Phosphine-catalyzed one-pot isomerization of 3-alkynoates and [2 + 3]-cycloaddition with imines: formal synthesis of Securinegaalkaloid (±)-allosecurinine†
Magesh
Sampath
,
Pei-Ying Beatrix
Lee
and
Teck-Peng
Loh
*
Division of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637616. E-mail: teckpeng@ntu.edu.sg; Fax: +65 6515 8229; Tel: +65 6513 8475
First published on 7th July 2011
Abstract
Trimethylphosphine-induced isomerization and cycloaddition of 3-alkynoates with imines for the synthesis of 2,5-syn di-substituted pyrrolines were described. In addition, the utility of this protocol was demonstrated in the efficient formal synthesis of the Securinegaalkaloid ‘allosecurinine’.
Heterocycles and carbocycles are known to be of great importance in identifying biologically significant molecules.1 Many natural products and drug molecules possess nitrogen heterocycles as the core structural unit. Amongst them, pyrrolines are featured widely in many natural products.2 Consistent with their biological importance, various methods have been developed for the synthesis of pyrrolines.
An efficient protocol to construct such heterocycles, using metal free catalysis, has been attracting much attention. Amongst the available methods, Lu's phosphine-catalyzed [2 + 3]-cycloaddition using electron-deficient allenoates/2-alkynoates and imines is considered to be an efficient strategy for the construction of pyrrolines.3 The asymmetric version of this reaction was successfully examined by Jacobsen,3q Marinetti3r–t and Gladysz.3u Inspired by this strategy, Kwon and Fu elegantly demonstrated the [4 + 2] approach in the synthesis of tetrahydropyridine derivatives.4 Although Lu's [3 + 2]-cycloaddition with olefins have been widely utilized in the synthesis of natural products,5 surprisingly, there is no documentation of this reaction with imines in the natural product synthesis. This is probably due to the difficulty of executing this reaction using aliphatic aldimines which contain α-C–H functionality. Another limitation is the difficulty of synthesizing the allenoate/2-alkynoate starting material and their limited substrate scope.6
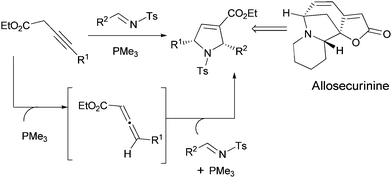
Inspired by our previous studies on the use of 3-alkynoates in [2 + 3]-cycloaddition reaction,6d in this report we describe trimethylphosphine catalyzed in situisomerization of 3-alkynoate to allenoate and cycloaddition with various activated aromatic and aliphatic imines in the synthesis of 2,5-disubsituted pyrrolines as single syn diastereomers and applied this methodology to the formal synthesis of Securinegaalkaloid ‘allosecurinine’ (Scheme 1).
 | ||
| Scheme 1 Proposed synthesis of 2,5-syn substituted pyrrolines and its application in formal synthesis of allosecurinine. | ||
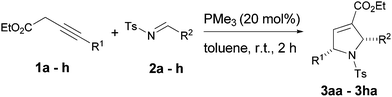
For the preliminary investigation, ethyl-4-phenylbut-3-ynoate 1a and N-tosylimine 2a were chosen as model substrates and the reactions were attempted with various commercially available phosphines (for detailed optimization studies please refer to supplementary information†). To our delight, the reaction proceeded smoothly with a catalytic amount of trimethylphosphine (20 mol%) to afford functionalized pyrrolines 3aa in 84% yield with excellent regio- and diastereoselectivities (Table 1, entry 1). However with nitrogen nucleophiles instead of phosphines, such as DABCO® (1, 4-diazabicyclo[2.2.2]octane) or triethylamine yields only the isomerized product, not the required cyclized product. To examine the generality of this protocol, various electron-deficient aldimines7a (Table 1; entries 1–8) and alkynoates8 (Table 1, entries 9–15) were screened, and in all the cases, excellent yields were observed. Electron withdrawing or releasing substituents on both aldimines and alkynoates provided products in moderate to good yields. Interestingly, the reaction with heteroaromatic containing 3-alkynoate 1f and imine 2h proceeded smoothly to afford product 3fh in 79% yield.
| Entry | R1 | R2 | Product | Yield (%) |
|---|---|---|---|---|
| a See the ESI for detailed experimental procedure;† 3-alkynoates (1a–1h) were contaminated with 2–5% of the corresponding allenoates. b Isolated yield. c The relative stereochemistry of product 3ad was assigned by 1H–1H Noesy. All other product was assigned by analogy. | ||||
| 1 | C6H5 (1a) | C6H5 (2a) | 3aa | 84 |
| 2 | C6H5 (1a) | 4-Et–C6H4 (2b) | 3ab | 76 |
| 3 | C6H5 (1a) | 4-Me–C6H4 (2c) | 3ac | 68 |
| 4c | C6H5 (1a) | 4-OMe–C6H4 (2d) | 3ad | 71 |
| 5 | C6H5 (1a) | 2-OMe–C6H4 (2e) | 3ae | 70 |
| 6 | C6H5 (1a) | 4-Br–C6H4 (2f) | 3af | 54 |
| 7 | C6H5 (1a) | 4-Cl–C6H4 (2g) | 3ag | 89 |
| 8 | C6H5 (1a) | 2-Furyl (2h) | 3ah | 64 |
| 9 | 4-Me–C6H4 (1b) | 2-Furyl (2h) | 3bh | 77 |
| 10 | 4-OMe–C6H4 (1c) | 2-Furyl (2h) | 3ch | 68 |
| 11 | 6-OMe–2-naphthyl (1d) | 2-Furyl (2h) | 3dh | 61 |
| 12 | 4-CF3–C6H4 (1e) | 2-Furyl (2h) | 3eh | 83 |
| 13 | Thien-2-yl (1f) | 2-Furyl (2h) | 3fh | 79 |
| 14 | H (1g) | 2-Furyl (2h) | 3gh | 70 |
| 15 | CO2Et (1h) | C6H5 (2a) | 3ha | 55 |
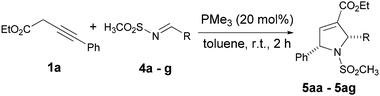
To further examine the synthetic value of the method, various methanesulfonamide protected imines7b were subjected to the previously optimized reaction conditions. In most of the cases, the results were similar with N-tosylaldimines. Both electron withdrawing and releasing groups on phenyl group of N-mesylimine proceeded smoothly to afford the cyclized product in good yields and excellent diastereoselectives (Table 2, entries 1–7). When using 4-nitro substituted N-mesylimines 4c, a considerable decrease in the yield was observed (Table 2, entry 3) due to the decomposition of imine in reaction condition.
| Entry | R | Product | Yield (%) |
|---|---|---|---|
| a See the ESI for detailed experimental procedure;† 3-butynoate (1a) was contaminated with 2% of corresponding allenoate. b Isolated yield. | |||
| 1 | Ph (4a) | 5aa | 84 |
| 2 | 4-CF3–C6H4 (4b) | 5ab | 76 |
| 3 | 4-NO2–C6H4 (4c) | 5ac | 47 |
| 4 | 4-Cl–C6H4 (4d) | 5ad | 71 |
| 5 | 4-CN–C6H4 (4e) | 5ae | 64 |
| 6 | 4-OMe–C6H4 (4f) | 5af | 70 |
| 7 | 2-Furyl (4g) | 5ag | 89 |
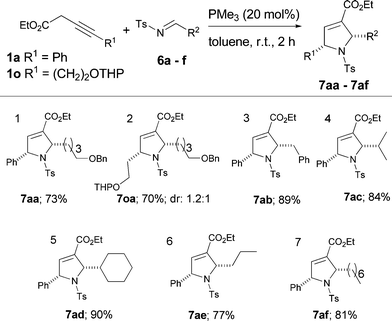
Pyrrolines with aliphatic substitutions are important building blocks featured widely in many natural products and drug molecules. With this in mind, we next turned our attention to using aliphatic aldimines with α-H functionality. Surprisingly, to the best of our knowledge, there is just one example where using such an aldimine (N-tosylcinnamaldimine) was reported.3b Under previously optimized reaction conditions, the aliphatic imines containing α-H functionality reacted smoothly to afford cyclized products in excellent yields (Table 3, entries 1–7). Moreover, THP protected hex-3-ynoates 1o and imine 6a provided 2,5-di-alkyl substituted pyrrolidine 7oa in 70% yield with 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric mixture (Table 3, entry 2).
1 diastereomeric mixture (Table 3, entry 2).
Encouraged by these results, next we sought application of this method in the synthesis of the Securinegaalkaloid.9 The most representative examples of this family includes securinine (1), its C-2 epimer allosecurinine (2) and their corresponding enantiomers, virosecurinine (3) and viroallosecurinine (4) (Scheme 2). Due to the interesting structural features and potential biological activities of this alkaloid, many research groups are continuing to focus on the synthesis.9 In 2008, Kerr et al. demonstrated the total synthesis of allosecurinine through the key intermediate 18.9h We envisioned the previously developed cycloaddition between aliphatic aldimines and alkyl substituted 3-alkynoates in the synthesis of pyrrolidine skeleton of key intermediate 18.
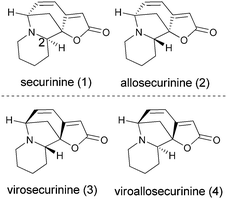 | ||
| Scheme 2 Structure of Securinegaalkaloids. | ||
Our retrosynthetic approach for the synthesis of intermediate 18 is shown in Scheme 3. The crucial steps involve (i) assembly of alkynoates 8 and imine 9 by PMe3-catalyzed [2 + 3]-cyclization to obtain 10 and (ii) reductive opening of epoxide to obtain the key intermediate 1,2-diol 18.
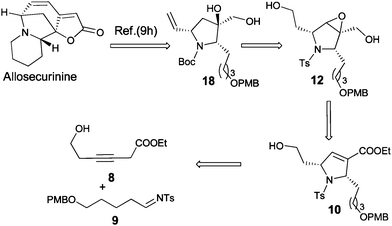 | ||
| Scheme 3 Proposed synthesis of 2,5-syn substituted pyrrolines and its application in formal synthesis of allosecurinine. | ||
To commence our synthetic plan, alkynoate 8 and N-tosylimine 9 were readily prepared following reported procedures.7,8 Using previously optimized conditions, the [2 + 3]-cycloaddition reaction was executed smoothly between 8 and 9 in the presence of a catalytic amount of trimethylphosphine to furnish the desired product 10 in 82% isolated yields as the single syn-diastereomers.
With the racemic product 10 in hand, aforethought, reduction of ethyl ester with DIBAL-H furnished the primary alcohol 11 and subsequent epoxidation with m-CPBA yields the required product 12.
In the next step, various reducing agents for epoxide opening such as Red-Al, lithium aluminum hydride and sodium hydride were tested. However, only a trace amount of products was obtained. To our delight, with di-isobutylaluminium hydride (DIBAL-H), the required product 13 was isolated in 64% yield. The resulting 1,2-diol moiety in product 13 was protected using 2,2′-dimethoxypropane to provide 14 and the remaining primary alcohol was iodinated to afford 15 in 87% yield with a trace amount of di-iodide product 15a (X-ray). The iodinated product 15 undergoes E2-elimination with sodium hydride to furnish 16. Although removal of the tosyl group failed with various mild protocols, eventually sodium naphthalide in DMSO was found to work smoothly to afford the product 17 in 69% yields.10 Finally, deprotection of the 1,2-diol and Boc protection were carried out in one-pot to furnish the key intermediate 18 (88% yield from 17; Scheme 4) and the structure was confirmed by comparison with a previous report.9h
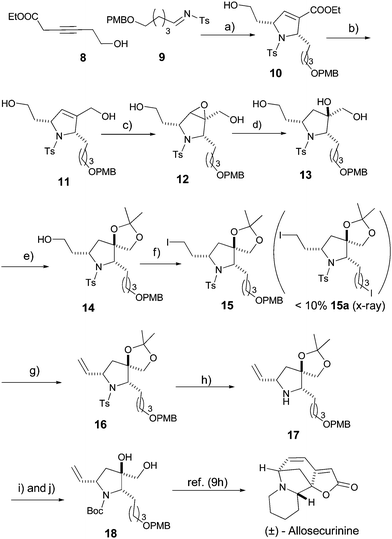 | ||
Scheme 4 Synthesis of key intermediate 18. Reaction conditions: a) PMe3 20 mol%, toluene, 2 h, 25 °C, 82%; b) 2.0 equiv. DIBAL-H (1.0 M in THF), −78 °C, CH2Cl2, 74%; c) m-CPBA, CH2Cl2, 12 h, 81%; d) 4.0 equiv. DIBAL-H (1.0 M in THF); −78 °C, −25 °C, CH2Cl2, 24 h, 64%; e) 10 equiv. 2-2′-dimethoxypropane, 0.1 equiv. CSA, CH2Cl2; 77% yield; f) I2, PPh3, imidazole; THF; 87%; g) NaH (60%), TBAI, THF, 78 °C, 88%; h) Na-naphthalenide, DME, 69%; i) 3M HCl, THF–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and j) (Boc)2O, TEA; THF, 88% (over 2 steps). CSA = camphorsulfonic acid, DME = dimethoxyethane, TEA = triethylamine. 1) and j) (Boc)2O, TEA; THF, 88% (over 2 steps). CSA = camphorsulfonic acid, DME = dimethoxyethane, TEA = triethylamine. | ||
In conclusion, an effective procedure for the synthesis of 2,5-syn-di-subsituted pyrrolines is described. This one-pot isomerization of 3-alkynoates and cycloaddition sequence with imines provided an efficient alternative to existing protocols for the synthesis of 2,5-syn-disubsituted pyrrolines. Moreover, pyrrolines with both aromatic and aliphatic substitutions were synthesized in excellent yields. In addition, the utility of this protocol was demonstrated in the efficient formal synthesis of the natural product allosecurinine. Further investigations on the asymmetric version for the synthesis of optically pure cis 2,5-disubstituted pyrrolines which enroute to enantioselective synthesis of both allosecurinine and viroallosecurinine are in progress.
Acknowledgements
We gratefully acknowledge Nanyang Technological University and Biomedical Research Council (A*STAR Grant No 05/1/22/19/408) for the funding support for this researchNotes and references
- (a) L. Fowden, Nature, 1955, 176, 347 CrossRef CAS; (b) Q. Cheng, H. Kiyota, M. Yamaguchi, T. Horiguchi and T. Oritani, Bioorg. Med. Chem. Lett., 2003, 13, 1075 CrossRef CAS; (c) A. W. Bannon, M. W. Decker, M. W. Holladay, P. Curzon, A. H. Dickenson, R. D. Porsolt, M. Williams and S. P. Arnerie, Science, 1998, 279, 77 CrossRef CAS; (d) J. P. Michael, Nat. Prod. Rep., 2004, 21, 625 RSC; (e) J. P. Michael, Alkaloids: Chem. Biol., 2001, 55, 91 CrossRef CAS; (f) J. K. Khalaf and A. Datta, J. Org. Chem., 2004, 69, 387–390 CrossRef CAS; (g) E. A. Severino and C. R. D. Correia, Org. Lett., 2000, 2, 3039–3042 CrossRef CAS; (h) J.-F. Poisson, A. Orellana and A. E. Greene, J. Org. Chem., 2005, 70, 10860–10863 CrossRef CAS; (i) J.-E. Joo, K.-Y. Lee, V.-T. Pham, Y.-S. Tian and W.-H. Ham, Org. Lett., 2007, 9, 3627–3630 CrossRef CAS.
- (a) D. O'Hagan, Nat. Prod. Rep., 2000, 17, 435 RSC; (b) M. P. Green, J. C. Prodger and C. J. Hayes, Tetrahedron Lett., 2002, 43, 6609 CrossRef CAS; (c) C. M. Huwe and S. Blechert, Tetrahedron Lett., 1995, 36, 1621 CrossRef CAS.
- (a) Z. Xu and X. Lu, Tetrahedron Lett., 1997, 38, 3461 CrossRef CAS; (b) Z. Xu and X. Lu, J. Org. Chem., 1998, 63, 5031–5041 CrossRef CAS; (c) Z. Xu and X. Lu, Tetrahedron Lett., 1999, 40, 549 CrossRef CAS; (d) X.-F. Zhu, C. E. Henry and O. Kwon, Tetrahedron, 2005, 61, 6276–6282 CrossRef CAS; (e) Y.-L. Shi and M. Shi, Org. Lett., 2005, 7, 3057–3060 CrossRef CAS; (f) G.-L. Zhao and M. Shi, J. Org. Chem., 2005, 70, 9975–9984 CrossRef CAS; (g) X.-Y. Guan and M. Shi, J. Org. Chem., 2009, 74, 1977–1981 CrossRef CAS; (h) L.-G. Meng, P. Cai, Q. Guo and S. Xue, J. Org. Chem., 2008, 73, 8491 CrossRef CAS; (i) S. Zheng and X. Lu, Org. Lett., 2008, 10, 4481–4484 CrossRef CAS; (j) Q. Zhang, L. Yang and X. Tong, J. Am. Chem. Soc., 2010, 132, 2550–2551 CrossRef CAS. For reviews, see: (k) X. Lu, C. Zhang and X. Xu, Acc. Chem. Res., 2001, 34, 535–544 CrossRef CAS; (l) J. L. Methot and W. R. Roush, Adv. Synth. Catal., 2004, 346, 1035 CrossRef CAS; (m) X. Tang, B. Zhang, Z. He, R. Gao and Z. He, Adv. Synth. Catal., 2007, 349, 2007–2017 CrossRef CAS; (n) L.-W. Ye, J. Zhou and Y. Tang, Chem. Soc. Rev., 2008, 37, 1140 RSC; (o) A. Voituriez, A. Panossian, F.-N. Brégeotzz, P. Retailleau and A. Marinetti, Adv. Synth. Catal., 2009, 351, 1968 CrossRef CAS; (p) A. Marinetti and A. Voituriez, Synlett, 2010, 0174–0194 CrossRef CAS. For asymmetric reactions, see: (q) Y.-Q. Fang and E. N. Jacobsen, J. Am. Chem. Soc., 2008, 130, 5660–5661 CrossRef CAS; (r) L. Jean and A. Marinetti, Tetrahedron Lett., 2006, 47, 2141–2145 CrossRef CAS; (s) F.-N. Brégeot, L. Jean, P. Retailleau and A. Marinetti, Tetrahedron, 2007, 63, 11920 CrossRef; (t) N. Pinto, F.-N. Brégeot and A. Marinetti, Eur. J. Org. Chem., 2009, 146–151 CAS; (u) A. Scherer and J. A. Gladysz, Tetrahedron Lett., 2006, 47, 6335–6337 CrossRef CAS. For mechanistic studies, see: (v) Y. Liang, S. Liu, Y. Xia, Y. Li and Z.-X. Yu, Chem.–Eur. J., 2008, 14, 4361–4373 CrossRef CAS; (w) Y. Xia, Y. Liang, Y. Chen, M. Wang, L. Jiao, F. Huang, S. Liu, Y. Li and Z.-X. Yu, J. Am. Chem. Soc., 2007, 129, 3470 CrossRef CAS. For library synthesis, See: (x) O. Kwon, F. Tamanoi, H. Fiji and M. Watanabe, PCT Int. Appl., 2010 Search PubMed , WO 2010014054; (y) F. Tamanoi, O. Kwon, M. Watanabe and H. Fiji, PCT Int. Appl., 2007 Search PubMed , WO 2007111948.
- (a) X.-F. Zhu, J. Lan and O. Kwon, J. Am. Chem. Soc., 2003, 125, 4716–4717 CrossRef CAS; (b) Y. S. Tran and O. Kwon, Org. Lett., 2005, 7, 4289–4291 CrossRef CAS; (c) R. P. Wurz and G. C. Fu, J. Am. Chem. Soc., 2005, 127, 12234–12235 CrossRef CAS.
- (a) Y. Du and X. Lu, J. Org. Chem., 2003, 68, 6463–6465 CrossRef CAS; (b) J.-C. Wang and M. J. Krische, Angew. Chem., Int. Ed., 2003, 42, 5855–5857 CrossRef CAS; (c) R. A. Jones and M. J. Krische, Org. Lett., 2009, 11, 1849 CrossRef CAS; (d) T. Q. Pham, S. G. Pyne, B. W. Skeleton and A. H. White, J. Org. Chem., 2005, 70, 6369–6377 CrossRef CAS; (e) H. Mizuno, K. Domon, K. Masuya, K. Tanino and I. Kuwajima, J. Org. Chem., 1999, 64, 2648–2656 CrossRef CAS.
- (a) F. Sato, T. Nakagawa and A. Kasatkin, Tetrahedron Lett., 1995, 36, 3207–3210 CrossRef; (b) U. Maeorg and A. Jogi, Molecules, 2001, 6, 964–968 CrossRef; (c) Y. Wu, X. Wu, Z. Yao, Y. Li, J. Li and Y. Xia, J. Org. Chem., 1995, 60, 3257 CrossRef; (d) M. Sampath and T.-P. Loh, Chem. Sci., 2010, 1, 739–742 RSC; (e) M. Sampath and T.-P. Loh, Chem. Commun., 2009, 1568–1570 RSC.
- For synthesis of imines, see: (a) T.-S. Jin, M.-J. Yu, Y. Liu, L.-B. Zhao and T.-S. Li, Synth. Commun., 2006, 36, 2339–2344 CrossRef CAS; (b) K. Y. Lee, C. G. Lee and J. N. Kim, Tetrahedron Lett., 2003, 44, 1231–1234 CrossRef CAS; (c) F. Chemla, V. Hebbe and J.-F. Normant, Synthesis, 2000, 75–77 CrossRef CAS.
- A. Suarez and G. C. Fu, Angew. Chem., Int. Ed., 2004, 43, 3580–3582 CrossRef CAS.
- For previous studies on securinine alkaloids, see: (a) S. M. Weinreb, Nat. Prod. Rep., 2009, 26, 758–775 RSC; (b) T. Honda, H. Namiki, M. Watanabe and H. Mizutani, Tetrahedron Lett., 2004, 45, 5211–5213 CrossRef CAS; (c) P. Liu, S. Hong and S. M. Weinreb, J. Am. Chem. Soc., 2008, 130, 7562–7563 CrossRef CAS; (d) A. Alibés, P. Bayón, P. de March, M. Figueredo, J. Font, E. Garcia-Garcia and D. González-Gálvez, Org. Lett., 2005, 7, 5107–5109 CrossRef; (e) T. Honda, H. Namiki, K. Kaneda and H. Mizutani, Org. Lett., 2004, 6, 87–89 CrossRef CAS; (f) T. Honda, H. Namiki, M. Kudoh, H. Nagase and H. Mizutani, Heterocycles, 2003, 59, 169–187 CrossRef CAS; (g) P. Liu, S. Hong and S. M. Weinreb, J. Am. Chem. Soc., 2008, 130, 7562–7563 CrossRef CAS; (h) A. B. Leduc and M. A. Kerr, Angew. Chem., Int. Ed., 2008, 47, 7945–7948 CrossRef CAS , and references cited therein.
- C. H. Heathcock, T. A. Blumenkopf and K. M. Smith, J. Org. Chem., 1989, 54, 1548–1562 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures analytical data. CCDC 829904 contains supplementary crystallographic data of 15a. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1sc00311a |
| This journal is © The Royal Society of Chemistry 2011 |