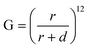Aptamer-based surface-enhanced Raman scattering detection of ricin in liquid foods
Lili
He
a,
Elise
Lamont
b,
Belamaranahally
Veeregowda
c,
Srinand
Sreevatsan
b,
Christy L.
Haynes
d,
Francisco
Diez-Gonzalez
a and
Theodore P.
Labuza
*a
aDepartment of Food Science and Nutrition, University of Minnesota, Saint Paul, Minnesota 55108, USA. E-mail: tplabuza@umn.edu; Fax: (612) 624-9701; Tel: (612) 625-5272
bDepartments of Veterinary Population Medicine and Veterinary and Biomedical Sciences, College of Veterinary Medicine, University of Minnesota, Saint Paul, Minnesota 55108, USA
cDepartment of Veterinary Microbiology, Veterinary College, Hebbal, Bangalore 560 024, India
dDepartment of Chemistry, University of Minnesota, Minneapolis, Minnesota 55455, USA
First published on 26th May 2011
Abstract
A “two-step” aptamer-based surface-enhanced Raman scattering (SERS) detection assay was developed for ricin in liquid foods. Ricin B chain was first captured out of food matrices by aptamer-conjugated silver dendrites and then the spectrum was directly read on the silver dendrites. Aptamer use in this assay promotes ease of manipulation as well as improved sensitivity compared to antibody-based approaches. The limit of detection for ricin B chain was 10 ng mL−1 in phosphate buffered saline (PBS), 50 ng mL−1 in orange juice, and 100 ng mL−1 in milk based on principal component analysis (PCA) of measured spectra. This assay shows great promise as a rapid (< 40 min), sensitive, and simple “Yes/No” method to detect bio-weapons like ricin in liquid foods.
Introduction
Surface-enhanced Raman scattering (SERS) techniques have shown great promise as rapid and sensitive analytical tools that yield molecular fingerprints and good sensitivity based on scattering amplification in the near vicinity of nanoscale-roughened noble metal substrates.1 However, few studies have applied SERS for chemo/biosensing in complex matrices such as food.2–7 Technical challenges in using SERS in complex food matrices include overwhelming fluorescence and interfering Raman signals from food components.8 Accordingly, successful detection in a complex food matrix requires an effective initial separation step. Antibodies and aptamers are the two major classes of agents employed for molecule-specific capture; however, most antibody- or aptamer-based SERS assays rely on secondary conjugation with a Raman-active molecule-linked antibody or aptamer as an extrinsic Raman reporter.6,7,9–15 The signal is from the Raman reporter molecule, rather than the target compound itself. In addition, secondary conjugation significantly increases sample processing time.Herein, we developed a simple, rapid, and sensitive “two-step” SERS assay using aptamer-conjugated silver dendrites to detect ricin B chain in liquid foods. Ricin is a protein toxin naturally present in the castor bean plant (Ricinus communis); it is classified as a “select” bioterror agent and was involved in a very recent bioterrorism attack plot targeting US hotels and restaurants at multiple locations, as reported by the Department of Homeland Security officials in December 2010.16 The ricin molecule is composed of two chains, A and B, connected by a disulfide bond. The A chain is responsible for the enzymatic activity of the toxin. The B chain is responsible for delivering the A chain into the cell. Both chains are needed for in vivo toxicity.17 It is estimated that the LD50 for ricin in humans is approximately 5–10 μg kg−1BW through inhalation and 1–20 mg kg−1BW or 8 castor beans through ingestion.18–20 In this study, we used the non-toxic ricin B chain and its specific aptamer to demonstrate the assay. During the assay, the ricin B chain was first captured out of food matrices by aptamer-conjugated silver dendrites and then the Raman spectrum was directly read on the silver dendrites. In this way, the measurement is based on the Raman “finger-print” of the target itself; combined with the specific capture agent, false positive results are extremely unlikely. In addition, direct reading on the Ag-aptamer-target complex reduces the analysis time, which is a critical factor for detection of bio-weapons such as ricin in foods. It is of great importance to have a rapid yes/no assay to catch a contaminated product before it enters the consumer food chain. The use of an aptamer in this SERS assay holds many advantages over the traditional antibody-based approach,3 including ease of manipulation, improved sensitivity, and decreased spectral congestion when detecting protein targets. To the best of our knowledge, this is the first report of aptamer-based SERS detection of a protein in complex food matrices on a practical time scale (within 40 min).
Materials and methods
Sample preparation
Ricin B chain was purchased from Vector Laboratories (Burlingame, CA). Orange juice (Tropicana Products, Chicago, IL) and whole milk fortified with Vitamin D (Roundy's, Milwaukee, WI) were purchased from a local grocery store. Ricin B chain was spiked into phosphate buffered saline (PBS), orange juice and milk at levels of 10, 25, 50, 100, 200 and 500 μg mL−1. PBS, orange juice and milk without ricin B chain were used as negative controls.Preparation of aptamer-modified silver dendrites
Silver (Ag) dendrites were prepared through a simple replacement reaction involving both zinc and silver nitrate (AgNO3) according to a previously published method.21 The prepared Ag dendrites can be kept in water and are stable for at least half a year. The Ag dendrite optical properties make them viable SERS substrates for excitation wavelengths ranging from 500 to 800 nm with a satisfactory and consistent enhancement factor.21 The aptamer against ricin B chain was selected and characterized in the laboratory of Dr Srinand Sreevatsan in the Departments of Veterinary Population Medicine and Veterinary and Biomedical Sciences, College of Veterinary Medicine, University of Minnesota. The aptamer has been shown to have exclusive specificity to ricin in multiple food matrices (data not shown). It is a 5′ thiol-modified DNA aptamer to promote affinity for the SERS substrate.Substrate modification was accomplished via overnight exposure of the Ag dendrites to the thiolated aptamer with consistent rotation. Different amounts of aptamer were tested with 20 μL (∼ 80 μg) of Ag dendrites to determine the optimum modification conditions. Then, the Ag-aptamer (Ag-Ap) complex was collected using centrifugation (6000 × g for 1 min) and the pellet was washed using 1 mL double distilled (DD) water.
Capture and detection of ricin B chain in liquid foods
The prepared Ag-Ap complex (20 μL) was incubated with 1 mL of ricin B chain solutions for 30 min under consistent agitation at room temperature. After centrifugation at 6000 × g for 1 min at room temperature, the pellet was washed using DD water three times, deposited onto a glass slide, and air-dried at room temperature ahead of SERS analysis.Raman instrumentation
A DXR Raman microscope (Thermo Fisher Scientific, Madison, WI) was used in this study. This instrument facilitates 780 nm excitation of SERS scattering through a 10× confocal microscope objective, resulting in a laser spot diameter of approximately 3 μm and 5 cm−1 spectral resolution. Quadruplicate SERS measurements were performed with 4 mW laser power and 25 μm slit width for 15 s integration time. Spectra were collected using the Thermo Fisher Scientific OMNIC™ Software with Array Automation function; this function automatically collects and processes data from groups of samples that are arranged in an ordered array. An array multiple (n = 25) was used to collect the data by randomly picking 25 spots on the Ag surface for each sample.Data analysis
SERS spectral data were analyzed by the TQ Analyst software (Thermo Fisher Scientific, Madison, WI). Principal component analysis (PCA) was applied to analyze the variance of spectral data and build the qualitative predictive model based on the standards. The information provided by PCA indicates any patterns or trends in the data, which may or may not be significant to a given application. Before PCA analysis, pre-data processing such as standard normal variant, second derivative transformation, and smoothing was applied when necessary to help PCA capture more significant variance.22Results and discussion
The concept of the “two-step” aptamer-based SERS assay is illustrated in Fig. 1. The aptamer was conjugated onto the surface of Ag through Ag–thiol binding ahead of sensor use. After capturing the ricin B chain, changes in SERS spectra were used as the base of sensing. Compared with antibody-based SERS methods which used protein G binding3 or biotin–avidin binding,23conjugation of aptamers onto Ag is much easier. The maximum binding capacity of 20 μL (∼ 80 μg) of Ag dendrites was found to be approximately 0.8 μmol aptamer (data not shown). The achieved SERS spectra following exposure to the DNA aptamer and ricin B chain are shown in Fig. 2, and the appropriate vibrational band assignments are listed in Table 1.24| Raman shift (cm−1) | Assignments24 | |
|---|---|---|
| aptamer to ricin B chain | 1316 | Adenine (ring stretching) |
| 1022 | DNA backbone (PO2− stretching) | |
| 792 | Thymine, cytosine | |
| 735 | Adenine (ring breathing) | |
| ricin B chain | 1001 | Phenylalanine (ring breathing) |
| 716 | Cystine, cysteine (C–S stretching) | |
| 633 | Cystine, cysteine (C–S stretching) |
 | ||
| Fig. 1 Illustration of the “two-step” aptamer-based SERS assay. | ||
 | ||
| Fig. 2 Average of raw spectra of Ag-Ap, Ag-Ap-R, and Ag-R (A) and the enlarged spectra processed with second derivative transformation in two different cm−1 shift regions (B and C). Ag: silver, Ap: aptamer, R: ricin B chain. | ||
After capturing the ricin B chain, the Ag-Ap-ricin (R) pellet was deposited and spectra were measured on the glass slide directly. As shown in Fig. 2A, the SERS spectra before and after capturing ricin B chain look similar at first glance. However, two small additional bands appear at 985 and 621 cm−1 shift after capturing the ricin B chain. Following preprocessing of the spectra using a second derivative transformation, these two spectral changes become much more prominent as shown in Fig. 2B and 2C. These two additional bands resulted from ricin vibrations that usually occur at 1001 cm−1 and 633 cm−1 in normal Raman spectra, with some degree of shift attributed to the conformational change of 3-dimensional structures or associated with the aptamer-modified surface. The appearance of these two bands at 985 and 621 cm−1 indicates the success of capturing and detecting of ricin B chain. The band at 1074 cm−1 shift is due to the NO3− residue on the Ag dendrites.21 Compared with previous work on Ag-antibody capture (when a protein is used to selectively capture another protein), where the spectral change was slight and somewhat unpredictable (in the general region of 1200 to 1700 cm−1 shift3), the spectral change is much clearer and easier to understand using the nucleic acid aptamer to capture the protein target.
The aptamer-modified Ag dendrite substrate was systematically exposed to varied concentrations of ricin B chain in PBS, and the data were analyzed by PCA. As shown in Fig. 3, the data points of all ricin B chain positive samples can be separated from negative controls which indicates that the limit of detection of this assay is at least 10 ng mL−1. However, using this platform, quantification within this range is not possible as the data points of different concentrations of ricin B chain were mixed together. This is due to the large size of the aptamer, which forces the ricin B chain to bind in the least sensitive region of the plasmonic electromagnetic fields. More specifically, the SERS electromagnetic enhancement mechanism has demonstrated distance dependence, expressed for an ideal spherical noble metal substrate, as
 | ||
| Fig. 3 A PCA plot from SERS spectra of Ag-Ap (open triangles), Ag-Ap-R (various concentrations, various shapes), and Ag-R (open circles). Ag: silver, Ap: aptamer, R: ricin B chain. | ||
We also evaluated this assay in orange juice and milk as these are appropriate complex liquid food matrices. Before measurement in orange juice, a stringer filter (0.22 μm) was used to remove the pulp. For milk, a fivefold dilution was performed before filtration to remove large casein micelles and fat globules. The lowest concentration of ricin B chain that can be captured and recognized using PCA was 50 ng mL−1 in orange juice and 100 ng mL−1 in milk (20 ng mL−1 in the test tube after dilution) as shown in Fig. 4. Compared with the Ag-antibody assay, which had a detection limit of 100 ng mL−1 in PBS and 5 μg mL−1 in milk,3 the Ag-Ap assay shows a lower limit of detection in both PBS and food matrices. As noted, if we consider the lowest dose as the most conservative one for ricin at 1 mg kg−1BW for a 20 kg child, the child needs to drink 250 ml of milk or orange juice (∼ 1 pint single serving size) containing ricin at 80 μg mL−1 level. Therefore, the required detection threshold for ricin in liquid foods is quite generous. Both antibody- and aptamer-based SERS methods are sufficiently sensitive to detect ricin in liquid foods.
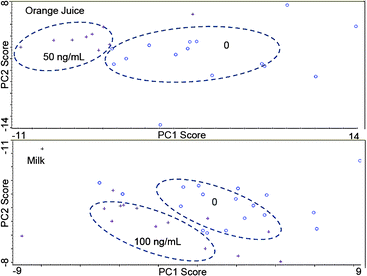 | ||
| Fig. 4 PCA plots of ricin B chain capture in orange juice and milk. In the top plot (orange juice), the 50 ng mL−1 data points are represented by crosses while the blanks are represented by open circles. In the bottom plot (milk), the 100 ng mL−1 data points are represented by crosses while the blanks are represented by open circles. | ||
Conclusions
In conclusion, this work demonstrated a simple, rapid, and sensitive assay using aptamer-based SERS measurements. If binding of the aptamer to the silver dendrites is done ahead of analysis, the total time for sample preparation and detection is less than 40 min. The success of this assay depends on a good aptamer that can specifically capture the target in the presence of other compounds. Technically, the base of the sensor relies on the distinct scattering spectra of aptamers and protein targets where we can expect the change of spectral patterns before and after capturing the target. With the aid of PCA, the pattern change can be easily analyzed and modeled. This assay works well for a protein target even though innate protein Raman scattering is relatively weak. It can be generalized to any kind of target detection based on the use of an appropriate capture aptamer. Compared with the Ag-antibody assay, the use of aptamer capture provides advantages of better stability, easier manipulation, and higher sensitivity. Future experiments will focus on continuing optimization and application of this assay for ricin whole molecule detection and fabricating commercially viable SERS substrates based on this assay.Acknowledgements
This research was supported by the U.S. Department of Homeland Security (DHS) through the National Center for Food Protection and Defense at the University of Minnesota (Grant number DHS-3002-11364-00021976). It has not been formally reviewed by DHS. The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of DHS. DHS does not endorse any products or commercial services mentioned in this publication.We appreciate that Thermo Fisher Scientific (Madison, WI) loaned us the DXR Raman microscope for the SERS study.
Notes and references
- C. L. Haynes, A. D. McFarland and R. P. Van Duyne, Anal. Chem., 2005, 77, 338a–346a CAS.
- L. He, T. Rodda, B. Deen, I. Ronningen, T. Blasius, C. Haynes, F. Diez-Gonzalez and T. P. Labuza, J. Food Sci., 2011 DOI:10.1111/j.1750-3841.2011.02196.x.
- L. He, T. Rodda, C. L. Haynes, T. Deschaines, T. Strother, F. Diez-Gonzalez and T. P. Labuza, Anal. Chem., 2010, 83(5), 1510–1513 Search PubMed.
- L. He, C. Haynes, F. Diez-Gonzalez and T. P. Labuza, J. Raman Spectrosc., 2010 DOI:10.1002/jrs.2880.
- M. Lin, L. He, J. Awika, L. Yang, D. R. Ledoux, H. Li and A. Mustapha, J. Food Sci., 2008, 73, T129–134 CrossRef CAS.
- Y. Wang, S. Ravindranath and J. Irudayaraj, Anal. Bioanal. Chem., 2011, 399, 1271–1278 CrossRef CAS.
- B. J. Yakes, R. J. Lipert, J. P. Bannantine and M. D. Porter, Clin. Vaccine Immunol., 2008, 15, 227–234 CrossRef CAS.
- L. G. Thygesen, M. M. Løkke, E. Micklander and S. B. Engelsen, Trends Food Sci. Technol., 2003, 14, 50–57 CrossRef CAS.
- Y. Cui, B. Ren, J.-L. Yao, R.-A. Gu and Z.-Q. Tian, J. Raman Spectrosc., 2007, 38, 896–902 CrossRef CAS.
- N. N. Yazgan, I. H. Boyaci, E. Temur, U. Tamer and A. Topcu, Talanta, 2010, 82, 631–639 Search PubMed.
- C. Song, Z. Wang, R. Zhang, J. Yang, X. Tan and Y. Cui, Biosens. Bioelectron., 2009, 25, 826–831 CrossRef CAS.
- M. H. Harpster, H. Zhang, A. K. Sankara-Warrier, B. H. Ray, T. R. Ward, J. P. Kollmar, K. T. Carron, J. O. Mecham, R. C. Corcoran, W. C. Wilson and P. A. Johnson, Biosens. Bioelectron., 2009, 25, 674–681 CrossRef CAS.
- Y. S. Huh, A. J. Lowe, A. D. Strickland, C. A. Batt and D. Erickson, J. Am. Chem. Soc., 2009, 131, 2208–2213 CrossRef CAS.
- C. C. Lin, Y. M. Yang, Y. F. Chen, T. S. Yang and H. C. Chang, Biosens. Bioelectron., 2008, 24, 178–183 CrossRef CAS.
- J. D. Driskell, K. M. Kwarta, R. J. Lipert, M. D. Porter, J. D. Neill and J. F. Ridpath, Anal. Chem., 2005, 77, 6147–6154 CrossRef CAS.
- CBS news, http://www.cbsnews.com/video/watch/?id = 7169294n&tag=contentMain;contentBody, 2010.
- S. Olsnes and A. Pihl, Biochemistry, 1973, 12, 3121–3126 CrossRef CAS.
- G. A. Balint, Toxicology, 1974, 2, 77–102 Search PubMed.
- S. M. Bradberry, K. J. Dickers, P. Rice, G. D. Griffiths and J. A. Vale, Toxicol. Rev., 2003, 22, 65–70 CrossRef CAS.
- A. Rauber and J. Heard, Vet. Hum. Toxicol., 1985, 27, 498–502 Search PubMed.
- L. He, M. Lin, H. Li and N.-J. Kim, J. Raman Spectrosc., 2009, 41, 739–744 Search PubMed.
- W. Wu, B. Walczak, D. L. Massarta, K. A. Prebbleb and I. R. Last, Anal. Chim. Acta, 1995, 315, 243–255 CrossRef CAS.
- X. X. Han, L. Chen, W. Ji, Y. Xie, B. Zhao and Y. Ozaki, Small, 2011, 7, 316–320 Search PubMed.
- A. Barhoumi, D. Zhang, F. Tam and N. J. Halas, J. Am. Chem. Soc., 2008, 130, 5523–5529 CrossRef CAS.
- T. M. Cotton, R. A. Uphaus and D. Mobius, J. Phys. Chem., 1986, 90, 6071–6073 Search PubMed.
- K. Kneipp, H. Kneipp, I. Itzkan, R. R. Dasari and M. S. Feld, Chem. Rev., 1999, 99, 2957–2976 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |