Extensive grinding and pressurized extraction with water are key points for effective and species preserving extraction of arsenic from rice
Pradeep
Alava
*ab,
Tom
Van de Wiele
b,
Filip
Tack
a and
Gijs
Du Laing
a
aGhent University, Faculty of Bioscience Engineering, Laboratory of Analytical Chemistry and Applied Ecochemistry, Coupure Links 653, B-9000 Gent, Belgium. E-mail: pradeep.alava@ugent.be; gijs.dulaing@ugent.be
bGhent University, Faculty of Bioscience Engineering, Laboratory of Microbial Ecology and Technology, Gent, Belgium
First published on 12th April 2012
Abstract
An adequate sample preparation is an essential prerequisite for an accurate assessment of exposure to arsenic (As) upon consumption of contaminated rice. Firstly, a well-defined amount of As must be released from the matrix following sample extraction. Secondly, given the toxicological importance of As species, the sample extraction procedure must preserve As speciation. We evaluated the effectiveness of closed and open microwave digestion procedures to extract As from a certified reference sample of rice and 3 commercial rice matrices. In addition, we investigated to what extent rice grain particle size after grinding, the ratio of rice over extraction liquid, hold time and temperature affect the release of different arsenic (As) species (AsIII, AsV, and DMAV) from rice samples. Particle size was found to have a major influence on arsenic extraction. Extraction efficiency of As was decreased to 75% when rice was treated as whole grain compared to powdered form. Extraction efficiency using microwave digestion in closed vessels was better than using microwave digestion in open vessels when the particle size was larger than 0.5 mm. For powdered samples, extraction efficiencies using both methods were similar. However, less time (30 min) was needed for complete extraction using microwave digestion in closed vessels compared to using microwave digestion in open vessels (180 min). The highest extraction efficiency for closed microwave digestion was obtained with powdered rice in 80 °C water at a liquid/solid ratio of 10 and a hold time of 30 min. The use of closed or open vessels during microwave digestion was indifferent to the speciation pattern. Extraction efficiencies of individual As species are affected by particle size to the same extent as that of total As, except for AsIII. We concluded that closed microwave digestion of powdered sample under the proposed conditions is the most successful technique for species-preserving quantitative extraction of As species from rice.
1 Introduction
Due to the use of arsenic-rich groundwater to flood rice fields in several regions of the world, and because of the conversion of arsenate to more bioavailable arsenite under reducing conditions in rice fields, arsenic (As) concentrations in rice are much higher than in other crops.1 Moreover, rice is widely consumed. Accordingly, rice has been demonstrated to be one of the major contributors of As in human diets. Threshold levels of exposure were previously set by the Joint FAO/WHO Expert Committee on Food Additives (JECFA). However, the provisional tolerable weekly intake (PTWI) of 15 μg kg−1 b.w. was considered by the CONTAM panel to be no longer appropriate as a range of adverse effects were reported to occur at exposures lower than those reviewed by the JECFA.2The bioavailability and toxicity of As are determined by its speciation, i.e. the chemical form in which it occurs. Inorganic As, i.e. arsenate (AsV) and arsenite (AsIII), and dimethylarsenic acid (DMAV) constitute the dominant As species present in rice while traces of monomethylarsonic acid (MMAV) are sometimes reported (National Research Council Update, 2001). Inorganic As is considered as a class I non-threshold carcinogen.3 The acute toxicity of AsIII for human cells is about 100 times higher than that of AsV, 150 times higher than that of DMAV and 300 times higher than that of MMAV.4
Given the variable toxicity of different As species as mentioned above, an appropriate analytical tool is needed to characterize As contaminated food. Previous efforts to determine total As and its speciation in rice samples and food in general were focused on using hyphenated systems. These hyphenated systems combine a separation technique such as liquid chromatography6–14 with a detector such as an atomic absorption spectrometer (AAS),15 an atomic fluorescence spectrometer (AFS)16 or an inductively coupled plasma mass spectrometer (ICP-MS).1,5 Among those, most successful data have been obtained using inductively coupled plasma mass spectrometry (ICP-MS).1,5 This has proven to be a suitable and sensitive method for As speciation analysis in biological,17 environmental18 and food samples19 including rice.20
An important prerequisite for arriving at an adequate speciation analysis is the preservation of As forms during sample preparation. Extraction procedures that have been used for arsenic speciation analysis include microwave21–23 or heating block procedures.24 The most valuable methods are those that use water as an extraction solvent,23 as they can preserve speciation and are economically and environmentally friendly. Extraction efficiencies of 97% were obtained while ensuring the preservation of the As speciation in the rice.23 In contrast, application of aggressive reagents like nitric acid, H2O2, TFA and TMAH25 typically results in the conversion of arsenic species during sample extraction. Several parameters may affect extraction efficiency and species stability during extraction. These include temperature and holding time,23 particle size24 and ratio of the amount of rice to the volume of extraction liquid.
Narukawa and Chiba24 postulated the effect of rice particle size on arsenic extraction only till 0.5 mm in the absence of pressure. They did not mention what will happen if the rice sample is treated as a particle with more than 0.5 mm size.
We aimed to evaluate to what extent these parameters affect the extractability of As from rice samples. Moreover, we studied whether the use of an open (open vessels) or a closed (closed vessels) microwave digestion system affects the extraction efficiency to assess the role of extraction pressure. In addition, by comparing polished (fiber poor (2%)), basmati (medium fiber (12%)) and parboiled (fiber rich (22%)) rice, we examined whether extraction efficiencies depend on the nature of the rice matrix.
2 Experimental
2.1 Reagents
Individual stock solutions containing 100 mg As L−1 as AsIII, AsV, DMAV and MMAV were prepared using Milli-Q water. Individual standards were prepared by diluting these stock solutions to 50 μg As L−1 using Milli-Q water.Compounds used to prepare stock solutions of the different arsenic species were NaAsO2 (VWR, Belgium) for arsenite (AsIII), Na2HAsO4·7H2O (Fluka, Switzerland) for arsenate (AsV), (CH3)2AsO2Na·3H2O (Fluka, Switzerland) for dimethylarsonic acid (DMAV), and (CH3)AsNa2O3·6H2O (Chemservice, Belgium) for monomethylarsonic acid (MMAV).
2.2 Certified reference material (CRM)
NIST SRM 1568a Rice Flour (National Institute of Standards and Technology, NIST, USA) was used to check the recovery of total arsenic. The certified value of total As in NIST SRM 1568a is 290 ± 0.03 μg kg−1, while the As speciation is not defined.2.3 Influence of extraction on speciation and recovery
An essential requirement for As speciation analysis is that the chemical forms of As remain unchanged during the extraction process. Recoveries of AsIII, AsV, MMAV, DMAV and MMTA were investigated using closed and open microwave extraction. The NIST 1568a sample was spiked with each of the As species standards (10 μg kg−1 as As) and their recoveries were determined (n = 3).2.4 Preparation of different particle size rice samples
Basmati, polished and parboiled rice were obtained from a local supermarket. All three types of rice (polished, basmati and parboiled) were ground using a mill followed by successive sieving through sieves with 1 and 0.5 mm mesh size. Thus, following fractions were collected: (1) whole grain (non-ground sample), (2) >1 mm–whole grain, (3) 0.5 mm–1 mm, (4) <0.5 mm. In addition, an aliquot of fraction (4) was additionally ground in a mortar, and is referred to as (5) fine powder.2.5 Experimental setup
2.6 Analysis procedure for total arsenic and its speciation in rice matrices
Microwave digestion procedures using open (Teflon tubes without lid) and closed vessels were used to assess the role of pressure during extraction.For microwave extraction, one gram dry weight of a sample (rice) was placed into 50 ml polypropylene centrifuge tubes and 10 ml of double distilled deionized water was added. For microwave using closed vessels, the vessels were closed and temperature was raised to 80 °C in 5 minutes, held at 80 °C for 30 minutes, after which vessels were cooled to room temperature in 10 minutes.
In the open microwave digestion procedure, the temperature was raised to 90 °C in 5 minutes and held for 180 minutes at 90 °C. The mixture was cooled to room temperature in 10 minutes.
Digested samples resulted in a nearly clear suspension and were filtered using a 0.45 μm syringe-type PVDF membrane filter and the filtrate was diluted to 25 ml using double distilled deionized water. This filtrate was analyzed for total arsenic content using ICP-MS. The same filtrate was used for speciation analysis using HPLC-ICP-MS.26 CRM NIST 1568a rice powder was used to check the recovery of total arsenic.
To check whether different particle size fractions of rice had the same amount of total arsenic, each particle size fraction was additionally extracted using a mixture of 2.5 ml of nitric acid and 4 ml of hydrogen peroxide instead of water. No speciation analysis was conducted on this extract.
2.7 Instrumentation
A MarsX system (CEM, Matthews, NC, USA) was used for the microwave-assisted extraction. High performance liquid chromatography hyphenated to an inductively coupled plasma mass spectrometer (HPLC-ICP-MS) was used to analyze the different food extracts for content in different As species (Perkin Elmer, Sunnyvale, CA, USA). It consisted of a P680 HPLC pump, an ASI-100 automated sample injector and an Elan DRC-e ICP-MS detector. A Hamilton PRP-X100 anion exchange column (Grace, Belgium) was used as the stationary phase. By using mass call solution the ICP-MS was tuned to maintain the ratio of oxides (CeO/Ce) and divalent/monovalent ions (140Ce2+/140Ce1+) less than or equal to 0.03, as prescribed by PerkinElmer, to minimize the potential interferences. Oxygen was used as reaction gas in both methods, resulting in detection of arsenic as AsO (arsenic oxide) at 91 amu. This procedure is designed to overcome the interference caused by ArCl+ and CaCl+ upon arsenic detection at 75 amu. Instrumental settings are summarized in Table 1.| Detection DRC mode (As) | |
|---|---|
| Instrument | Perkin Elmer Elan DRC-e |
| Plasma RF power | 1250 W |
| Nebulizer flow rate | 0.7–1.1 ml min−1 (optimized daily) |
| Lens voltage and autolens | Optimized for AsO daily |
| Dwell times m/z | 91 (AsO 500 ms) |
| Reaction gas | O2, 0.75 ml min−1 |
| Cell parameters Rpq | 0.6 |
3 Results and discussion
3.1 Effect of different extraction solvents on arsenic speciation
Similar patterns of speciation were observed when NIST 1568a was extracted using water by both closed and open microwave digestion (Table 2). Recoveries of all spiked species standards (10 μg kg−1 as As each) were close to 100% for the closed microwave extraction procedure while using water as the extraction solvent (Table 2). This proves that they are not chemically altered to any significant extent during the extraction, whereas with the aggressive reagents like nitric acid (HNO3) + hydrogen peroxide (H2O2), a different pattern of speciation was observed (Table 2). This can be attributed to interconversion of As species while using aggressive reagents for extraction.25,27 Using TFA as an extraction solvent has been reported to result in conversion of arsenate to arsenite,5 so this can be used only to quantify total inorganic content. Raab et al.28 used HNO3 + H2O2 extraction for total arsenic content and 1% HNO3 + 1% H2O2 for speciation. They stated that usage of 1% nitric acid can preserve speciation with minimum changes. However, we found that performing extraction using 1% HNO3 + 1% H2O2 resulted in remarkable interspecies conversions (Table 3). Arsenic speciation content for different rice samples is presented in Table 3. Huang et al.29 extracted inorganic arsenic using 0.28 M nitric acid and postulated recoveries of 101–105% for spiked AsIII and 95–97% for spiked AsV. Using further diluted nitric acid decreased the total extraction efficiency of As.29 However, in our study extraction recovery for spiked inorganic As species is 100–101%. Compared to the extraction method using 0.28 M nitric acid, the present method using water as the extraction solvent obtained a similar extraction recovery for total arsenic. From these results we conclude that using aggressive reagents will not give the precise characterization of species even though they extract the total arsenic from the sample. Water extraction method is the best method for species preserving extraction of arsenic from rice and economic compared to methods using chemical reagents.| Method | Sample | AsIII (μg kg−1) | DMAV (μg kg−1) | MMAV (μg kg−1) | AsV (μg kg−1) | Sum of species (μg kg−1) | Recovery (%) |
|---|---|---|---|---|---|---|---|
| Closed microwave (water) | NIST 1568a | 53.3 ± 1.0 | 172.3 ± 1.7 | 13.2 ± 0.3 | 46.1 ± 0.7 | 284.9 ± 2.9 | 98.3 (30 min) |
| Open microwave (water) | NIST 1568a | 52.1 ± 1.1 | 175.2 ± 3.1 | 13.1 ± 0.2 | 46.8 ± 0.9 | 287.2 ± 2.7 | 98.9 (3 h) |
| Closed microwave (water) | Spike recovery | 10.1 ± 0.2 | 9.3 ± 1.9 | 10.1 ± 0.3 | 10.1 ± 0.3 | 39.5 ± 0.5 | 98.7 |
| Closed microwave (HNO3 + H2O2) | NIST 1568a | 72.6 ± 1.1 | 63.3 ± 3.1 | 21.4 ± 0.2 | 130.9 ± 0.9 | 288.2 ± 2.7 | 99.3 |
| Method | Sample | AsIII (μg kg−1) | DMAV (μg kg−1) | MMAV (μg kg−1) | AsV (μg kg−1) | Species total (μg kg−1) | Total content (μg kg−1) | Chromatographic recovery (%) |
|---|---|---|---|---|---|---|---|---|
| Closed microwave (water) | Polished | 1.6 ± 0.02 | 125.9 ± 4.1 | <0.6 (detection limit) | 35.1 ± 0.8 | 162.6 ± 3.1 | 163 ± 1.7 | 99.4 |
| Closed microwave (1% HNO3 + 1% H2O2) | Polished | 2.1 ± 0.08 | 62.6 ± 1.5 | <0.6 (detection limit) | 98.2 ± 1.1 | 162.9 ± 3.1 | 163.4 ± 3.1 | 99.5 |
| Closed microwave (water) | Basmati | 2.4 ± 0.03 | 149.2 ± 2.9 | <0.6 (detection limit) | 44.7 ± 0.9 | 196.3 ± 4.1 | 197.8 ± 1.8 | 99.2 |
| Closed microwave (1% HNO3 + 1% H2O2) | Basmati | 3.2 ± 0.04 | 67 ± 1.2 | <0.6 (detection limit) | 127.1 ± 3.1 | 197.3 ± 4.3 | 198.1 ± 3.6 | 99.5 |
| Closed microwave (water) | Parboiled | 2.6 ± 0.03 | 132.8 ± 2.3 | <0.6 (detection limit) | 40.7 ± 0.9 | 176.1 ± 3.3 | 177.6 ± 1.9 | 99.2 |
| Closed microwave (1% HNO3 + 1% H2O2) | Parboiled | 3.1 ± 0.04 | 67 ± 3.2 | <0.6 (detection limit) | 107.2 ± 2.2 | 177.3 | 178.1 ± 3.3 | 99.4 |
3.2 Determination of total arsenic
Using NIST 1568a extraction efficiencies were calculated. Extraction efficiencies of closed and open microwave digestion while using water as the extraction solvent were 98.3% and 99%, respectively (Table 2). Total As content of different rice types is presented in Table 3. Even though both extraction procedures have similar extraction efficiencies, closed microwave digestion attained it quickly (30 minutes). This and the above-mentioned results lead us to conclude that closed microwave digestion using water was the best approachable method to assess both total and speciation content of As.3.3 Extraction at different liquid to solid (l/s) ratios
Higher extraction efficiencies were obtained with higher l/s ratio values. Extraction efficiency decreased from 95% to 83% by decreasing the l/s ratio from 10 to 6 (Fig. 1). This decrease can be attributed to the decreased extraction efficiency of DMAV at low l/s ratio (Fig. 1). At low l/s ratio, DMAV may have reached an equilibrium stage, with no more DMAV being extracted from the sample. Narukawa et al.23 also stated that an l/s ratio of at least 10 is needed to achieve higher extraction efficiencies. From our data, an l/s ratio of 10 was found to be optimal. At even higher l/s ratios sensitivity of the analytical equipment may not be high enough to deal with the lower extract concentrations.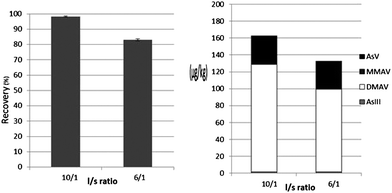 | ||
| Fig. 1 Effect of liquid/solid (l/s) ratio on extraction efficiencies of total arsenic (left figure) and individual arsenic species (right figure) in closed microwave extraction while using a powdered sample and water as the extracting solvent. Number of replicates (n) = 3. | ||
3.4 Extraction at different temperatures
Higher extraction efficiencies were obtained with increasing temperatures, provided the temperature remained below 100 °C (Fig. 2). At 100 °C, lower efficiencies of about 88% were obtained. A similar decrease of arsenic extraction efficiency at 100 °C was observed by Narukawa et al.23 This can be attributed to escape of arsenic due to aerosol formation and difficulties in dissolving As in the solvent during extraction. Extraction efficiencies further decreased at 120 °C which may be attributed to charring of the sample.23 So it can be concluded that extraction at 80 °C results in the optimal extraction efficiency. | ||
| Fig. 2 Effect of temperature on As extraction efficiencies during closed microwave digestion while using a powdered sample and water as the extracting solvent. Number of replicates (n) = 3. | ||
3.5 Extraction at different hold times
Higher extraction efficiencies were obtained when increasing the hold time up to 30 minutes at 80 °C (Fig. 3) using closed microwave digestion. Narakuwa et al.23 found that the extraction efficiency could not be increased when further increasing the hold time. Narukawa and Chiba24 achieved extraction efficiencies of 98% only after 3 h at 90 °C while using a heating block. In addition, we observed that open digestion took 3 h to achieve 99% extraction efficiency using microwave at 90 °C (Fig. 4), whereas closed microwave digestion attained 98% extraction efficiency by 30 minutes. This difference can be attributed to the absence of pressure, as their experiment was conducted in an open heating block. This supports the fact that pressure plays a key role in extracting arsenic from the sample. From our results, we conclude that a hold time of 30 minutes is most optimal to extract As from rice samples during a closed microwave extraction technique. | ||
| Fig. 3 Effect of hold time on As extraction efficiencies during closed microwave digestion at 80 °C while using a powdered sample and water as the extracting solvent. Number of replicates (n) = 3. | ||
 | ||
| Fig. 4 Effect of hold time on As extraction efficiencies during open microwave digestion at 90 °C while using a powdered sample and water as the extracting solvent. Number of replicates (n) = 3. | ||
3.6 Extraction of sample with different particle sizes
Extraction of different particle size fractions using nitric acid and hydrogen peroxide was also performed to check whether all fractions of rice had the same amount of total arsenic content. Results revealed that all fractions contain more or less similar amounts of total arsenic content. This is the case for all rice matrices (Table 4).| Method | Sample | Powder (μg kg−1) | <0.5 mm (μg kg−1) | 0.5–1 mm (μg kg−1) | 1 mm-whole grain (μg kg−1) | Whole grain (μg kg−1) |
|---|---|---|---|---|---|---|
| Closed microwave (HNO3 + H2O2) | Polished | 162.4 ± 3.3 | 162.7 ± 4.3 | 162.9 ± 4.1 | 163.1 ± 3.3 | 163.4 ± 3.1 |
| Closed microwave (HNO3 + H2O2) | Basmati | 196.3 ± 5.7 | 196.9 ± 4.8 | 197.3 ± 4.8 | 197.6 ± 4.1 | 198.1 ± 3.6 |
| Closed microwave (HNO3 + H2O2) | Parboiled | 176.4 ± 5.1 | 176.8 ± 4.5 | 177.4 ± 3.9 | 177.8 ± 3.7 | 178.1 ± 3.3 |
Higher extraction efficiencies were obtained with decreasing particle size for all types of rice when using water as the extraction solvent. For closed microwave extraction the extraction efficiencies were 98%, 93%, 92%, 90% and 85%, when using polished rice as powder, particles of <0.5 mm size, 0.5 mm–1 mm, 1 mm–whole grain and whole grain, respectively. In open microwave extraction, the extraction efficiency was 98% when analyzing powder, but decreased to 50% at particle size of 0.5 mm and more (Fig. 5). For closed microwave extraction, these efficiencies were 98%, 92%, 90%, 86% and 74% when analyzing basmati rice as powder, particles of <0.5 mm size, 0.5 mm–1mm, 1 mm–whole grain and whole grain, respectively. In open microwave extraction, the extraction efficiency was 98% when analyzing powder, but decreased to 48% at a particle size of 0.5 mm and more (Fig. 5). Closed microwave extraction efficiencies were 97%, 93%, 89%, 84% and 72%, when preparing parboiled rice as powder, particle with a size of <0.5 mm, 0.5 mm–1 mm, 1 mm–whole grain, or analyzing whole grain, respectively. In open microwave extraction, this extraction efficiency was 98% when working with powder, but decreased to 47% with particle size 0.5 mm and more (Fig. 5).
 | ||
| Fig. 5 Effect of particle size on As extraction efficiencies for different rice samples treated by closed and open microwave extraction using water as the extracting solvent. Number of replicates (n) = 3. | ||
Open and closed microwave extraction exhibited similar extraction efficiencies for all types of rice when the rice was prepared as powder, i.e. also ground in a mortar after grinding using a mill. Closed microwave extraction achieved this efficiency more rapidly (30 min) compared to open microwave extraction (180 min). The decrease of extraction efficiency when using open microwave digestion while treating a rice sample of 0.5 mm and more particle size can be attributed to the absence of pressure. In the literature20,23 it is stated that inorganic arsenic species are tightly bound to proteins in rice and for extracting them, strong conditions are required. Narukawa and Chiba24 observed less recovery of arsenic for rice sample at particle size 0.5 mm compared to present data. This was due to the absence of pressure and also uneven distribution of temperature in the heating block. All the above results postulated that pressure and particle size of the sample play key roles in effective extraction of arsenic from rice. From our data, we conclude that closed microwave digestion of a powdered matrix rather than the whole grain product results in better extraction efficiencies.
3.7 Arsenic species content in different particle size samples
Speciation analysis was also performed on all three types of rice extracts obtained by closed microwave digestion. DMAV represented the most abundant species in all types of rice, followed by AsV and AsIII (Table 3). DMAV was found to be the abundant species in NIST156a by other research groups also,23,24 with some exceptions.27 The extraction of As from powdered rice samples revealed a 70% organic As content versus a 30% inorganic As content in all rice types (Fig. 6), whereas rice samples with higher inorganic content compared to organic content have also been reported in some other studies.23,27 This observation postulates the speciation pattern that we found was not representative for all rice types. Differences in speciation patterns in rice samples can be explained by differences in origin and growth conditions of rice. Although the total extraction efficiency was lower for whole grain samples (75%), the speciation pattern, i.e. the ratio between the different species, did not change significantly. The only exception observed was a slightly lower extraction efficiency for AsIII in the whole grain sample of parboiled rice (Fig. 6). This can be attributed to the fact that this species is well bound to the matrix.24 From our data, it can be concluded that extraction efficiencies of individual Arsenic species are affected by particle size to the same extent, except for AsIII. | ||
| Fig. 6 Fraction of different arsenic species relative to total arsenic content in different rice matrices (when extracted using a closed microwave system with water as the extracting solvent), as a function of particle size. | ||
4 Conclusion
Open and closed microwave digestion methods performed similarly when rice is treated as a powder. When rice particle size is above or equal to 0.5 mm, closed microwave digestion performed better. Extraction efficiencies of individual arsenic species are affected by particle size to the same extent, except for AsIII.Even though aggressive reagents are commonly used for total arsenic extraction, water is a suitable choice while extracting a powdered sample. The water extraction method provides a similar quantitative extraction efficiency and also preserves the speciation compared to other extraction methods using aggressive reagents. In addition, water is the cheapest and environmentally friendly solvent. Sample particle size plays a very crucial role in the extraction efficiency of arsenic. The sample needs to be finely ground to powder form to achieve the best extraction efficiency while using the water extraction method. Closed microwave digestion with water and rice as a powdered sample at 80 °C and a hold time of 30 min is the best condition for rice digestion for effective and As species preserving extraction.
Acknowledgements
This study was funded by the Federal Public Service of Health, Food Chain Safety and Environment Contract (BIOTRAS RF 6247).References
- P. N. Williams, A. Villada, C. Deacon, A. Raab, J. Figuerola, A. J. Green and A. A. Meharg, Environ. Sci. Technol., 2007, 41, 6854–6859 CrossRef CAS.
- http://www.efsa.europa.eu/en/scdocs/scdoc/1351.htm .
- NRC (National Research Council), Arsenic in Drinking Water – 2001 Update, National Academy Press, Washington, D.C, 2001 Search PubMed.
- IARC. 84, Some Drinking-Water Disinfectants and Contaminants, Including Arsenic, Geneva, 2004 Search PubMed.
- P. N. Williams, A. H. Price, A. Raab, S. A. Hossain, J. Feldmann and A. A. Meharg, Environ. Sci. Technol., 2005, 39, 5531–5540 CrossRef CAS.
- K. A. Francesconi and D. Kuehnelt, Analyst, 2004, 129, 373–395 RSC.
- P. L. Buldini, S. Cavalli and A. Trifirò, J. Chromatogr., A, 1997, 789, 529–548 CrossRef CAS.
- V. G. Mihucz, E. Tatar, I. Virag, C. Zhao, Y. Jao and G. Zaray, Food Chem., 2007, 105, 1718–1725 CrossRef CAS.
- M. K. Engupta, M. A. Hossain, A. Mukherjee, S. Ahamed, B. Das, B. Nayak, A. Pal and D. Chakraborti, Food Chem. Toxicol., 2006, 44, 1823–1829 CrossRef.
- A. H. Ackerman, P. A. Creed, A. N. Parks, M. W. Fricke, C. A. Schwegel, J. T. Creed, D. T. Heitkemper and N. P. Vela, Environ. Sci. Technol., 2005, 39, 5241–5246 CrossRef CAS.
- M. Ae, C. Watanabe, T. Inaoka, M. Sekiyama, N. Sudo, M. H. Bokul and R. Ohtsuka, Lancet, 2002, 360, 1839–1840 CrossRef.
- J. G. Aparra, D. Velez, R. Barbera, R. Farre and R. Montoro, J. Agric. Food Chem., 2005, 53, 8829–8833 CrossRef.
- S. Torres-Escribano, M. Leal, D. Velez and R. Montoro, Environ. Sci. Technol., 2008, 42, 3867–3872 CrossRef CAS.
- A. Ignes, K. Mita, F. Burlo and A. A. Carbonell-Varrachina, Food Addit. Contam., 2007, 25, 41–50 CrossRef.
- J. Gailer and K. J. Irgolic, J. Chromatogr., A, 1996, 730, 219–229 CrossRef CAS.
- P. Terasahde, M. Pantsar-Kallio and P. K. G. Manninen, J. Chromatogr., A, 1996, 750, 83–88 CrossRef.
- M. F. Hughes, V. Devesa, B. M. Adair, S. D. Conklin, J. T. creed, M. Styblo, E. M. Kenyon and D. J. Thomas, Toxicol. Appl. Pharmacol., 2007, 227, 26–35 CrossRef.
- D. Wallschlager and J. London, Environ. Sci. Technol., 2008, 42, 228–234 CrossRef.
- Y. Nakamura, T. Narukawa and J. Yoshinaga, J. Agric. Food Chem., 2008, 56, 2536 CrossRef CAS.
- E. Sanz, R. Muñoz-Olivas, C. Camara, M. K. Sengupta and S. Ahamed, J. Environ. Sci., 2007, 42(12), 1695–1705 CAS.
- Y.-G. Zhu, G.-X. Sun, M. Lei, M. Teng, Y.-X. Liu, N.-C. Chen, L. H. Wang, A. M. Carey, C. Deacon, A. Raab, A. A. Meharg and P. N. Williams, Environ. Sci. Technol., 2008, 42, 5008–5013 CrossRef CAS.
- I. Pizarro, M. Gomez, C. Camara and M. A. Palacios, Anal. Chim. Acta, 2003, 495, 85 CrossRef CAS.
- T. Narukawa, K. Inagaki, T. Kuroiwa and K. Chiba, Talanta, 2008, 77, 427–432 CrossRef CAS.
- T. Narukawa and K. Chiba, J. Agric. Food Chem., 2010, 58, 8183–8188 CrossRef CAS.
- M. Quaghebeur, Z. Rengel and M. Smirk, J. Anal. At. Spectrom., 2003, 18, 128–134 RSC.
- P. Alava, F. Tack, G. D. Laing and T. Van De Wiele, Biomed. Chromatogr., 2012, 26(4), 524–533 CrossRef CAS.
- E. Sanz, R. Muñoz-Olivas and C. Camara, J. Chromatogr., A, 2005, 1097, 1 CrossRef CAS.
- A. Raab, C. Baskaran, J. Feldmann and A. A. Meharg, J. Environ. Monit., 2009, 11, 41–44 RSC.
- J. H. Huang, P. Fecher and G. Ilgen, Food Chem., 2012, 130(2), 453–459 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
