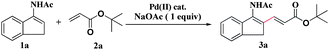DOI:
10.1039/C1SC00262G
(Edge Article)
Chem. Sci., 2011,
2, 1822-1825
Synthesis and characterization of a cyclic vinylpalladium(II) complex: vinylpalladium species as the possible intermediate in the catalytic direct olefination reaction of enamide†
Received
27th April 2011
, Accepted 13th June 2011
First published on 27th June 2011
Abstract
A six-membered cyclic vinylpalladium complex was synthesized via alkenyl C–H bond direct palladation, which was probed in detail by 1H NMR spectroscopy and characterized by X-ray analysis. The cyclic vinylpalladium complex was propounded to be the intermediate during the olefination reaction of enamides. An efficient catalytic system for oxidative cross-coupling reaction of enamides with electron-deficient olefins catalyzed by palladium(II) acetate and 1 atm oxygen as sole oxidant was developed. The corresponding products were obtained in moderate to good yields under mild conditions.
Introduction
During the last few decades, palladium-catalyzed coupling reaction has grown to become an attractive and powerful method for the formation of new C–C and C–heteroatom bonds.1 Particularly, the atom economical merits conferred upon direct C–H bond functionalization catalyzed by palladium catalyst emerged in recent years, spurred intense development in this arena.2 Among the various direct C–H bond functionalization examples, chelation-assisted sp2 (aryl) and sp3 C–H bonds activation is one of the most well-studied (eqn (1)).2l,3 Evidences of palladacyclic intermediate generated during coupling process have been disclosed.4 On the other hand, the formation of analogous vinylpalladium complex via alkenyl C–H bond direct functionalization lies in obscurity due to lack of detailed investigation.4m,5 Recently our group presented a direct arylation of cyclic enamides at their β-position with aryl boronic acids or aryl silanes by using palladium(II) acetate as catalyst.6 A cyclic vinylpalladium intermediate was invoked in the catalytic cycle proposed for the coupling reaction (eqn (2)), although we were unfortunately not able to conclusively establish the vinylpalladium species at that moment. In this communication, we describe the independent synthesis, characterization, and reactivity studies of the vinylpalladium complex that is propounded to be the coupling intermediate in the above-said reaction.| | 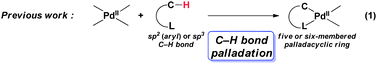 | (1) |
| | 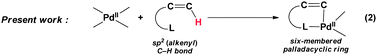 | (2) |
Results and discussion
Initial studies and reaction optimization
A mixture of N-[1-(naphthalen-2-yl)vinyl]acetamide (1k) and palladium(II) acetate (1 equiv) in DMSO-d6 was stirred in a round-bottom flask under an atmosphere of air for 12 h at 25 °C (or 10 min at 80 °C). Subsequent analysis by 1H NMR revealed that the signal of one of the terminal alkenyl protons disappeared from the spectrum completely.7 The new complex was isolated as a solid via deposition with dry diethyl ether. A crystal suitable for X-ray analysis was prepared by recrystallization and a six-membered vinylpalladium cyclic intermediate (1k′) was unveiled. Next tert-butyl acrylate was introduced into the vinylpalladium solution and the mixture was heated at 80 °C for another 16 h. The desired product 3k was observed and isolated in 53% yield. (Scheme 1)8
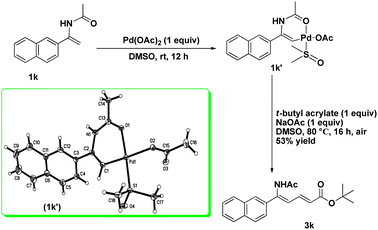 |
| | Scheme 1 Formation of cyclic vinylpalladium complex and its reactivity study with tert-butyl acrylate. | |
Inspired by the observed results, our efforts were focused on the optimization of the reaction conditions and developing an efficient catalytic system for olefination of enamide. To this end, the reaction of N-(3H-inden-1-yl) enamide 1a and tert-butyl acrylate (2a) was screened under various reaction conditions using different palladium catalysts and oxidants. The results are shown in Table 1. When the reaction was carried out under the previously reported conditions, the desired product was obtained in 50% yield (Table 1, entry 1).5b The reaction cannot proceed in DMSO solvent, wherein the starting material was recovered after 24 h (Table 1, entry 2). It was found that using acetic acid alone as solvent, with 10 mol% of palladium(II) acetate as catalyst, oxygen as the sole oxidant and with 1 equivalent of sodium acetate as additive under 80 °C, the desired product can be obtained in 80% yield (Table 1, entry 3). However, decreasing the catalyst loading to 5 mol% will lower the yield dramatically (Table 1, entry 4), while increasing the amount of NaOAc has no significant effect (Table 1, entry 5). Other palladium catalysts were also investigated in this reaction and Pd(TFA)2, PdCl2, Pd(CH3)2Cl2 and Pd(PhCN)2Cl2 were all found to give the product in moderate yields (Table 1, entries 6, 8, 9 and 10); but no desired product was formed when Pd(PPh3)2Cl2 was used as catalyst (Table 1, entry 7). We also observed that only 37% yield was achieved when the ratio of 1a and 2a was changed to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, while no desired product was obtained in the absence of palladium catalyst. The absence of oxygen as the oxidant resulted in the rapid decomposition of the substrate and therefore trace amount of product formation (Table 1, entry 12).
4, while no desired product was obtained in the absence of palladium catalyst. The absence of oxygen as the oxidant resulted in the rapid decomposition of the substrate and therefore trace amount of product formation (Table 1, entry 12).
| Entry |
Oxidant |
Pd(II)/mol (%) |
Solvent
|
Yieldb(%) |
Reaction conditions unless otherwise specified: 1a (2 equiv), 2a (1 equiv, 0.4 M) and Pd(II) (0.1equiv), oxygen (1 atm) at 80 °C in acetic acid.
Isolated yields.
without NaOAc as additive.
No reaction.
4 equivalent of NaOAc was used.
The starting material decomposed.
Mole ratio 1a:2a = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. HOAc: acetic acid; TFA: trifluoroacetate. 4. HOAc: acetic acid; TFA: trifluoroacetate.
|
| 1c |
Cu(OAc)2 + O2 |
Pd(OAc)2/10 |
DMSO/AcOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
50 |
| 2d |
O2 |
Pd(OAc)2/10 |
DMSO
|
- |
| 3 |
O2 |
Pd(OAc)2/10 |
HOAc
|
80 |
| 4 |
O2 |
Pd(OAc)2/5 |
HOAc
|
55 |
| 5e |
O2 |
Pd(OAc)2/10 |
HOAc
|
81 |
| 6 |
O2 |
Pd(TFA)2/10 |
HOAc
|
58 |
| 7f |
O2 |
Pd(PPh3)2Cl2/10 |
HOAc
|
- |
| 8 |
O2 |
PdCl2/10 |
HOAc
|
35 |
| 9 |
O2 |
Pd(PhCN)2Cl2/10 |
HOAc
|
73 |
| 10 |
O2 |
Pd(CH3)2Cl2/10 |
HOAc
|
51 |
| 11g |
O2 |
Pd(OAc)2/10 |
HOAc
|
37 |
| 12 |
— |
Pd(OAc)2/10 |
HOAc
|
<10 |
In addition to tert-butyl acrylate, different electron-deficient coupling partners were tested in this cross-coupling reaction using 10 mol% loading of Pd(OAc)2. methyl acrylate, ethyl acrylate, n-butyl acrylate and phenyl acrylate all furnished the desired product in high yields. As for acrylonitrile, only 12% yield was obtained, while styrene afforded the corresponding desired product in 55% yield. Therefore tert-butyl acrylate was the best coupling partner in our system.9
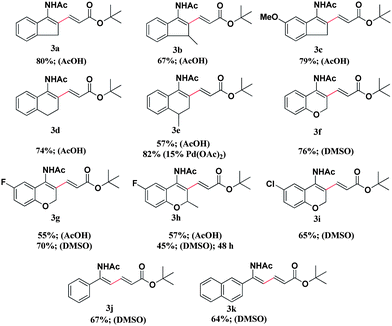
Substrate scope
Next, a variety of enamide derivatives were screened with tert-butyl acrylate using 10 mol% Pd(OAc)2 catalyst. The results are shown in Table 2. It can be seen that both cyclic enamides (Table 2, 3a–3i) and acyclic enamides (Table 2, 3j, 3k) can all generate the products in moderate to high yields. The effect of the substituents on the benzene ring was not obvious; an electron-donating group was well-tolerated (Table 2, 3c), while moderately electron-withdrawing groups slightly decreased the product yield (Table 2, 3g–3i). The presence of methyl group on the acyclic ring diminished the yield in acetic acid solvent (Table 2, 3b, 3h). Notably, halide substituents were tolerated, which is very useful for further transformation (Table 2, 3g–3i). The choice of solvent was found to be crucial for the success of this coupling reaction. For indanone- and tetralone-derived enamides, the desired products can be obtained in acetic acid solvent (Table 2, 3a–3e). On the other hand, 4-chromanone-derived enamide 1f completely decomposed in acetic acid, while a high yield of 76% can be achieved in DMSO. Substrates 1g and 1h can furnish the desired product in moderate yields either in acetic acid or in DMSO. Acyclic substrates were only compatible in DMSO, providing the corresponding products in moderate yields (Table 2, 3j, 3k).
Proposed mechanism
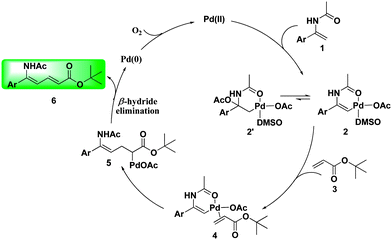
Based on the X-ray structure, a plausible mechanism is proposed in Scheme 2. The alkenyl C–H bond of enamide 1 is firstly activated by the Pd(II) complex to form the six-membered palladacycle intermediate 2 (a possible σ–C(sp3)–Pd intermediate 2′ cannot be ruled out in this step). Next, coordination of the acrylate to Pd followed by migratory insertion gives intermediate 5. The desired product 6 is finally obtained after β-hydride elimination. The Pd(0) that was generated is then recycled back to Pd2+ by the oxidant.
Conclusions
In summary, a mechanism of direct coupling reaction between enamide and acrylate was well-studied by 1H NMR spectroscopy and X-ray analysis. A vinylpalladium cyclic complex was isolated and proved to be the possible coupling intermediate with acrylate. We also have developed the first successful catalytic olefination reaction of enamides with electron-poor alkenes catalyzed by Pd(OAc)2 and 1 atm oxygen as sole oxidant. The corresponding products were obtained in moderate to high yields and with excellent regioselectivities. The use of oxygen as sole oxidant makes the method potentially appealing for the chemical industry in view of its environmental and economical advantages. This novel method produces highly functionalized, versatile compounds which can be converted to a wide variety of building blocks and complex molecules. Work along this line and detailed mechanistic studies are in progress.
Acknowledgements
We gratefully acknowledge the Nanyang Technological University and Singapore Ministry of Education Academic Research Fund Tier 2 (No. T207B1220RS) for the funding of this research. We thank Prof. K. Narasaka for his helpful discussions.
References
-
(a)
R. F. Heck, Palladium Reagents in Organic Synthesis; Academic Press: New York, 1985 Search PubMed;
(b)
J. J. Li, G. W. Gribble, Palladium in Heterocyclic Chemistry; Pergamon: New York, 2000 Search PubMed;
(c)
Handbook of organopalladium chemistry for organic synthesis; E. Negishi, Ed.; Wiley and Sons: New York, 2002 Search PubMed;
(d)
J. Tsuji, Palladium Reagents and Catalysts: Innovations in Organic Synthesis; Wiley and Sons: New York, 1995 Search PubMed;
(e)
J. Tsuji, Palladium Reagents and Catalysts: New Perspectives for the 21st Century; Wiley and Sons: New York, 2003 Search PubMed;
(f)
Palladium in Organic Synthesis; J. Tsuji. Ed.; Springer: Berline, 2005 Search PubMed.
- For reviews see:
(a) C. Jia, T. Kitamura and Y. Fujiwara, Acc. Chem. Res., 2001, 34, 633 CrossRef CAS;
(b) J.-Q. Yu, R. Giri and X. Chen., Org. Biomol. Chem., 2006, 4, 4041 RSC;
(c) T. Satoh and M. Miura, Chem. Lett., 2007, 36, 200 CrossRef CAS;
(d) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173 RSC;
(e) L.-C. Campeau, D. R. Stuart and K. Fagnou, Aldrichimica Acta, 2007, 40, 35 CAS;
(f) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS;
(g) F. Kakiuchi and T. Kochi, Synthesis, 2008, 3013 CrossRef CAS;
(h) B.-J. Li, S.-D. Yang and Z.-J. Shi, Synlett, 2008, 949 CAS;
(i) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef CAS;
(j) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS;
(k) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS;
(l) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS;
(m) L.-M. Xu, B.-J. Li, Z. Yang and Z.-J. Shi, Chem. Soc. Rev., 2010, 39, 712 RSC;
(n) P. Sehnal, R. J. K. Taylor and I. J. S. Fairlamb, Chem. Rev., 2010, 110, 824 CrossRef CAS;
(o) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS.
- For reviews of palladium-catalyzed aryl C–H bond functionalization see:
(a) A. D. Pyabov, Chem. Rev., 1990, 90, 403 CrossRef;
(b) G. Dyker, Angew. Chem., Int. Ed., 1999, 38, 1698 CrossRef;
(c) J. Dupont, C. S. Consorti and J. Spencer, Chem. Rev., 2005, 105, 2527 CrossRef CAS, and references therein. For recent advance of palladium-catalyzed alkyl C–H bond functionalization see:
(d) R. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer and O. Baudoin, Chem.–Eur. J., 2010, 16, 2654 CrossRef CAS, and references cited therein;
(e) M. Wasa, K. M. Engle and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 3680 CrossRef CAS;
(f) L.-C. Campeau, D. J. Schipper and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 3266 CrossRef CAS;
(g) J. J. Mousseau, A. Larivée and A. B. Charette, Org. Lett., 2008, 10, 1641 CrossRef CAS;
(h) D.-H. Wang, M. Wasa, R. Giri and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 7190 CrossRef CAS;
(i) M. Lafrance, S. I. Gorelsky and K. Fagnou, J. Am. Chem.
Soc., 2007, 129, 14570 CAS;
(j) D. Kalyani and M. S. Sanford, Top. Organomet. Chem., 2007, 24, 85 CrossRef CAS, and references cited therein;
(k) R. Giri, N. L. Maugel, J.-J. Li, D.-H. Wang, S. P. Breazzano, L. B. Saunders and J.-Q. Yu, J. Am. Chem. Soc., 2007, 129, 3510 CrossRef CAS;
(l) D.-H. Wang, D.-F. Wu and J.-Q. Yu, Org. Lett., 2006, 8, 3387 CrossRef CAS;
(m) X. Chen, C. E. Goodhue and J.-Q. Yu, J. Am. Chem. Soc., 2006, 128, 12634 CrossRef CAS;
(n) R. Giri, X. Chen and J.-Q. Yu, Angew. Chem., Int. Ed., 2005, 44, 2112 CrossRef CAS;
(o) L. V. Desai, K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2004, 126, 9542 CrossRef CAS;
(p) B. D. Dangel, K. Godula, S. W. Youn, B. Sezen and D. Sames, J. Am. Chem. Soc., 2002, 124, 11856 CrossRef CAS.
-
(a) B. Xiao, Y. Fu, T.-J. Gong, J.-J. Dai, J. Yi and L. Liu, J. Am. Chem. Soc., 2010, 132, 468 CrossRef CAS;
(b) N. D. Ball, J. W. Kampf and M. S. Sanford, J. Am. Chem. Soc., 2010, 132, 2878 CrossRef CAS;
(c) P. S. Hanley, D. Marković and J. F. Hartwig, J. Am. Chem. Soc., 2010, 132, 6302 CrossRef CAS;
(d) D. C. Powers, M. A. L. Geibel, J. E. M. N. Klein and T. Ritter, J. Am. Chem. Soc., 2009, 131, 17050 CrossRef CAS;
(e) N. D. Ball and M. S. Sanford, J. Am. Chem. Soc., 2009, 131, 3796 CrossRef CAS;
(f) V. S. Thirunavukkarasu, K. Parthasarathy and C.-H. Cheng, Angew. Chem., Int. Ed., 2008, 47, 9462 CrossRef CAS;
(g) B. P. Fors, D. A. Watson, M. R. Biscoe and S. L. Buchwald, J. Am. Chem. Soc., 2008, 130, 13552 CrossRef CAS;
(h) R. Giri, N. Mauge, B. M. Foxman and J.-Q. Yu, Organometallics, 2008, 27, 1667 CrossRef CAS;
(i) S. H. Cho, S. J. Hwang and S. Chang, J. Am. Chem. Soc., 2008, 130, 9254 CrossRef CAS;
(j) D. Tanaka, S. P. Romeril and A. G. Myers, J. Am. Chem. Soc., 2005, 127, 10323 CrossRef CAS;
(k) R. Giri, J. Liang, J.-G. Lei, J.-J. Li, D.-H. Wang, X. Chen, I. C. Naggar, C. Guo, B. M. Foxman and J.-Q. Yu, Angew. Chem., Int. Ed., 2005, 44, 7420 CrossRef CAS;
(l) P. A. van de Schaaf, J.-P. Sutter, M. Grellier, G. M. P. van M, A. L. Spek, G. van Koten and M. Pfeffer, J. Am. Chem. Soc., 1994, 116, 5134 CrossRef;
(m) G. R. Newkome, K. J. Tharlot, B. K. Cheskin, D. W. Evans and G. R. Baker, Organometallics, 1990, 9, 1375 CrossRef CAS;
(n) S. Yang, B. Li, X. Wan and Z. Shi, J. Am. Chem. Soc., 2007, 129, 6066 CrossRef CAS;
(o) H. Horino and N. Inuoe, J. Org. Chem., 1981, 46, 4416 CrossRef CAS.
- For palladium-catalyzed direct cross-coupling reactions to form diene products, see:
(a) Y.-H. Xu, W.-J. Wang, Z.-K. Wen, J. J. Hartley and T.-P. Loh, Tetrahedron Lett., 2010, 51, 3504 CrossRef CAS;
(b) H. Yu, W. Jin, C. Sun, J. Chen, W. Du, S. He and Z. Yu, Angew. Chem., Int. Ed., 2010, 49, 5792 CrossRef CAS;
(c) Y.-H. Xu, J. Lu and T.-P. Loh, J. Am. Chem. Soc., 2009, 131, 1372 CrossRef CAS;
(d) Y. Hatamoto, S. Sakaguchi and Y. Ishii, Org. Lett., 2004, 6, 4623 CrossRef CAS. For other examples including vinylpalladium species generated via C–H bonds direct functionalization, see:
(e) M. Li, L. Li and H. Ge, Adv. Synth. Catal., 2010, 352, 2445 CrossRef CAS;
(f) X. Han and X. Lu, Org. Lett., 2009, 11, 2381 CrossRef CAS;
(g) H. Ge, M. J. Niphakis and G. I. Georg, J. Am. Chem. Soc., 2008, 130, 3708 CrossRef CAS;
(h) R. Giri and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 14082 CrossRef CAS;
(i) S. Würtz, S. Rakshit, J. J. Neumann, T. Dröge and F. Glorious, Angew. Chem., Int. Ed., 2008, 47, 7230 CrossRef;
(j) M. J. Silva, J. A. Gonçalves, R. B. Alves, O. W. Howarth and E. V. Gusevskaya, J. Organomet. Chem., 2004, 689, 302 CrossRef;
(k)
P. Maitlis, The Organic Chemistry of PalladiumAcademic Press, New York, 1971, vol. 2, p. 62 Search PubMed;
(l) For a new example of rhodium(III)-catalyzed olefination of vinylic C–H bonds to form diene products, see: T. Besset, N. Kuhl, F. W. Paturean and F. Glorious, Chem.–Eur. J., 2011, 17, 7167 CrossRef CAS.
-
(a) H. Zhou, Y.-H. Xu, W. J. Chung and T. P. Loh, Angew. Chem., Int. Ed., 2009, 48, 5355 CrossRef CAS;
(b) H. Zhou, W. J. Chung, Y.-H. Xu and T. P. Loh, Chem. Commun., 2009, 3472 RSC. See the examples of arylation of enamides at α-position with boronic acids:
(c) M. M. S. Andappan, P. Nisson, H. van Schenck and M. Larhed, J. Org. Chem., 2004, 69, 5212 CrossRef CAS;
(d) P.-A. Enquist, P. Nisson, P. Sjöberg and M. Larhed, J. Org. Chem., 2006, 71, 8779 CrossRef CAS.
- See the detailed results in the supporting information.
- The structure of 1k′ was identified by spectroscopic and X-ray crystallographic analysis. See the detailed results in the supporting information. CCDC 806122 (1k′) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre viahttp://www.ccdc.cam.ac.uk./data_request/cif.
- See Table 1 in the supporting information.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, while no desired product was obtained in the absence of palladium catalyst. The absence of oxygen as the oxidant resulted in the rapid decomposition of the substrate and therefore trace amount of product formation (Table 1, entry 12).
4, while no desired product was obtained in the absence of palladium catalyst. The absence of oxygen as the oxidant resulted in the rapid decomposition of the substrate and therefore trace amount of product formation (Table 1, entry 12).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. HOAc: acetic acid; TFA: trifluoroacetate.
4. HOAc: acetic acid; TFA: trifluoroacetate.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)
1)