Polyselenophenes with distinct crystallization properties†
Lianshan
Li
,
Jon
Hollinger
,
Ashlee A.
Jahnke
,
Srebri
Petrov
and
Dwight S.
Seferos
*
Department of Chemistry, University of Toronto, 80 St. George Street, Toronto, Ontario M5S 3H6, Canada. E-mail: dseferos@chem.utoronto.ca
First published on 13th September 2011
Abstract
The polythiophene derivative poly(3-hexyl)thiophene (P3HT) has become one of the most well studied organic materials due to its interesting and important chemical and physical properties. Two different crystal structures have been observed for P3HT, type-1 and type-2, however a pure type-2 structure has never been obtained. Herein, we investigate the crystal structure of polyselenophene analogs (P3HS), and discover that a pure type-2 phase is formed in low molecular weight P3HS (Mn = 5.9 kg mol−1). Wide-angle X-ray scattering shows that the type-2 phase is readily formed and stable at room temperature, which is very distinct from what is observed in control experiments with P3HT. Absorption spectra of P3HS films with the pure type-2 phase lack the typical shoulder peaks indicating that π–π stacking is relatively poor in the type-2 phase. Scanning transmission electron microscopy (STEM) images, however, show that large nanofibers are formed by type-2 crystallization thereby demonstrating the potential of P3HS to drive unique types of self-assembled structures through crystallization, and should motivate continued efforts on selenophene analogs of P3HT for a variety of studies and uses.
Introduction
The conjugated polymer poly(3-hexyl)thiophene (P3HT) has become one of the most well studied organic materials, finding use in a variety optoelectronic devices.1–8 Of equal importance to device characterization are the supramolecular structure and morphology of this important macromolecule. This is because device properties are greatly affected by film morphology.9–16 In this vein, the structural characteristics of several poly(3-alkyl)thiophenes (P3ATs) have been intensively studied.17–24X-Ray diffraction investigations have shown that the common crystalline polymorph of these macromolecules consists of lamellar structures with regions filled by alkyl side-chains separated by parallel stacks of polymer main-chains. More specifically, each layer consists of stacked arrays of main-chains that are 3.8 Å apart and are alternately oriented, resulting in a staggered nesting of the side-chains. The layer thickness in this phase, which is the distance between main-chains, varies almost linearly with the length of the aliphatic side-chains.20 This three-dimensional structure is often referred to as the type-1 polymorph of P3ATs (Fig. 1b). In addition, a second polymorph (type-2) has been reported for the longer side-chain derivatives (octyl, decyl, and dodecyl) of the P3AT series.25 This phase is characterized by a substantially shorter layer thickness. Structural models for the type-2 polymorph are less clearly defined. In the literature there are suggestions that this phase presents a more significant interdigitation of the side-chains when compared to what is now generally accepted for the type-1 structure.25 A second explanation is that the side-chains are more tilted, which allows the main-chains to pack more closely.26 | ||
| Fig. 1 Wide-angle X-ray scattering of P3HS films prepared from different molecular weight samples before (a) and after (c) annealing at 140 °C. The insert in c shows the magnification of Mn = 5.9 kg mol−1 P3HS from 3°–10°. Scattering of an un-annealed P3HT film (d). Proposed supramolecular structure of the type-1 and type-2 polymorphs (b). | ||
Unlike the longer side-chain derivatives, the type-2 polymorph of the hexyl derivative P3HT is more elusive. For P3HT, type-2 phases have been observed in films composed of very low molecular weight samples, however, the type-1 phase is still the dominant polymorph, even at molecular weights as low as Mn = 2.6 kg mol−1.19 Additionally, reports thus far have shown that in P3HT the type-2 phase is much less crystalline than the type-1 as evidenced by a far less intense diffraction peak. Recently, our work on selenophene-thiophene block copolymers lead us to conclude that the type-2 polymorph is more crystalline than the type-1 in low molecular weight drop-cast samples of these novel block copolymers.27 As well, it appears that in a low molecular weight sample, a pure type-2 phase is formed, which has never been observed in P3HT.
Because a pure type-2 phase has never been obtained in P3HT, we hypothesized that this pure type-2 phase results from the selenophene as opposed to the thiophene block in the sample. In the case of polyselenophene homopolymers, however, this polymorph has not been reported. Herein, we explicitly study the crystal structure of P3HS with different molecular weights and show that, unlike P3HT, pure type-2 phases can be obtained in low molecular weight samples. Having obtained a pure type-2 polymorph, we study the optical and morphological properties of this homopolymer in this distinct crystal structure for the first time.
Results and discussion
P3HS and P3HT were synthesized according to literature procedures28–31 and characterized by NMR spectroscopy (Fig. S1, S2, S3, S4, S5†) and gel permeation chromatography by comparison to polystyrene standards (Fig. S4†). P3HS polymer samples have relative number average molecular weight values (Mn) of 19, 9.9 or 5.9 kg mol−1, P3HT polymer samples have (Mn) of 19.5 or 3.5 kg mol−1. In order to verify the molecular weight, matrix assisted laser desorption ionization mass spectrometry (MALDI) was used to determine the Mn of low weight P3HS. The MALDI spectrum of the P3HS sample with Mn of 5.9 kg mol−1 shows that the molecular weight lies between 2.0–3.0 kg mol−1 (Fig. S9†), which is consistent with GPC over-estimation by a factor of 1.2–2.3.32,33 For clarity we will refer to these three distributions of P3HS as high, medium, and low molecular weight samples. Drop-cast films of P3HS samples were investigated by wide-angle X-ray scattering (WAXS; Fig. 1a). This analysis shows that the as-cast high molecular weight P3HS film is poorly crystalline, exhibiting a weak broad diffraction peak at 2θ = 5.64°, corresponding to an interlayer spacing of 15.5 Å (Fig. 1a, green line). This is very close to an earlier reported crystal structure of P3HS, which had an interlayer spacing of 15.2 Å.29 This value is also very close to the reported 16.0 Å in type-1 P3HT.34 Because P3HS and P3HT have identical side-chains, and thus similar size repeat units, we concluded that this 15.5 Å diffraction peak is due to the type-1 phase of P3HS.As we turn attention to the medium molecular weight sample, the crystallinity of the film is noticeably improved and a second, new peak at 2θ = 7.30° appears. This new peak corresponds to a 12.1 Å spacing, indicating that a second polymorph with a shorter interlayer spacing is present in the medium molecular weight polymer. We define this diffraction pattern as the type-2 polymorph in P3HS. Although the type-1 phase coexists with this type-2 polymorph, (Fig. 1a, red line), the type-2 phase appears to be more crystalline as indicated by the sharpness of the diffraction peak. Moreover, when we decrease the molecular weight further and investigate the low molecular weight sample, the type-1 peak disappears, leaving only the sharp peak at 2θ = 7.30° showing that a pure type-2 phase is obtained (Fig. 1a, black line). The peak corresponding to the pure type-2 phase in the low molecular weight sample is much sharper than that corresponding to the type-1 phase in the high molecular weight sample, which indicates better crystallinity in the type-2 phase of P3HS in drop-cast films. In control experiments, P3HT with Mn = 19 kg mol−1 and 3.5 kg mol−1 were investigated by WAXS under analogous experimental conditions. Only type-1 phases are observed in both of these samples (Fig. 1c). Although the side-chain regioregularity of P3ATs has a significant impact on solid-state packing, the pure type-2 phase in low molecular weight P3HS is not caused by lower regioregularity as only type-1 phase is observed in both high and low molecular weight P3HT with nearly identical regioregularity as the P3HS samples (the RR was calculated from the ratio of peaks at 2.73 and 2.53 of the NMR spectra; Table S1†). Overall, the experimental data show that the crystallization of P3HS is strikingly distinct from P3HT.
We next designed experiments to investigate the stability of the type-2 structure in P3HS. In these studies, samples were annealed at 140 °C for 2 h. After annealing all the P3HS samples transferred to the type-1 phase (Fig. 1b). Interestingly, in annealed films, increasing molecular weight improves the relative crystallinity of the sample, which is different than in the as-cast films and the type-2 phase. Overall, annealing experiments show that the type-2 phase in P3HS is thermodynamically unstable, and for low molecular weight P3HS, the pure type-2 phase is the kinetic product.
Having determined that a pure type-2 structure can be obtained in low molecular weight P3HS, we next investigated the optical properties of these polymers in chlorobenzene and as thin films before and after annealing. The solution absorption spectra show that medium and high molecular weight samples have a very similar absorption maximum, occurring at 499 nm (Fig. 2a, black and green lines). For the low molecular weight polymer however, the absorption maximum appears at 450 nm (Fig. 2a, red line), which is significantly blue-shifted compared to the other samples. This difference is likely due to the chains being shorter than the chromophoric unit of the polymer (the polymer consists of about 13 repeat units after normalizing for GPC overestimation, a factor of 1.2–2.3 for rigid rod polymers).32,33 These differences in optical properties are even more pronounced in the solid state, where the high molecular weight sample exhibits a maximum at 610 nm, and in comparison the low molecular weight sample exhibits a maximum at 520 nm (Fig. 2b). Interestingly, shoulder peaks are observed in the medium and high molecular weight polymer films, but are missing in low molecular weight sample films (Fig. 2b, red line). The shoulder peak in the polymer thin film is indicative of organization and π–π stacking in the solid state.35 The lack of a well-defined shoulder peak in the absorption spectra of the low molecular weight sample suggests that π–π stacking in the type-2 phase is poor compared to the type-1 phase. After annealing for 2 h at 140 °C under nitrogen, the shoulder peaks in the high and medium molecular weight polymer become more pronounced, meaning that annealing and converting to a pure type-1 crystal structure improves π–π stacking in these two long polymers (Fig. 2c). A shoulder peak is still missing in the annealed low molecular weight polymer film, and is consistent with the X-ray data and the observation that a strong type-1 crystal structure is required for π–π stacking.
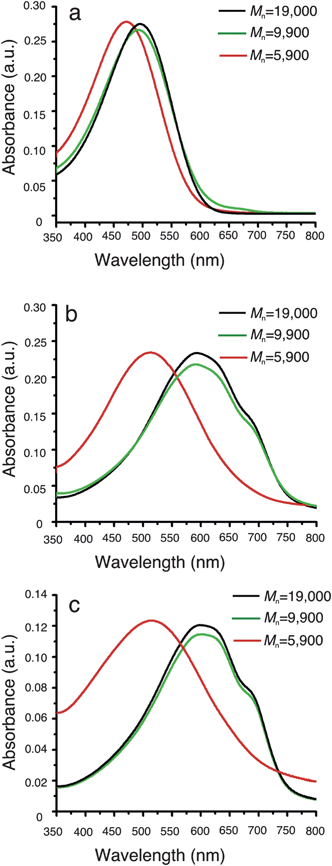 | ||
| Fig. 2 Absorption spectra of P3HS in chlorobenzene (a) and as a thin film before (b) and after (c) annealing at 140 °C. | ||
We next hypothesized that differences in crystal type would cause a difference in the microstructure of the film. The self-assembled structure of low molecular weight P3HS was investigated first using scanning transmission electron microscopy (STEM). Networks that consist of numerous fibers are formed from low molecular weight P3HS (Fig. 3a). Magnified STEM images show that the diameters of these fibers are about 100 nm and that they do not contain any obvious ends (Fig. 3a insert). This regular microstructure is consistent with the relatively high crystallinity in the type-2 phase at room temperature. As the molecular weight of the sample is increased to 9.9 kg mol−1, the film becomes very uniform and without any distinct features (Fig. 3b). As molecular weight is increased further to 19 kg mol−1, spherical domains that are 200–500 nm in diameter are observed (Fig. 3c), however these domains do not have regular features, which is consistent with the amorphous character of the un-annealed high molecular weight samples.
 | ||
| Fig. 3 STEM images of P3HS films formed from polymers with Mn = 5.9 kg mol−1 (a, d), 9.9 kg mol−1 (b, e) and 19 kg mol−1 (c, f) before (a, b, c) and after (d, e, f) annealing. | ||
Another polymerization method reported by Kiriy and co-workers36 was also used to synthesize low molecular weight P3HS without a tail-to-tail defect as this is expected to have an effect on polymer packing, especially for low molecular weight samples. This alternate P3HS was also characterized by NMR spectroscopy (Fig. S6†) and gel permeation chromatography by comparison to polystyrene standards. GPC measurement shows that the relative number average molecular weight is 4.5 kg mol−1, and very similar to the previously described low molecular weight sample (Table S1†). Images of drop-coated films of this sample are nearly identical to the previous low molecular weight P3HS (Fig. S7†), which indicates that the morphology we observe is not simply because of the high percentage of defects in low molecular weight polymer.
To compare these images with those in which the sample has a purely type-1 structure, we investigated the morphology of the samples after annealing at 140 °C for 2 h. STEM images of the annealed low molecular weight P3HS film shows that the large fibers vanish and the film appears very flat with several pinhole features (Fig. 3d). This is consistent with the destruction of the type-2 phase and lack of crystallinity in low molecular weight annealed films. For medium molecular weight films, annealing causes the formation of new large domains (Fig. 3e). Finally, for the high molecular weight films, annealing causes the formation of nanofibers that are 20–30 nm in diameter (Fig. 3f). Compared with the un-annealed film, it seems apparent that the crystallinity of the high molecular weight film is greatly improved by annealing, and the crystallinity of the low molecular weight film is greatly diminished by annealing, which is consistent with the X-ray diffraction studies noted above.
Conclusions
In summary, we have discovered a pure type-2 crystal phase in P3HS and examined the optical and morphological properties of this phase for the first time. X-ray studies show that this pure type-2 phase is more crystalline than the type-1 phase in un-annealed samples. Absorption spectroscopy indicates that the π–π stacking in the type-2 phase is relatively poor, when compared to the type-1 phase. Electron microscopy performed on kinetically formed pure type-2 films, however, reveal an interesting metastable microstructure that consists of large fibers, and is very prominent in the pure type-2 films. These studies are important because they highlight the differences in the self-assembly and crystallization of P3HS as opposed to P3HT polymers. Because the chemical structures of these two macromolecules are very similar, these differences were not expected. These observations demonstrate the potential of P3HS to drive distinct types of self-assembled structures in conjugated polymer films through distinct crystallization, and should motivate future research on P3HS-type polymers including diblock and gradient copolymers.Acknowledgements
We thank the University of Toronto, the Natural Sciences and Engineering Research Council of Canada, the Canadian Foundation for Innovation, and the Ontario Research Fund for supporting this work.Notes and references
- Y. Kim, S. Cook, S. M. Tuladhar, S. A. Choulis, J. Nelson, J. R. Durrant, D. D. C. Bradley, M. Giles, I. McCulloch, C.-S. Ha and M. Ree, Nat. Mater., 2006, 5, 197 CrossRef CAS.
- G. Li, V. Shrotriya, J. Huang, Y. Yao, T. Moriarty, K. Emery and Y. Yang, Nat. Mater., 2005, 4, 864 CrossRef CAS.
- W. Ma, C. Yang, X. Gong, K. Lee and A. J. Heeger, Adv. Funct. Mater., 2005, 15, 1617 CrossRef CAS.
- Y.-J. Cheng, C.-H. Hsieh, Y. He, C.-S. Hsu and Y. Li, J. Am. Chem. Soc., 2010, 132, 17381 CrossRef CAS.
- C. H. Woo, B. C. Thompson, B. J. Kim, M. F. Toney and J. M. J. Fréchet, J. Am. Chem. Soc., 2008, 130, 16324 CrossRef CAS.
- Z. Xu, L.-M. Chen, G. Yang, C.-H. Huang, J. Hou, Y. Wu, G. Li, C.-S. Hsu and Y. Yang, Adv. Funct. Mater., 2009, 19, 1227 CrossRef CAS.
- R. M. Beal, A. Stavrinadis, J. H. Warner, J. M. Smith, H. E. Assender and A. A. R. Watt, Macromolecules, 2010, 43, 2343 CrossRef CAS.
- J. F. Mike, K. Nalwa, A. J. Makowski, D. Putnam, A. L. Tomlinson, S. Chaudhary and M. Jeffries-EL, Phys. Chem. Chem. Phys., 2011, 13, 1338 RSC.
- R. Zhang, B. Li, M. C. Iovu, M. Jeffries-EL, G. Sauvé, J. Cooper, S. Jia, S. Tristram-Nagle, D. M. Smilgies, D. N. Lambeth, R. D. McCullough and T. Kowalewski, J. Am. Chem. Soc., 2006, 128, 3480 CrossRef CAS.
- R. J. Kline, M. D. McGehee, E. N. Kadnikova, J. Liu, J. M. J. Fréchet and M. F. Toney, Macromolecules, 2005, 38, 3312 CrossRef CAS.
- V. Ho, B. W. Boudouris, B. L. McCulloch, C. G. Shuttle, M. Burkhardt, M. L. Chabinyc and R. A. Segalman, J. Am. Chem. Soc., 2011, 133, 9270 CrossRef CAS.
- L. F. Drummy, R. J. Davis, D. L. Moore, M. Durstock, R. A. Vaia and J. W. P. Hsu, Chem. Mater., 2011, 23, 907 CrossRef CAS.
- R. A. Marsh, J. M. Hodgkiss, S. Albert-Seifried and R. H. Friend, Nano Lett., 2010, 10, 923 CrossRef CAS.
- F. S. Kim, G. Ren and S. A. Jenekhe, Chem. Mater., 2011, 23, 682 CrossRef CAS.
- G. Ren, P.-T. Wu and S. A. Jenekhe, ACS Nano, 2011, 5, 376 CrossRef CAS.
- E. Lee, B. Hammer, J.-K. Kim, Z. Page, T. Emrick and R. C. Hayward, J. Am. Chem. Soc., 2011, 133, 10390 CrossRef CAS.
- Z. Wu, A. Petzold, T. Henze, T. Thurn-Albrecht, R. H. Lohwasser, M. Sommer and M. Thelakkat, Macromolecules, 2010, 43, 4646 CrossRef CAS.
- S. Joshi, S. Grigorian, U. Pietsch, P. Pingel, A. Zen, D. Neher and U. Scherf, Macromolecules, 2008, 41, 6800 CrossRef CAS.
- A. Zen, M. Saphiannikova, D. Neher, J. Grenzer, S. Grigorian, U. Pietsch, U. Asawapirom, S. Janietz, U. Scherf, I. Lieberwirth and G. Wegner, Macromolecules, 2006, 39, 2162 CrossRef CAS.
- T. J. Prosa, M. J. Winokur, J. Moulton, P. Smith and A. J. Heeger, Macromolecules, 1992, 25, 4364 CrossRef CAS.
- M. E. Kose, J. Phys. Chem. C, 2011, 115, 13076 CAS.
- W. C. Tsoi, S. J. Spencer, L. Yang, A. M. Ballantyne, P. G. Nicholson, A. Turnbull, A. G. Shard, C. E. Murphy, D. D. C. Bradley, J. Nelson and J.-S. Kim, Macromolecules, 2011, 44, 2944 CrossRef CAS.
- K. Zhao, L. Xue, J. Liu, X. Gao, S. Wu, Y. Han and Y. Geng, Langmuir, 2010, 26, 471 CrossRef CAS.
- M. Aryal, K. Trivedi and W. Hu, ACS Nano, 2009, 3, 3085 CrossRef CAS.
- T. J. Prosa, M. J. Winokur and R. D. McCullough, Macromolecules, 1996, 29, 3654 CrossRef CAS.
- S. V. Meille, V. Romita, T. Caronna, A. J. Lovinger, M. Catellain and L. Belobrzeckaja, Macromolecules, 1997, 30, 7898 CrossRef CAS.
- L. Li, J. Hollinger, N. Coombs, S. Petrov and D. S. Seferos, Angew. Chem., Int. Ed., 2011, 50, 8148 CrossRef CAS.
- J. Hollinger, A. A. Jahnke, N. Coombs and D. S. Seferos, J. Am. Chem. Soc., 2010, 123, 8546 CrossRef.
- M. Heeney, W. Zhang, D. J. Crouch, M. L. Chabinyc, S. Gordeyev, R. Hamilton, S. J. Higgins, I. McCulloch, P. J. Skabara, D. Sparrowea and S. Tierneya, Chem. Commun., 2007, 5061 RSC.
- J. R. Locke and A. J. McNeil, Macromolecules, 2010, 43, 8709 CrossRef CAS.
- Z. Q. Wu, R. J. Ono, Z. Chen and C. W. Bielawski, J. Am. Chem. Soc., 2010, 132, 14000 CrossRef CAS.
- J. Liu, R. S. Loewe and R. D. McCullough, Macromolecules, 1999, 32, 5777 CrossRef CAS.
- S. Holdcroft, J. Polym. Sci., Part B: Polym. Phys., 1991, 29, 1585 CrossRef CAS.
- R. D. McCullough, S. Tristram-Nagle, S. P. Williams, R. D. Lowevt and M. Jayaramant, J. Am. Chem. Soc., 1993, 115, 4910 CrossRef CAS.
- Z. Zhou, X. Chen and S. Holdcroft, J. Am. Chem. Soc., 2008, 130, 11711 CrossRef CAS.
- V. Senkovskyy, M. Sommer, R. Tkachov, H. Komber, W. T. S. Huck and A. Kiriy, Macromolecules, 2010, 43, 10157 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1sc00415h |
| This journal is © The Royal Society of Chemistry 2011 |
