution. Majority of peptides were enriched on the matrix after sample loading and only a few peptides with low intensity were detected in the 90% ACN/0.1% FA fraction (Fig. 2B), demonstrating the suitable loading condition of 90% ACN/0.1% FA. Many non-phosphopeptides with high abundance could be removed with 80% ACN/0.1% FA solution (Fig. 2C), such as peptides bearing m/z 979.37(2+), 1158.32(2+), 1267.46(1+), 1384.45(1+). Most phosphopeptides could be eluted with 70% ACN/0.1% FA solution (Fig. 2D). However, the relative intensities of phosphopeptides to those of non-phosphopeptides were low, for example the intensity of phosphopeptide bearing m/z 830.74(2+) was lower than that of non-phosphopeptide bearing m/z 880.29(2+), implying that phosphopeptides might not be eluted thoroughly under merely acetonitrile gradient. Except for several peptides, no phosphopeptides were eluted with 50% ACN/0.1% FA solution (Fig. 2E). The results indicated that phosphopeptides could be enriched on Click OEG-CD and sequentially eluted from the matrix with acetonitrile gradient. Although some non-phosphopeptides could be removed from phosphopeptide fraction, phosphopeptides could not be thoroughly eluted merely under acetonitrile gradient.
 | ||
| Fig. 2 Nano-ESI-MS characterization of α-casein digest fraction desalted with C18 (A); fractions from Click OEG-CD eluted with ACN gradient under HILIC mode: (B) Mass spectrum of α-casein digest rinsed with 40 μL of 90% ACN/0.1% FA. (C) Mass spectrum of α-casein digest rinsed with 40 μL of 80% ACN/0.1% FA. (D) Mass spectrum of α-casein digest rinsed with 40 μL of 70% ACN/0.1% FA. (E) Mass spectrum of α-casein digest rinsed with 40 μL of 50% ACN/0.1% FA. Peaks marked with * are phosphopeptides. Other Peaks are non-phosphopeptides. | ||
To evaluate the effect of salt concentration, peptide mixture in 90% ACN/0.1% FA was loaded onto Click OEG-CD microcolumn, and the bound peptides were rinsed with 80% ACN/0.1% FA aqueous solution (40 μL), 5 mM NH4FA/80% ACN/0.1% FA aqueous solution (40 μL), and 10 mM NH4FA/80% ACN/0.1% FA aqueous solution (40 μL), sequentially. The mass spectrum of each desalted fraction is shown in Fig. 3. Most phosphopeptides were eluted when the salt (NH4FA) concentration increased to 5 mM (Fig. 3B), indicating that captured phosphopeptides could be eluted with the increase of salt concentration. Meanwhile, the phosphopeptide intensity ratio of fraction eluted with 5 mM NH4FA/80% ACN/0.1% FA aqueous solution to that with 10 mM NH4FA/80% ACN/0.1% FA aqueous solution was more than 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, indicating the suitable condition of 10 mM NH4FA for elution step. It can also be seen that the relative intensity of phosphopeptide bearing m/z 830.74(2+) to that of non-phosphopeptide bearing m/z 880.29(2+) was higher with salt gradient (Fig. 3B) than with acetonitrile gradient enrichment (Fig. 2D), suggesting that the captured phosphopeptides on Click OEG-CD could be eluted more thoroughly with salt gradient than with acetonitrile gradient. The result demonstrated the anion exchange interaction's contribution to phosphopeptide enrichment on Click OEG-CD matrix. Nevertheless, some non-phosphopeptides bearing m/z 979.75(2+), 1158.29(2+), 1267.42(1+), 1384.43(1+) were co-eluted with phosphopeptides (Fig. 3B and C), which could be removed from phosphopeptide fraction with acetonitrile gradient. The result indicated that acetonitrile gradient should be applied in the elution step in order to remove non-phosphopeptides with low polarity and enhance the phosphopeptide enrichment selectivity.
1, indicating the suitable condition of 10 mM NH4FA for elution step. It can also be seen that the relative intensity of phosphopeptide bearing m/z 830.74(2+) to that of non-phosphopeptide bearing m/z 880.29(2+) was higher with salt gradient (Fig. 3B) than with acetonitrile gradient enrichment (Fig. 2D), suggesting that the captured phosphopeptides on Click OEG-CD could be eluted more thoroughly with salt gradient than with acetonitrile gradient. The result demonstrated the anion exchange interaction's contribution to phosphopeptide enrichment on Click OEG-CD matrix. Nevertheless, some non-phosphopeptides bearing m/z 979.75(2+), 1158.29(2+), 1267.42(1+), 1384.43(1+) were co-eluted with phosphopeptides (Fig. 3B and C), which could be removed from phosphopeptide fraction with acetonitrile gradient. The result indicated that acetonitrile gradient should be applied in the elution step in order to remove non-phosphopeptides with low polarity and enhance the phosphopeptide enrichment selectivity.
 | ||
| Fig. 3 Nano-ESI-MS characterization of α-casein digest fractions from Click OEG-CD eluted with the salt gradient under HILIC mode. (A) Mass spectrum of α-casein digest rinsed with 40 μL of 80% ACN/0.1% FA. (B) Mass spectrum of α-casein digest rinsed with 40 μL of 5 mM NH4FA/80% ACN/0.1% FA. (C) Mass spectrum of α-casein digest rinsed with 40 μL of 10 mM NH4FA/80% ACN/0.1% FA. Each fraction was desalted before MS analysis. Peaks marked with * are phosphopeptides. Other peaks are non-phosphopeptides. | ||
According to the above investigations, to improve phosphopeptide enrichment selectivity and elute most captured phosphopeptides by using Click OEG-CD matrix, both acetonitrile and salt concentrations in loading and elution steps should be optimized.
3.3 Phosphopeptide enrichment
The SPE enrichment conditions were optimized aiming at enhancing phosphopeptide enrichment selectivity of Click OEG-CD matrix. Under the optimized conditions, 90% ACN/0.1% FA was set as loading condition. Under this condition, most non-phosphopeptides could be removed, while phosphopeptides could be well retained on the matrix. In the elution step, 10 mM NH4FA/70% ACN/0.1% FA was applied as elution buffer. Under the condition, most phosphopeptides could be eluted. After enrichment by using Click OEG-CD matrix, a large number of non-phosphopeptides were removed from phosphopeptide fraction in the washing step (Fig. 4A), indicating the high enrichment selectivity of Click OEG-CD matrix. Meanwhile, phosphopeptides with different hydrophilicity could be integrated in the elution fraction. Control experiment was also carried out with commercial TiO2 material (Fig. 4B). As is shown in Fig. 4, most phosphopeptides detected after TiO2 enrichment could also be detected after Click OEG-CD enrichment from the α-casein digest, indicating the similar phosphopeptide enrichment selectivity of Click OEG-CD to that of TiO2. Besides, phosphopeptides are suspected to degrade under the high pH elution step.11 The problem could be avoided on Click OEG-CD as the whole process was carried out in acidic environment. | ||
| Fig. 4 Nano-ESI-MS characterization of α-casein digest fractions from: (A) mass spectrum enriched by Click OEG-CD. (B) Mass spectrum enriched by TiO2, desalted by ZipTip™ C18. Peaks marked with * are phosphopeptides. Other peaks are non-phosphopeptides. | ||
The capability of Click OEG-CD matrix to enrich phosphopeptides from complex sample was also evaluated, with a digest mixture of α-casein and HSA at the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. As is shown in Fig. 5A, phosphopeptides were almost submerged by non-phosphopeptides before enrichment. After enrichment with Click OEG-CD matrix, a lot of non-phosphopeptides were excluded from the phosphopeptide fraction, and the relative abundance of phosphopeptides was enhanced (Fig. 5B). The result indicated the high phosphopeptide enrichment selectivity of Click OEG-CD matrix from relative complex samples. Click OEG-CD has the potential in real sample analysis.
5. As is shown in Fig. 5A, phosphopeptides were almost submerged by non-phosphopeptides before enrichment. After enrichment with Click OEG-CD matrix, a lot of non-phosphopeptides were excluded from the phosphopeptide fraction, and the relative abundance of phosphopeptides was enhanced (Fig. 5B). The result indicated the high phosphopeptide enrichment selectivity of Click OEG-CD matrix from relative complex samples. Click OEG-CD has the potential in real sample analysis.
 | ||
Fig. 5 Nano-ESI-MS characterization of tryptic digest mixture of α-casein and HSA with the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. (A) Mass spectrum of fraction desalted with C18. (B) Mass spectrum enriched by Click OEG-CD matix. Peaks marked with * are phosphopeptides. Other peaks are non-phosphopeptides. 5. (A) Mass spectrum of fraction desalted with C18. (B) Mass spectrum enriched by Click OEG-CD matix. Peaks marked with * are phosphopeptides. Other peaks are non-phosphopeptides. | ||
3.4 Phosphopeptide fractionation
Although SPE approach is efficient and facile for sample preparation prior to MS, fractionation is necessary to reduce the complexity of peptide mixtures in global peptide analysis. In addition to phosphopeptide enrichment, Click OEG-CD matrix packed in column was also applied in phosphopeptide fractionation. However, as far as the α-casein digest is concerned, around half of the phosphopeptides are singly phosphorylated peptides. Separation of these phosphopeptides based on mere charge difference is insufficient, polar difference between peptides should be considered in method development. In the work, a constant salt concentration was applied in mobile phase and fractionation of α-casein digest was under acetonitrile gradient. Under optimized condition, phosphopeptides with different charges and polarity were expected to be separated. It can be seen from Fig. 6 that phosphopeptides were not eluted until fraction 6. Phosphopeptide bearing 976.46(2+) was eluted in the fraction 14. Longer retention time of phosphopeptides than non-phosphopeptides is the consequence of their stronger interaction with Click OEG-CD. Notably, under different conditions, the separation selectivity of Click OEG-CD could be changed. For an example, a doubly charged acidic peptide at m/z 1158.07 (EPMIGVNQELAYFYPELFR) was eluted in fraction 3 and well separated from phosphopeptides in fractionation mode, which was co-eluted with phosphopeptides under SPE mode (Fig. 4). Besides, the selectivity of Click OEG-CD matrix is different from that of conventional SAX matrix. For example, two mono-phosphopeptides bearing 830.68(2+) and 976.46(2+) were eluted in fraction 6 and 14, which are often co-eluted on SAX column.21 The result also indicated that Click OEG-CD could be complementary to conventional SAX under proper chromatography condition.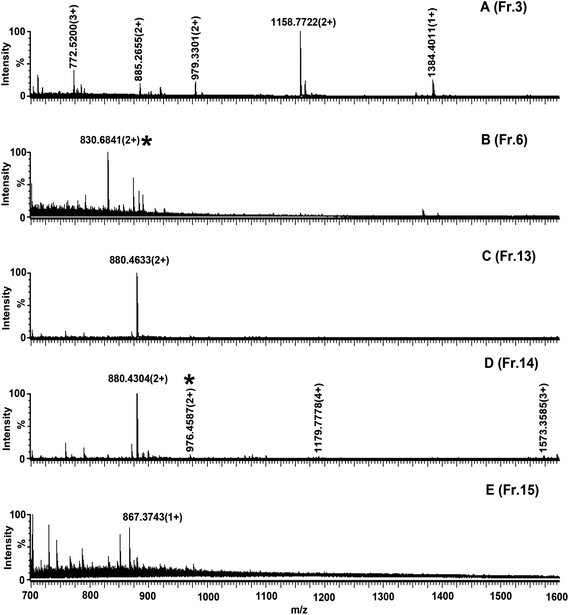 | ||
| Fig. 6 Nano-ESI-MS characterization of α-casein digest fractions from Click OEG-CD by fractionation: (A), (B), (C), (D), and (E) are spectra of fraction 3, 6, 13, 14, and 15, respectively. Peaks marked with * are phosphopeptides. Other peaks are non-phosphopeptides. | ||
4. Conclusions
Click OEG-CD matrix demonstrated itself as a useful material for phosphopeptide enrichment and fractionation. The provided hydrogen bonding and anion-exchange interactions of Click OEG-CD contributed to the retention of phosphopeptide. Under the optimized conditions, the selectivity on Click OEG-CD for phosphopeptide isolation could be modulated to meet the different requirement. Click OEG-CD showed similar selectivity in phosphopeptide enrichment as commercial TiO2. Click OEG-CD showed different selectivity to conventional SAX in phosphopeptide fractionation under proper condition. Meanwhile, the dephosphorylation could be avoided as the whole process was performed under low pH. Therefore, Click OEG-CD is expected to show high performance in both phosphopeptide enrichment and fractionation in the investigation of complex proteomic sample.Acknowledgements
This work was supported by “Project of National Science Foundation of China (20975100, 20825518, 21105100 and 21105007)”.References
- G. H. Han, M. L. Ye and H. F. Zou, Development of phosphopeptide enrichment techniques for phosphoproteome analysis, Analyst, 2008, 133, 1128–1138 RSC.
- M. Gilar, Y. Q. Yu, J. Ahn, J. Fournier and J. C. Gebler, Mixed-mode chromatography for fractionation of peptides, phosphopeptides, and sialylated glycopeptides, J. Chromatogr., A, 2008, 1191, 162–170 CrossRef CAS.
- A. Motoyama, T. Xu, C. I. Ruse, J. A. Wohlschlegel and J. R. Yates, Anion and cation mixed-bed ion exchange for enhanced multidimensional separations of peptides and phosphopeptides, Anal. Chem., 2007, 79, 3623–3634 CrossRef CAS.
- J. Y. Yan, X. L. Li, S. Y. Cheng, Y. X. Ke and X. M. Liang, Facile synthesis of titania-zirconia monodisperse microspheres and application for phosphopeptides enrichment, Chem. Commun., 2009, 2929–2931 RSC.
- S. Feng, C. S. Pan, X. G. Jiang, S. Y. Xu, H. J. Zhou, M. L. Ye and H. F. Zou, Fe3+ immobilized metal affinity chromatography with silica monolithic capillary column for phosphoproteome analysis, Proteomics, 2007, 7, 351–360 CrossRef CAS.
- Y. M. Ndassa, C. Orsi, J. A. Marto, S. Chen and M. M. Ross, Improved immobilized metal affinity chromatography for large-scale phosphoproteomics applications, J. Proteome Res., 2006, 5, 2789–2799 CrossRef CAS.
- M. C. Posewitz and P. Tempst, Immobilized gallium(III) affinity chromatography of phosphopeptides, Anal. Chem., 1999, 71, 2883–2892 CrossRef CAS.
- T. E. Thingholm, T. J. D. Jorgensen, O. N. Jensen and M. R. Larsen, Highly selective enrichment of phosphorylated peptides using titanium dioxide, Nat. Protoc., 2006, 1, 1929–1935 CrossRef CAS.
- M. R. Larsen, T. E. Thingholm, O. N. Jensen, P. Roepstorff and T. J. D. Jorgensen, Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns, Mol. Cell. Proteomics, 2005, 4, 873–886 CAS.
- H. Y. Lin, C. T. Chen and Y. C. Chen, Detection of phosphopeptides by localized surface plasma resonance of titania-coated gold nanoparticles immobilized on glass substrates, Anal. Chem., 2006, 78, 6873–6878 CrossRef CAS.
- S. T. Sun, H. T. Ma, G. H. Han, R. A. Wu, H. F. Zou and Y. W. Liu, Efficient enrichment and identification of phosphopeptides by cerium oxide using on-plate matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis, Rapid Commun. Mass Spectrom., 2011, 25, 1862–1868 CrossRef CAS.
- J. Dai, W. H. Jin, Q. H. Sheng, C. H. Shieh, J. R. Wu and R. Zeng, Protein phosphorylation and expression profiling by Yin-yang multidimensional liquid chromatography (Yin-yang MDLC) mass spectrometry, J. Proteome Res., 2007, 6, 250–262 CrossRef CAS.
- J. Villén, S. A. Beausoleil, S. A. Gerber and S. P. Gygi, Large-scale phosphorylation analysis of mouse liver, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 1488–1493 CrossRef.
- A. Gruhler, J. V. Olsen, S. Mohammed and P. Mortensen, et al., Quantitative phosphoproteomics applied to the yeast pheromone signaling pathway, Mol. Cell. Proteomics, 2005, 4, 310–327 CAS.
- J. Dai, L. S. Wang, Y. B. Wu, Q. H. Sheng, J. R. Wu, C. H. Shieh and R. Zeng, Fully automatic separation and identification of phosphopeptides by continuous pH-gradient anion exchange online coupled with reversed-phase liquid chromatography mass spectrometry, J. Proteome Res., 2009, 8, 133–141 CrossRef CAS.
- G. H. Han, M. L. Ye, H. J. Zhou, X. N. Jiang, S. Feng, X. G. Jiang, R. J. Tian, D. F. Wan, H. F. Zou and J. R. Gu, Large-scale phosphoproteome analysis of human liver tissue by enrichment and fractionation of phosphopeptides with strong anion exchange chromatography, Proteomics, 2008, 8, 1346–1361 CrossRef CAS.
- P. J. Boersema, S. Mohammed and A. J. R. Heck, Hydrophilic interaction liquid chromatography (HILIC) in proteomics, Anal. Bioanal. Chem., 2008, 391, 151–159 CrossRef CAS.
- D. E. McNulty and R. S. Annan, Hydrophilic interaction chromatography reduces the complexity of the phosphoproteome and improves global phosphopeptide isolation and detection, Mol. Cell. Proteomics, 2008, 7, 971–980 CAS.
- P. J. Boersema, N. Divecha, A. J. R. Heck and S. Mohammed, Evaluation and optimization of ZIC-HILIC-RP as an alternative MudPIT strategy, J. Proteome Res., 2007, 6, 937–946 CrossRef CAS.
- C. S. Gan, T. N. Guo, H. M. Zhang, S. K. Lim and S. K. Sze, A comparative study of electrostatic repulsion-hydrophilic interaction chromatography (ERLIC) versus SCX-IMAC-based methods for phosphopeptide isolation/enrichment, J. Proteome Res., 2008, 7, 4869–4877 CrossRef CAS.
- A. J. Alpert, Separations in poly(dimethylsiloxane) microchips coated with supported bilayer membranes, Anal. Chem., 2008, 80, 9756–9762 CrossRef.
- Z. M. Guo, Y. Jin, T. Liang, Y. F. Liu, Q. Xu, X. M. Liang and A. W. Lei, Synthesis, chromatographic evaluation and hydrophilic interaction/reversed-phase mixed-mode behavior of a “Click beta-cyclodextrin” stationary phase, J. Chromatogr., A, 2009, 1216, 257–263 CrossRef CAS.
- Z. M. Guo, A. W. Lei, Y. P. Zhang, Q. Xu, X. Y. Xue, F. F. Zhang and X. M. Liang, “Click saccharides”: novel separation materials for hydrophilic interaction liquid chromatography, Chem. Commun., 2007, 2491–2493 RSC.
- Y. Y. Zhao, Z. M. Guo, Y. P. Zhang, X. Y. Xue, Q. Xu, X. L. Li, X. M. Liang and Y. K. Zhang, Retention properties of novel β-CD bonded stationary phases in reversed-phase HPLC mode, Talanta, 2009, 78, 916–921 CrossRef CAS.
- Y. Y. Zhao, L. Yu, Z. M. Guo, X. L. Li and X. M. Liang, Reversed phase depletion coupled with hydrophilic affinity enrichment for the selective isolation of N-linked glycopeptides by using Click OEG-CD matrix, Anal. Bioanal. Chem., 2011, 399, 3359–3365 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
